Abstract
Subtle sexual dimorphism and its perception in apparently monomorphic bird species warrant assessment of how birds identify the sex of conspecifics, particularly of prospective mates. Visual sensitivity and its potential co-variation with cryptic sexual dichromatism are still uninvestigated in most avian taxa. Using molecular sexing, reflectance spectrometry and perceptual modelling based on the sequencing of short wavelength visual pigments, we assessed the sex-specificity of coloration and colour perception in the red-tailed tropicbird Phaethon rubricauda. We also measured morphological dimorphism at a previously unstudied breeding locality for this species. Our data are in line with both physical and avian-perceived monochromatism with a potential indication of achromatic sex differences in plumage reflectance. The moderate extent of size dimorphism is consistent with reports from other Pacific breeding populations, and morphological measurements from live specimens in this study are in line with reports on museum specimens from the same sample location. Potential differences between individuals of the same sex in size and coloration warrant the assessment of sexual dimorphism in larger sample sizes of this species.
Introduction
Sexual dimorphism in avian taxa commonly co-varies with social mating systems and, also, with genetic mating system. Thus, the assessment of sex differences advances our understanding of the evolutionary mechanisms underlying sexual selection (Owens & Hartley Citation1998; Webster et al. Citation2007). Cryptic or subtle sexual dimorphism in particular, including the effects of ultra-violet colours (Andersson et al. Citation1998; Hunt et al. Citation1998), have been the focus of extensive research interest in avian species (Vorobyev et al. Citation1998; Eaton Citation2005, Citation2007; Hofmann et al. Citation2007; Murphy Citation2007; Igic et al. Citation2010) and other lineages (Stuart-Fox et al. Citation2004; Stuart-Fox et al. Citation2008; Font et al. Citation2009; Kekäläinen et al. Citation2010). Describing the extent of cryptic or subtle phenotypic dimorphism (as perceived by human observers) in monomorphic seabird taxa is central to understanding the behavioural basis of sex identification, which, if erroneous, could inflict fitness cost (Hamer et al. Citation2006; Lewis et al. Citation2006). Erroneous sex identification could for example, be costly through delaying timely pair-bonding, which can be critical for reproductive success (Ismar et al. Citation2010). Many recent analyses of gender differences in phenotypic attributes mainly focus on behavioural assessments (Lewis et al. Citation2002; Huyvaert et al. Citation2006; Matthews et al. Citation2008). Yet, presumably sex-specific behaviours may be erroneous sexing tools as shown by discrepancies between observational sexing in the field and molecular results (Daniel et al. Citation2007; Young et al. Citation2008).
The assessment of sexual dimorphism in apparently monomorphic taxa has become more feasible owing to both (1) the emergence of reliable, non-invasive sexing methods of living animals (Longmire et al. Citation1993; Griffiths et al. Citation1998), and (2) with the application of physical measures to objectively characterise phenotypic features such as coloration (Finger & Burkhardt Citation1992; Burkhardt Citation1996; Cuthill et al. Citation1999), as well as its perception by the visual system of the birds themselves (Goldsmith Citation1994; Vorobyev & Osorio Citation1998; Endler & Mielke Citation2005; Cassey et al. Citation2008, Cassey et al. Citation2010). The combination of genetic sexing methods, physical colour measurements, and sensory modelling still awaits investigation in disparate and diverse avian lineages (Avilés & Soler Citation2009; Lenouvel et al. Citation2009; Igic et al. Citation2010).
Tropicbirds (Phaethontidae) are classically treated as pelecaniform seabirds (e.g. Le Corre Citation1997; Brewer & Hertel Citation2007) due to shared morphological and behavioural characteristics (Cracraft Citation1985; Fedducia 1999). However, the phylogenetic position of tropicbirds within this order has been repeatedly questioned on the basis of both DNA hybridization and mitochondrial sequencing (Hedges & Sibley Citation1994; Kennedy & Spencer Citation2004), with emerging evidence that tropicbirds may belong to a lineage unrelated to other extant seabirds (Ericson et al. Citation2006; Morgan-Richards et al. Citation2008; Hackett et al. Citation2008).
Violet sensitivity (VS) is the pancestral state for the Aves (Hunt et al. Citation2001), whereas avian ultraviolet sensitivity (UVS) has evolved independently in several avian lineages (Ödeen & Håstad Citation2003), including oscines (e.g. Burkhardt Citation1996; Andersson Citation1999), parrots (Pearn et al. Citation2001; Hausmann et al. Citation2003), and shorebirds (Håstad et al. Citation2005, Citation2009, Ödeen et al. Citation2010). Evidence of violet-sensitive vision is now available for diverse seabirds (Ödeen & Håstad Citation2003; Hart Citation2004; Håstad et al. Citation2005; Wright & Dearborn Citation2009), yet findings of UV reflectance of plumage and integument traits also exist for various seabird species (Jouventin et al. Citation2005). Information on the phaethontid visual system is therefore still needed to verify where this group fits in this avian plumage-colour perception framework.
The Kermadec Island Group represents the sole New Zealand breeding location for the red-tailed tropicbird Phaethon rubricauda at the southern margin of its species distribution (Schreiber & Schreiber Citation1993).
Contrary to colour patterns in the congeneric white-tailed tropicbird Phaethon lepturus breeding on Ascension Islands in the Atlantic Ocean (Stonehouse Citation1962), the pink plumage suffusion reported in P. rubricauda is mostly presumed not to be sexually dichromatic (Fleet Citation1974), with contrasting reports on whether or not the conspicuousness of suffusion correlates with breeding stage (Fleet Citation1974; Gould et al. Citation1974). Yet, an intensity gradient of the pink suffusion among subspecies is reported, with the highest coloration intensity (as judged by the human eye) found in Kermadec Island Group breeding birds P. r. roseotincta (Tarburton Citation1989). The distinctness of the nominate subspecies of Northern and Southern Pacific P. rubricauda, with the Kermadec breeding P. r. roseotincta as the largest subspecies, and Eastern Pacific breeding P. r. rothschildi as the smallest, and intermediately-sized P. r. melanorhynchos, is called into question based on morphometric measurements and human perception of coloration from museum specimens, since these data were not significantly different from those of birds across similar latitudes along the cline (Tarburton Citation1989). Yet, museum skins are known to undergo shrinkage, which can affect measurements (Pitman et al. Citation1998), and coloration of plumage can be subject to fading (McNett et al. Citation2005).
Here, we assess three morphological features regarding sex differences (wing length, culmen and coloration of plumage) which are traditionally used to distinguish between the subspecies in P. rubricauda (Peters Citation1931; Tarburton Citation1989), presenting the first quantitative study of coloration and perception in a tropicbird taxon. We also provide further sex-specific morphometric information from breeding birds occupying a remote and potentially genetically isolated South-West Pacific breeding site. To objectively characterise coloration, and its perception and potential avian perceived dimorphism in the red-tailed tropicbird, we describe plumage reflectance using spectrophotometry, deploy visual modelling and present short-wavelength light receptor opsin gene sequencing for both sexes in the species.
Methods
Study site and sampling
Fourteen adult and eight nestling red-tailed tropicbirds Phaethon rubricauda were captured on their nest at their breeding sites on North Meyer Islet, Kermadec Group, New Zealand (29° 14′ S, 177° 52′ W), between 1 and 14 April 2008, as part of a research and restocking expedition to the Department of Conservation station on nearby Raoul Island. A variety of breeding stages were available, spanning from incubation to late parental care (). Morphological measurements of bill, head, tarsus, wing, tail, and, where present, tail streamers were taken (). Blood samples of 30 µL were collected from the metatarsal vein of all adult birds and six chicks, and stored in Seutin buffer (Seutin et al. Citation1991). Two nape feathers were collected from all adult birds and two large chicks by swiftly pulling with a pair of tweezers, and stored at −20 °C in darkness upon return to the laboratory at the University of Auckland. Additionally, the reflectance spectra of two recently shed tail-streamers, which were collected from two different nest sites, were recorded.
Figure 1 Red-tailed tropicbirds Phaethon rubricauda at breeding sites on North Meyer Islet, Kermadec Group, New Zealand (photos taken by SMHI, April 2008).

Table 1 Morphological measurements [mm] (mean±standard deviation) of chicks and adults, and sexual size dimorphism (SD=[xbar] male/[xbar] female *100) of adult Phaethon rubricauda breeding on North Meyer Islet, Kermadec Group, measured in April 2008.
Molecular sexing and opsin sequencing
Total genomic DNA was extracted from blood stored in Seutin buffer using standard phenol/chloroform techniques. All individuals were sexed based on genetic markers using the method outlined in Fridolfsson & Ellegren (Citation1999). Individuals were scored as female if the PCR products had both the 600 bp CHD1Z band and the 450 bp CHD1W band and as male if only the 600 bp CHD1Z band was present (Griffiths et al. Citation1998). The sexing protocol was repeated twice and confirmed the molecular assignment of sex of all individuals.
To assess ultraviolet (UVS) or violet sensitivity (VS) in the species, a 119 bp fragment of the UVS/VS opsin gene (SWS1) was amplified and sequenced for two male and two female P. rubricauda. The opsin gene was amplified using the primers SU149a and SU306b (Ödeen & Håstad Citation2003), modified to include M13-tails. PCR amplifications were carried out in 25 µL reaction volumes containing 60 mM Tris-HCl pH 8.5, 15 mM (NH4)2SO4, 2.5 mM MgCl2, 0.3 uM of each dNTP, 0.2 uM of each primer and 0.5 U of Platinum Taq polymerase (Invitrogen). Thermal cycling was conducted using an ABI 9700 following conditions outlined in Ödeen & Håstad (Citation2003). PCR products were purified using Exo/SAP treatment (Silva Jr. et al. Citation2001). Five µL of PCR product was added to 0.2 µL of Exo I (GE Healthcare), 0.1 µL Shrimp Alkaline Phosphotase (GE Healthcare) and 1.7 µL UltraPure water (Invitrogen) and incubated at 37 °C for 30 min, then at 80 °C for 15 min to inactivate the enzymes. Samples were sequenced in both directions using Big-Dye Terminator Cycle Sequencing v3.1 kit (Applied Biosystems) with M13 forward and reverse primers and analysed using an ABI 3100 automated sequencer. Sequences were edited using Chromas Pro (Technelysium Pty. Ltd.) and exported to BioEdit (Hall Citation1999) where they were aligned, translated and compared to other seabird SWS1 sequences downloaded from GenBank.
Reflectance measurement and spectral analysis
Spectral analyses were conducted using an Ocean Optics USB2000 Miniature Fiber Optic Spectrometer, connected to a portable computer, illuminated by a DT mini-lamp and OOIBase32TM operating 136 software (Ocean Optics, Inc., Dunedin, FL, USA). All measurements were taken using a probe maintained at a 90° angle. White and dark standard reflection calibration measurements were taken every four measurements using an Ocean Optics WS-1 diffuse reflectance standard and a miniature cardboard box, with black interior, used to block out all external light, respectively. Reflectance measurements were taken from the distal part of the nape and tail feathers, which would have been exposed in the birds’ plumage. A non-reflecting background across the recorded wavelengths was chosen for measurements. Feathers were not touched at any stage during collection, storage in sterile vials, or subsequent handling (again with tweezers) for spectral measurements. For measurements, the distal part of the feather was gently bundled next to the rachis using tweezers, so that a consistent surface was obtained for placing of the probe. Each recorded feather reflectance spectrogram consisted of an average of three measurements from different spots of the distal part of the feather, each in turn consisting of three technical replicates over a 1500 ms integration time for each measurement. Total brightness (sum of reflectance values R300–700 nm), UV (R300–400 nm/R300–700 nm), blue (R400–475 nm/ R300–700 nm), green (R475–550 nm/R300–700 nm), yellow (R550–625 nm/R300–700 nm) and red (R625–700 nm/R300–700 nm) relative reflectances, and chroma were calculated from each spectrogram following Honza et al. (Citation2007).
Perceptual modelling of plumage coloration
To assess the visual perception of potential sexual differences of red-tailed tropicbird plumage coloration, the model developed by Vorobyev & Osorio (Citation1998) for tetrachromatic vision was employed using Avicol v2 software (Gomez Citation2007; Doutrelant et al. Citation2008). The software requires the spectral sensitivity (300–700 nm) of the bird's own retinal photoreceptors and the irradiance spectra (300–700 nm) which describe the illumination conditions of the visual environment. Due to the lack of data on red-tailed tropicbird photoreceptor sensitivity, sensitivity data used in the analysis were those for a violet sensitive species, the rock pigeon Columba livia. Sensitivity data for the pigeon were extracted from Vorobyev & Osorio (Citation1998) using VistaMetrix v1.35.0 data extraction software (http://www.skillcrest.com/). As tropicbirds at the Kermadec Islands breed on open cliffs without a canopy, sun-exposed irradiance spectra were extracted from Stuart-Fox et al. (Citation2003) in the 300–700 nm range using VistaMetrix software. Model specific parameters were set as in Igic et al. (Citation2010).
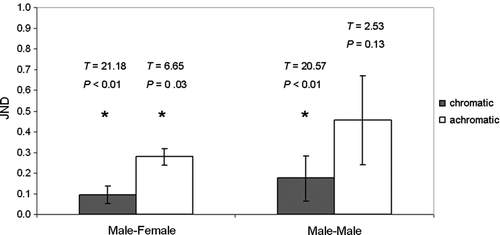
The perceptual chromatic distance between male and female spectra (ΔS) or the ‘just noticeable difference’ (JND) were extracted from this software to assess the tropicbird's ability to distinguish plumage coloration between the sexes. The software also extracts the bird's ability to distinguish plumage brightness as a JND for the achromatic contrast (ΔfQ). JND values >1 indicate that the bird is able to discriminate plumage colour differences between the reflectances of two classes of feathers, in our case male and female (Osorio & Vorobyev Citation1996; Igic et al. 2010). JND values between all possible male-male comparisons and all possible male-female comparisons were calculated for chromatic and achromatic contrast, to provide an exploratory analysis of whether sex in red-tailed tropicbirds can be identified from plumage reflection by conspecifics (). Further statistical analysis was conducted on output data from the software (see below).
Table 2 Means and medians of perceived differences between all possible male-male and male-female comparisons of red-tailed tropicbirds Phaethon rubricauda in our sample.
Statistical analyses
Sexual dimorphism indices were calculated as SD=[xbar] male/[xbar] female*100 as in Boland et al. (Citation2004) for the adult tropicbirds for both morphological and spectral measurements ().
Table 3 Chromatic and achromatic plumage reflectance in male and female tropicbirds Phaethon rubricauda breeding on the Kermadec Islands.
Female and male morphological and physical reflectance measurements were also compared using unpaired t-tests at a confidence level of α=0.05 (SigmaPlot 11.0). Furthermore, patterns of sex-specificity in brightness were assessed in a combined effect size model in JMP 7.0 to investigate the effects of sex, bird identity nested within sex and sample identity on brightness as the role variable. Sexual dichromatism in physical reflectance was tested using principal component analysis to reduce the correlated UV, blue, green, yellow and red chroma to fewer orthogonal variables. To test for sexual dichromatism using perceptual modelling, three randomly assigned male-male and male-female pairs were assessed for consistent differences in chromatic as well as achromatic components of plumage reflectance using one-sample t-tests (with random expectation of JND=1). In addition, the perceived chromatic and achromatic differences between three randomly assigned male-male and three male-female pairs were compared using a paired t-test.
Results
Morphometrics and sexual size dimorphism
Six of the sampled adult Phaethon rubricauda were sexed as female, and eight as male. Three of the six sampled nestlings were male and three were female; one chick of each sex was within the first week from hatching, while two males and two females were among older chicks starting to develop the typical white-and-black fledgling plumage (). Adult mean culmen length was larger in males than in females, with a sexual dimorphism SD=105.9 (). With our limited sample size, this P. rubricauda population appeared monomorphic in all other measurements, with the exception of head length (tip of bill to occipital protuberance: SD=106.0), which, as it includes the length of the culmen, is positively linked with culmen size. Large effect sizes (Thalheimer & Cook Citation2002) of Cohen's d>0.8 further indicated sexual dimorphism in head width, and reversed dimorphism in tail length (). In particular, wing measurements yielded SD=101.5, which, while non-significant with our small sample size, corresponds well with the direction of differences reported from other breeding populations (SD=100.9, Boland et al. Citation2004).
Opsin sequence and short-wave length sensitivity
We successfully amplified the SWS1 opsin sequence in four individuals, two males and two females, aligned, translated and compared them to SWS1 sequences of the Pelecaniformes downloaded from GenBank (). The sequences were identical for male and female tropicbird specimens (Genbank accession numbers HM212420–HM212423). Sequence length was 119 bp, translating to the 39 amino acid residue sequence VKYKKLRQPLNYILVNISFSGFMA¯CIFS¯VFT¯VFVSSSQG. Three of the key spectral tuning sites 86, 90 and 93 (Wilkie et al. Citation2000), shown underlined above, were identified by alignment with published pelecaniform sequences (Ödeen & Håstad Citation2003; Håstad et al. Citation2005; Wright & Dearborn Citation2009) and λmax values were estimated following Ödeen & Håstad (Citation2003). Our results indicate that the red-tailed tropicbird Phaethon rubricauda is violet-sensitive (VS) with λmax=406 nm.
Table 4 Translated amino acid sequence of SWS1 opsin of illustrative species of Phaethontidae (red-tailed tropicbird Phaethon rubricauda), Laridae, Columbidae, Phoenicopteridae, Accipitridae, and seabird families of the Pelecaniformes; SWS1 region shown is that which contains three residues critical in spectral tuning.
Monochromatism of plumage
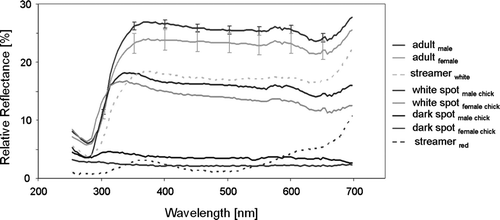
The only indication of sexual dimorphism in physical plumage reflectance () was found for brightness (P=0.065, t12=2.053; ), with males brighter than females. Brightness was also the only reflectance parameter that showed a large effect of size sensu Thalheimer & Cook Citation2002 (Cohen's d>0.8). Yet, no increase in effect significance was found in a combined model including all unaveraged brightness values for all measured feathers while controlling for bird identity as a random factor in the model (leverage psex=0.176). Red-tailed tropicbirds were found to be monochromatic, with the first two principal component axes (comparing UV against blue, green and yellow chroma; PC1 explaining 59.16%; and UV, blue, and green against yellow and red chroma; PC2 explaining 35.14%) explaining 94.3% of total variance in chroma. One-way analyses of variance of PC scores for these first two principal component axes showed no sex specific differences on either axis, respectively (P >F=0.569, P >F=0.656). Just noticeable differences for chromatic parameters of all possible pairs were<1, as were all JNDs for possible male-male achromatic comparisons; one of the six female birds sampled was distinguishable against all males (JNDs consistently >1) by achromatic parameters, but none of the other five females was distinguishable against any of the males. Both means and medians for JNDs of all four test groups (male-male chromatic and achromatic, male-female chromatic and achromatic) were <1 (). Equally, there were statistically no differences in the outputs of the avian sensory model generated for the comparison between the plumage reflectances of the two sexes in either chromatic (ΔS male-female: 0.097, male-male: 0.176) or achromatic (ΔfQ male-female: 0.279, male-male: 0.455) parameters, resulting in all JND values consistently<1 (). There were non-significant differences in perceived chromatic parameters between same-sex (male-male) and opposite-sex pairs (t2=2.26, P=0.15); similarly, differences in perceived achromatic parameters were also non-significant between same-sex and opposite-sex pairs, in line with findings from one-sampled JND analyses (t2=1.14, P=0.37).
Figure 2 Plumage spectrograms from male and female, adult and chick Phaethon rubricauda breeding on the Kermadec Islands; adult spectrograms plotted as means from eight males and six females, respectively, standard errors plotted every 50 nm; averages for white and dark spots from two feathers shown for one male and one female chick.
Discussion
Our sequencing results suggested violet sensitivity for the red-tailed tropicbird, which is in line with the relatively basal position of the phaethontid clade in the neognath avian phylogeny (Ödeen & Håstad Citation2003; Hackett et al. Citation2008). Estimates of maximum opsin sensitivity from amino acid sequences can be associated with an error of about 16 nm (Ödeen et al. Citation2009), less than the >23 nm difference between VS- and UVS single-cone opsins (Hart et al. Citation1999).
The extent and direction of sexual size-dimorphism in our sample of Kermadec Islands red-tailed tropicbirds was consistent with reports of larger males than females in this species from the Hawaiian Islands (Veit & Jones Citation2003). Overall, physical measures of feather coloration and sensory estimates of perceivable plumage variation did not show consistent sex differences in our decidedly small sample sizes, which were nonetheless representative of the total researcher-accessible breeding population on the Meyer Islets, Kermadec Group.
Even though examples of trans-oceanic migration are reported for marked individuals of this species (Le Corre et al. Citation2003), gene flow with other breeding populations may be restricted as is the case with other trans-Pacific breeding seabirds also nesting on remote islands of the North Tasman Sea (Steeves et al. Citation2005). Morphological distinction from other subspecies might be indicative of genetic divergence of the Kermadec Island breeding population, as morphometric differences have recently been found to be associated with genetic divergence in other seabird species breeding on the archipelago, including the Kermadec little shearwater Puffinus assimilis kermadecensis (Austin et al. Citation2004) and the Tasman masked booby Sula dactylatra tasmani (Steeves et al. Citation2010), thus confirming distinct subspecies status of several Kermadec, or Tasman and Southwest Pacific breeding populations.
Importantly, our measurements from live red-tailed tropicbirds of both sexes breeding on the Kermadec Islands were consistently greater than measurements from live specimens of cross-Pacific conspecifics with culmen, heads, and wings all appearing to be longer in Kermadec Island breeding birds (Veit & Jones 2003), in line with reports on comparative subspecies morphology from museum specimens (Fleet 1974; Diamond Citation1975; Tarburton Citation1989). The degree of dimorphism in culmen length (SD=105.9) may be slightly higher than that of a breeding population on North East Herald Cay, another West Pacific site (SD=102.2, Boland et al. Citation2004). As museum skins are known to undergo shrinkage, which can affect size measurements (Pitman et al. Citation1998), the confirmation of overall dimensions from living birds reported here contributes novel information for this breeding population, as does the previously unassessed sex-specific morphology for this locality.
From spectral measurement results, our sample suggests physical monomorphism in chromatic parameters of plumage reflectance in P. rubricauda. Although there was indication that males in red-tailed tropicbirds might be brighter, necessitating future assessment of a larger sample size, our perceptual modelling did not support dichromatism in that both chromatic and achromatic sensitivities fell consistently below the visual discriminability threshold of 1 JND. More extensive and, if possible, in situ measurements of reflectance are needed to assess individual, seasonal and geographic scales of variation in plumage of red-tailed tropicbirds, also providing samples for the future exploration of chemical and structural basis of plumage coloration (Shawkey et al. Citation2006). In red-tailed tropicbirds, mate behaviour, rather than physiological condition has recently been shown to trigger, for example, foraging and breeding behaviour (Sommerfeld & Hennicke Citation2010). Thus, characterising the signalling cues used for mate gender assessment by conspecifics may further the understanding of the behavioural and communication ecology this species. Future molecular analyses of mitochondrial and nuclear genetic structure will also be required to describe the population genetics and systematic status of the Kermadec breeding red-tailed tropicbirds within this conspicuous and far-ranging pelagic seabird complex.
Acknowledgements
We thank the New Zealand Department of Conservation (DoC) for research and landing permits, the New Zealand Navy for transport, the DoC crew on Raoul for their kind hospitality and technical support. We would like to acknowledge Chris Gaskin and Sandra Anderson as well as DoC volunteers for their help in the field. Craig Millar, Selina Patel, Donald Dearborn, Phill Cassey, Emma Marks, Vivian Ward, and anonymous reviewers provided helpful discussions. Funding was given by Education New Zealand (to SMHI), by a Massey University Vice-Chancellors Scholarship and a CONACyT Doctoral Scholarship, Mexico (to LOC), and the Research Committee of the University of Auckland Science Faculty and the Human Frontier Science Program (to MEH).
References
- Andersson , S . 1999 . Morphology of UV reflectance in the whistling thrush: implications for the study of structural colour signaling in birds . Journal of Avian Biology , 30 : 193 – 204 .
- Andersson , S , Örnborg , J and Andersson , M . 1998 . Ultraviolet sexual dimorphism and assortative mating in blue tits . Proceedings of the Royal Society B: Biological Sciences , 265 : 445 – 450 .
- Austin , JJ , Bretagnolle , V and Pasquet , E . 2004 . A global molecular phylogeny of the small Puffinus shearwaters and implications for systematics of the little-Audubon's shearwater complex . The Auk , 121 : 847 – 864 .
- Avilés , JM and Soler , JJ . 2009 . Nestling colouration is adjusted to parent visual performance in altricial birds . Journal of Evolutionary Biology , 22 : 376 – 386 .
- Boland , CRJ , Double , MC and Baker , GB . 2004 . Assortative mating by tail streamer length in Red-tailed Tropicbirds Phaethon rubricauda breeding in the Coral Sea . Ibis , 146 : 687 – 690 .
- Brewer , ML and Hertel , F . 2007 . Wing morphology and flight behavior of pelecaniform seabirds . Journal of Morphology , 268 : 866 – 877 .
- Burkhardt , D . 1996 . Die Ultraviolett-Tüchtigkeit des Vogelauges und einige Konsequenzen . Naturwissenschaften , 83 : 492 – 497 .
- Cassey , P , Honza , M , Grim , T and Hauber , ME . 2008 . The modeling of avian visual perception predicts behavioural rejection responses to foreign egg colours . Biology Letters , 4 : 515 – 517 .
- Cassey , P , Portugal , SJ , Maurer , G , Ewen , JG , Boulton , RL , Hauber , ME and Blackburn , TM . 2010 . Variability in avian eggshell colour: a comparative study of museum eggshells . PloS ONE , 5 : 1 – 9 .
- Cracraft , J . 1985 . Monophyly and the phylogenetic relationships of the Pelecaniformes: a numerical cladistic analysis . The Auk , 102 : 834 – 853 .
- Cuthill , IC , Bennett , ATD , Partridge , JC and Maier , EJ . 1999 . Plumage reflectance and the objective assessment of avian sexual dichromatism . American Naturalist , 153 : 183 – 200 .
- Daniel , C , Millar , CD , Ismar , SMH , Stephenson , BM and Hauber , ME . 2007 . Evaluating molecular and behavioural sexing methods for the Australasian gannet (Morus serrator) . Australian Journal of Zoology , 55 : 377 – 382 .
- Diamond , AW . 1975 . The biology of tropicbirds at Aldabra Atoll, Indian Ocean . The Auk , 92 : 16 – 39 .
- Doutrelant , C , Grégoire , A , Grnac , N , Gomez , D , Lambrechts , MM and Perret , P . 2008 . Female coloration indicates female reproductive capacity in blue tits . Journal of Evolutionary Biology , 21 : 226 – 233 .
- Eaton , MD . 2005 . Human vision fails to distinguish widespread sexual dichromatism among sexually ‘monochromatic’ birds . Proceedings of the National Academy of Sciences of the United States of America , 102 : 10942 – 10946 .
- Eaton , MD . 2007 . Avian visual perspective on plumage coloration confirms rarity of sexually monochromatic North American passerines . The Auk , 124 : 155
- Endler , JA and Mielke , PW Jr . 2005 . Comparing entire colour patterns as birds see them . Biological Journal of the Linnean Society , 86 : 405 – 431 .
- Ericson , PGP , Anderson , CL , Britton , T , Elzanowski , A , Johansson , US , Källersjö , M , Ohlson , JI , Parsons , TJ , Zuccon , D and Mayr , G . 2006 . Diversification of the Neoaves: Integration of molecular sequence data and fossils . Biology Letters , 2 : 543 – 547 .
- Feduccia A 1999 . The origin and evolution of birds , 2nd ed Yale University Press , Hew Haven, C.T .
- Finger , E and Burkhardt , D . 1992 . Avian plumage colours. Origin of UV reflection in a black parrot . Naturwissenschaften , 79 : 187 – 188 .
- Fleet , RR . 1974 . The red-tailed tropicbird on Kure Atoll . Ornithological Monographs , 16 : 1 – 64 .
- Font , E , Perez , I , De Lanuza , G and Sampedro , C . 2009 . Ultraviolet reflectance and cryptic sexual dichromatism in the ocellated lizard, Lacerta (Timon) lepida (Squamata: Lacertidae) . Biological Journal of the Linnean Society , 97 : 766 – 780 .
- Fridolfsson , AK and Ellegren , H . 1999 . A simple and universal method for molecular sexing of non-ratite birds . Avian Biology , 30 : 116 – 121 .
- Goldsmith , TH . 1994 . Ultraviolet receptors and color vision: evolutionary implications and a dissonance of paradigms . Vision Research , 34 : 1479 – 1487 .
- Gomez D 2007 . AVICOL v2, a program to analyse spectrometric data . (Available upon request from the author at [email protected] )
- Gould , PJ , King , WB and Sanger , GA . 1974 . Red-tailed tropicbird (Phaethon rubricauda). In Pelagic studies of seabirds in the Central and Eastern Pacific Ocean . Smithsonian Contributions to Zoology , 158 : 206 – 231 .
- Griffiths , R , Double , MC , Orr , K and Dawson , RJG . 1998 . A DNA test to sex most birds . Molecular Ecology , 7 : 1071 – 1075 .
- Hackett , SJ , Kimball , RT , Reddy , S , Bowie , RCK , Braun , EL , Braun , MJ , Chojnowski , JL , Cox , WA , Han , KL , Harshman , J , Huddleston , CJ , Marks , BD , Miglia , KJ , Moore , WS , Sheldon , FH , Steadman , DW , Witt , CC and Yuri , T . 2008 . A phylogenomic study of birds reveals their evolutionary history . Science , 320 : 1763 – 1767 .
- Hall , TA . 1999 . BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT . Nucleic Acids Symposium Series , 41 : 95 – 98 .
- Hamer , KC , Quillfeldt , P , Masello , JF and Fletcher , KL . 2006 . Sex differences in provisioning rules: responses of Manx shearwaters to supplementary chick feeding . Behavioral Ecology , 17 : 132 – 137 .
- Hart , NS . 2004 . Microspectrophotometry of visual pigments and oil droplets in a marine bird, the wedge-tailed shearwater Puffinus pacificus: topographic variations in photoreceptor spectral characteristics . Journal of Experimental Biology , 207 : 1229 – 1240 .
- Hart , NS , Partridge , JC and Cuthill , IC . 1999 . Visual pigments, cone oil droplets, ocular media and predicted spectral sensitivity in the domestic turkey (Meleagris gallopavo) . Vision Research , 39 : 3321 – 3328 .
- Håstad , O , Ernstdotter , E and Ödeen , A . 2005 . Ultraviolet vision and foraging in dip and plunge diving birds . Biology Letters , 1 : 306 – 309 .
- Håstad , O , Partridge , JC and Ödeen , A . 2009 . Ultraviolet photopigment sensitivity and ocular media transmittance in gulls, with an evolutionary perspective . Journal of Comparative Physiology A , 195 : 585 – 590 .
- Hausmann , F , Arnold , KE , Marshall , NJ and Owens , IPF . 2003 . Ultraviolet signals in birds are special . Proceedings of the Royal Society B , 270 : 61 – 67 .
- Hedges , SB and Sibley , CG . 1994 . Molecules vs morphology in avian evolution – the case of the pelecaniform birds . Proceedings of the National Academy of Sciences USA , 91 : 9861 – 9865 .
- Hofmann , C , Lo , W-S , Yao , C-T and Li , S-H . 2007 . Cryptic sexual dichromatism occurs across multiple types of plumage in the green-backed tit Parus monticolus . Ibis , 149 : 264 – 270 .
- Honza , M , Polačiková , L and Procházka , P . 2007 . Ultraviolet and green parts of the colour spectrum affect egg rejection in the song thrush . Biological Journal of the Linnean Society , 92 : 269 – 276 .
- Hunt , DM , Wilkie , SE , Bowmaker , JK and Poopalasundaram , S . 2001 . Vision in the ultraviolet . Cellular and Molecular Life Sciences , 58 : 1583 – 1598 .
- Hunt , S , Bennett , AT , Cuthill , IC and Griffiths , R . 1998 . Blue tits are ultraviolet tits . Proceedings of the Royal Society B: Biological Sciences , 265 : 451 – 455 .
- Huyvaert , KP , Anderson , DJ and Parker , PG . 2006 . Mate opportunity hypothesis and extrapair paternity in waved albatrosses (Phoebastria irrorata) . The Auk , 123 : 524 – 536 .
- Igic B , Leuschner N , Parker KA , Ismar SMH , Gill BJ , Lovegrove TG , Millar CD , Mark ME 2010 . Size dimorphism and avian-perceived sexual dichromatism in a New Zealand endemic bird, the Whitehead Mohoua albicilla . Journal of Morphology 271 : 697 – 704 .
- Ismar , SMH , Daniel , C , Stephenson , BM and Hauber , ME . 2010 . Mate replacement entails a fitness cost for a socially monogamous seabird . Naturwissenschaften , 97 : 109 – 113 .
- Jouventin , P , Nolan , PM , Örnborg , J and Dobson , FS . 2005 . Ultraviolet beak spots in king and emperor penguins . The Condor , 107 : 144 – 150 .
- Kekäläinen , J , Huuskonen , H , Kiviniemi , V and Taskinen , J . 2010 . Visual conditions and habitat shape the colouration of the Eurasian perch (Perca fluviatilis): a trade-off between camouflage and communication? . Biological Journal of the Linnean Society , 99 : 47 – 59 .
- Kennedy , A and Spencer , HG . 2004 . Phylogenies of the frigatebirds (Fregatidae) and tropicbirds (Phaethontidae), two divergent groups of the traditional order Pelecaniformes, inferred from mitochondrial DNA sequences . Molecular Phylogenetics and Evolution , 31 : 31 – 38 .
- Le Corre , M . 1997 . Diving depths of two tropical Pelecaniformes: the red-tailed tropicbird and the red-footed booby . The Condor , 99 : 1004 – 1007 .
- Le Corre , M , Salamolard , M and Portier , MC . 2003 . Transoceanic dispersion of the red-tailed tropicbird in the Indian Ocean . Emu , 103 : 183 – 184 .
- Lenouvel , P , Gomez , D , Théry , M and Kreutzer , M . 2009 . Do grooming behaviours affect visual properties of feathers in male domestic canaries, Serinus canaria? . Animal Behaviour , 77 : 1253 – 1260 .
- Lewis , S , Benvenuti , S , Dall'Antonia , L , Griffiths , R , Money , L , Sherratt , TN , Wanless , S and Hamer , KC . 2002 . Sex-specific foraging behaviour in a monomorphic seabird . Proceedings of the Royal Society B , 269 : 1687 – 1693 .
- Lewis , S , Wanless , S , Elston , DA , Schultz , MD , Mackley , E , du Toit , M , Underhill , JG and Harris , MP . 2006 . Determinants of quality in a long-lived colonial species . Journal of Animal Ecology , 75 : 1304 – 1312 .
- Longmire , JL , Maltbie , M , Pavelka , RW , Smith , LM , Witte , SM , Ryder , OA , Ellsworth , DL and Baker , RJ . 1993 . Gender identification in birds using microsatellite DNA fingerprint analysis . The Auk , 110 : 378 – 381 .
- Matthews , JL , Ismar , SMH and Hauber , ME . 2008 . Seaweed provisioning behaviour confers thermal benefit for nesting Australasian gannets (Morus serrator) . Behaviour , 145 : 1823 – 1837 .
- McNett , GD , Marchetti , K and Zink , RM . 2005 . Ultraviolet degradation in carotenoid patches: Live versus museum specimens of wood warblers (Parulidae) . Auk , 122 : 793 – 802 .
- Morgan-Richards , M , Trewick , SA , Bartosch-Härlid , A , Kardailsky , O , Phillips , MJ , McLenachan , PA and Penny , D . 2008 . Bird evolution: testing the Metaves clade with six new mitochondrial genomes . Evolutionary Biology , 8 : 20
- Murphy , MT . 2007 . A cautionary tale: cryptic sexual size dimorphism in a socially monogamous passerine . The Auk , 124 : 515 – 525 .
- Ödeen , A , Hart , NS and Håstad , O . 2009 . Assessing the use of genomic DNA as a predictor of the absorbance wavelength of avian SWS1 opsin visual pigments . Journal of Comparative Physiology A: Neuroethology Sensory Neural and Behavioral Physiology , 195 : 167 – 173 .
- Ödeen , A and Håstad , O . 2003 . Complex distribution of avian color vision systems revealed by sequencing the SWS1 opsin from total DNA . Molecular Biology and Evolution , 20 : 855 – 861 .
- Ödeen , A , Håstad , O and Alström , P . 2010 . Evolution of ultraviolet vision in shorebirds (Charadriiformes) . Biology Letters , 6 : 370 – 374 .
- Osorio , D and Vorobyev , M. 1996 . Colour vision as an adaptation to frugivory in primates . Proceedings of the Royal Society B: Biological Sciences , 263 : 593 – 599 .
- Owens , IPF and Hartley , IR . 1998 . Sexual dimorphism in birds: why are there so many different forms of dimorphism? . Proceedings of the Royal Society B , 265 : 397 – 407 .
- Pearn , SM , Bennett , ATD and Cuthill , IC . 2001 . Ultraviolet vision, fluorescence and mate choice in a parrot, the budgerigar Melopsittacus undulates . Proceedings of the Royal Society B , 268 : 2273 – 2279 .
- Peters JL 1931 . Checklist of birds of the world . Vol. 1 . Harvard University Press , Cambridge, MA .
- Pitman , RL and Jehl , JR Jr . 1998 . Geographic variation and reassessment of species limits in the “masked” boobies of the eastern Pacific Ocean . The Wilson Bulletin , 110 : 155 – 170 .
- Schreiber EA , Schreiber RW 1993 . Red-tailed tropicbird (Phaethon rubricauda) . In: Poole A The birds of North America Online . Cornell Lab of Ornithology , Ithaca .
- Seutin , G , White , BN and Boag , PT . 1991 . Preservation of avian blood and tissue samples for DNA analyses . Canadian Journal of Zoology , 69 : 82 – 90 .
- Shawkey , MD , Hauber , ME , Estep , LK and Hill , GE . 2006 . Evolutionary transitions and mechanisms of matte and iridescent plumage coloration in grackles and allies (Icteridae) . Journal of the Royal Society Interface , 3 : 777 – 786 .
- Silva , WA Jr , Costa , MCR , Valente , V , de Freitas Sousa , J , Paçó-Larson , ML , Espreafico , EM , Camargo , SS , Monteiro , E , de Jesus Holanda , A , Zago , MA , Simpson , AJG and Neto , ED . 2001 . PCR template preparation for capillary DNA sequencing . BioTechniques , 30 : 537 – 542 .
- Sommerfeld , J and Hennicke , J . 2010 . Comparison of trip duration, activity pattern and diving behaviour between incubating and chick-rearing red-tailed tropicbirds (Phaethon rubricauda) . Emu , 110 : 78 – 86 .
- Steeves , TE , Anderson , DJ and Friesen , VL . 2005 . A role for nonphysical barriers to gene flow in the diversification of a highly vagile seabird, the masked booby (Sula dactylatra) . Molecular Ecology , 14 : 3877 – 3887 .
- Steeves , T , Holdaway , RN , Hale , ML , McLay , E , McAllan , IAW , Christian , M , Hauber , ME and Bunce , M . 2010 . Merging ancient and modern DNA: extinct seabird taxon rediscovered in the North Tasman Sea . Biology Letters , 6 : 94 – 97 .
- Stonehouse , B . 1962 . The tropic birds (genus Phaethon) of Ascension Island . Ibis , 103 : 124 – 161 .
- Stuart-Fox , DM , Moussalli , A , Johnston , GR and Owens , IPF . 2004 . Evolution of colour variation in dragon lizards: quantitative tests of the role of crypsis and local adaptation . Evolution , 58 : 1549 – 1559 .
- Stuart-Fox , D , Moussalli , A , Marshall , JN and Owens , IPF . 2003 . Conspicuous males suffer higher predation risk: visual modelling and experimental evidence from lizards Animal Behaviour , 66 : 541 – 550 .
- Stuart-Fox , D , Moussalli , A and Whiting , MJ . 2008 . Predator-specific camouflage in chameleons . Biology Letters , 4 : 326 – 329 .
- Tarburton , MK . 1989 . Subspeciation in the red-tailed tropicbird . Notornis , 36 : 39 – 49 .
- Thalheimer W , Cook S 2002 . How to calculate effect sizes from published research: A simplified methodology . http://work-learning.com/effect_sizes.htm (accessed 11 August 2010) .
- Veit , AC and Jones , IL . 2003 . Function of tail streamers of red-tailed tropicbirds (Phaethon rubricauda) as inferred from patterns of variation . The Auk , 120 : 1033 – 1043 .
- Vorobyev , M and Osorio , D . 1998 . Receptor noise as a determinant of colour thresholds . Proceedings of the Royal Society B , 265 : 351 – 358 .
- Vorobyev , M , Osorio , D , Bennett , ATD , Marshall , NJ and Cuthill , IC . 1998 . Tetrachromacy, oil droplets and bird plumage colours . Journal of Comparative Physiology A , 183 : 621 – 633 .
- Webster , MS , Tarvin , KA , Tuttle , EM and Pruett-Jones , S . 2007 . Promiscuity drives sexual selection in a socially monogamous bird . Evolution , 61 : 2205 – 2211 .
- Wilkie , SE , Robinson , PR , Cronin , TW , Poopalasundaram , S , Bowmaker , JK and Hunt , DM . 2000 . Spectral tuning of avian violet- and ultraviolet-sensitive visual pigments . Biochemistry , 39 : 7895 – 7901 .
- Wright , SG and Dearborn , DC . 2009 . Male ornament variation in a sexually dimorphic seabird with variable mating success . Evolutionary Ecology Research , 11 : 759 – 770 .
- Young , LC , Zaun , BJ and VanderWerf , EA . 2008 . Successful same-sex pairing in Laysan albatross . Biology Letters , 4 : 323 – 325 .