Abstract
Ayersacarus woodi sp. n. (Acari: Leptolaelapidae) is described from Westland petrel (Procellaria westlandica Falla) burrows at Punakaiki, South Island, New Zealand. The genus has previously been reported only from sub-Antarctic islands. The new species is most like A. plumapilus Hunter (type species) but differs from it in females in the size and shape of the epigynal shield; much larger metapodal shields; and a wider than long anal shield with lateral pores. The ecology of the new species was explored using stable isotope (13C/12C; 15N/14N) analysis of females alongside contextual data from the site. From the 13C/12C results, the new species is dependent on marine C from petrels rather than terrestrial photosynthetic C. In terms of trophic level, the isotopic data are consistent with consumption of guano decomposers or their eggs.
Introduction
Many invertebrates, including mites, have been described from the burrows and surface nests of seabirds, but rarely are ecological parameters included in the taxonomic descriptions. Seabirds vector marine nutrients into terrestrial ecosystems (Polis et al. Citation2004) through their breeding and roosting sites, so understanding the ecology of associated invertebrates is fundamental to understanding this marine–terrestrial interface.
The direct observation of trophic interactions within soil and biogenic litter is difficult. However, the successful application of stable isotope analysis (typically carbon, C, and nitrogen, N isotopes; 13C/12C and 15N/14N: Scheu Citation2002; Pollierer et al. Citation2009) to soil biology over the past decade now offers a way to include ecological information with taxonomic descriptions. In most applications, 13C/12C indicates C source and 15N/14N indicates trophic level (Scheu Citation2002).
The genus Ayersacarus was established to hold two species; A. plumapilus Hunter, 1964 (type species) and A. gressitti Hunter 1964 (Hunter Citation1964a). Ayersacarus gelidus Hunter 1964 and A. strandtmanni Hunter 1964 were added later (Hunter Citation1964b). Those species were collected from Campbell I., Auckland I. and Macquarie I. in Zealandia's sub-Antarctic; A.tilbrooki Hunter, Citation1967 was added later from South Georgia, with a change to the generic diagnosis (Hunter Citation1967). All species were collected near procellarid or other seabird burrows or nests. Hunter (Citation1964a) originally assigned Ayersacarus to the Laelapidae. Spain & Luxton (Citation1971) placed the genus in the Dermanyssidae. Karg (Citation1978) moved Ayersacarus and three other genera to a new sub-family, the Leptolaelapinae: Macrochelidae: Eviphidoidea and in 1983 he added more genera to the subfamily and elevated it to family status, Leptolaelapidae (Karg Citation1983). He added two new further genera Pulchraplaga and Indutolaelaps from New Caledonia (Karg Citation1997). Lindquist et al. (Citation2009) did not accept the Leptolaelapidae as a valid family and consider that the familial position of Ayersacarus is unresolved. The later authors diagnose Pachylaelapidae as having one anterolateral setae on tibia III and genua and tibia IV. Ayersacarus has one anterolateral on tibiae III but two anterolateral setae on both the genua and tibia of leg IV; therefore Ayersacarus passes better in the alternate option of their key's couplet 52 but fails to run further in their key to families.
In this paper, we report a new species of Ayersacarus collected from a Westland petrel (Procellaria westlandica Falla, 1946) burrow on the South Island West Coast, bringing the genus from the sub-Antarctic to mainland New Zealand. We then use stable isotopes to test the hypothesis that Ayersacarus harvests marine rather than terrestrial C and N in the burrows of its host.
Methods
About 1 L of forest litter and soil was collected by hand from the floor of a Westland petrel burrow into a plastic box on 5 January 2010, immediately after the end of the petrel breeding season (Heather & Robertson Citation2005). Access to the burrow floor was provided by a plastic screw-top inspection lid that allowed collection from near what had been an incubation chamber. These inspection holes had been inserted by previous workers on the biology of the birds. The burrow floor litter and soil were removed to a laboratory where the mites were collected by hand or separated from soil with a Tullgren funnel; then cleared in Nesbitt's fluid and mounted in Hoyer's medium for initial study. More detailed studies were made by dissecting the dorsal shield; legs III and IV and chelicerae from specimens before slide mounting them in Hoyer's media and viewing under phase contrast optics. Type series are slide mounted in Hoyer's gum chloral.
Drawings were made with the aid of a camera lucida. The new species was compared with the holotypes of Ayersacarus plumapilus and A. gressitti. Idiosomal setal names follow Lindquist & Evans (Citation1965) and dorsal shield poroidotaxy follow Johnston & Moraza (Citation1991) and Moraza (Citation2004). All measurements are in micrometers (µm); s=standard deviation where n>2; n=sample size; brackets hold (range of data). Leg chaetotaxy follows Evans (Citation1963).
For stable isotope analysis, mites were confined to glass vials and starved to eliminate gut material that could influence the isotopic signatures. Eight ‘large’ (mean dry weight, 130 µg) and 15 ‘small’ (mean dry weight, 99 µg) females were sent for analysis. Individual animals were too small for analysis, so animals were pooled for duplicate analysis. For the large animals, each duplicate consisted of four different pooled individuals; for the small animals, the two duplicates consisted of seven and eight individuals, respectively. Other burrow material submitted for isotopic analysis included sieved (0.6 mm mesh) burrow soil, tree fern (Cyathea spp.) and supplejack (Ripogonum scandens) leaf fragments from the burrow litter, and a fragment of petrel egg membrane. Isotopic data for Westland petrel guano and other contextual material came from previous research at the site (Hawke Citation2005; Hawke & Holdaway Citation2005). Stable isotope ratios were measured on finely ground material using a DeltaPlus (Thermo-Finnigan, Bremen, Germany) continuous flow isotope ratio mass spectrometer at the NIWA Stable Isotope Laboratory (Wellington, New Zealand). The isotope ratios were calculated as a per mil (‰) deviation from the international limestone standard VPDB (13C/12C, reported as δ13C) and atmospheric N (δ15N) using DL leucine as secondary standard. The standard deviations of repeated analyses of secondary standard were 0.16‰ (δ13C) and 0.15‰ (δ15N).
Systematics
Ayersacarus woodi sp. n. Clark
Figs. 1–37
DIAGNOSIS: Large rufous mite. All dorsal shield setae pilose. Tibia II with 10 setae (2,3/1,2/1,1); tibia and genua III each with 9 setae (1,2/1,2/1,2 and 2,2/1,2/1,1); genua IV with 9 (2,2/0,3/1,1). Males variable in size and leg armature; larger males with larger leg apophyses and with ventral apophyses on tarsus II and IV. Spermatodactyl simple, hinged to movable digit; at least twice length of movable digit. Epigynal shield flask shaped, angular rather than rounded behind coxa IV; female with large metapodal shield, much longer (260–320) than wide (100–120); with scale-like pattern bearing one pilose setae and two poroids. Anal shield wider than long with glandular pores laterally. Exopodal shield large encircling coxa IV and fused to peritremal shield.
FEMALE (Figs. 1–10, 26–34): Holotype female length of dorsal shield x width 1160 x 710. Smaller females pale brown with cerotegument covered in detritus on ventral plates and posterior dorsum. Larger females rufous with mostly clean cuticles.
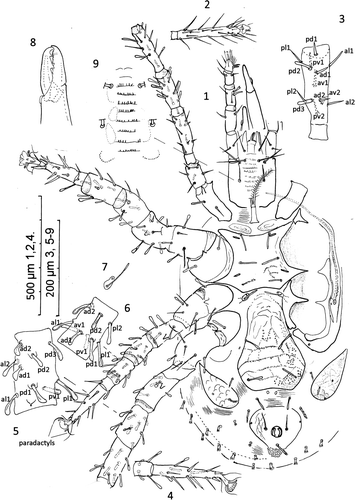
Figures. 1–9 Ayersacarus woodi sp. n. 1, female venter; 2, ventral view of tarsus I; 3, dorsal view left femur leg I; 4, tarsus IV, ventral view; 5, genua IV, dorsal view; 6, left tibia III, dorsal view showing shape of the 9 setae; 7, metasternal shield; 8, chelicerae, medial view; 9, another paratype female with 8 rows of deutosternal denticles.
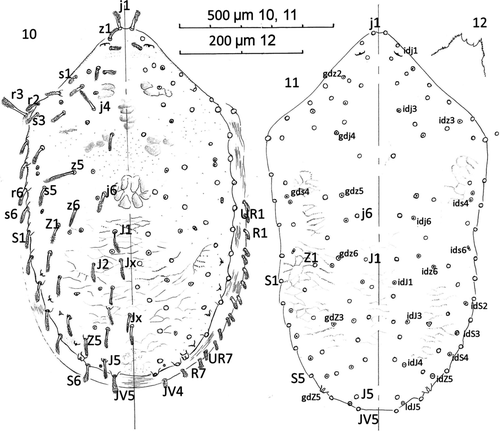
Dorsum (Figs. 10–11): Holodorsal shield with anterior drawn to right angled blunt point. Shield length mean 1177 (1010–1300), s=97, n=9 and with mean width of 700 (650–780), s=42, n=9. Shield length:width ratio 1.68 bearing 23 pairs of pilose setae in the following series; j1-6, z1-6, s1-6, r2-6; and 17 pairs in the following series; J1-5, Z1-5, S1-6, JV5, and five unpaired seta as Jx between the Jseries – two inline with J2, and three between J3 and J4. Post-lateral R1-7 series on striate cuticle with UR1-7, both series as short plumose setae 50 long. Three longer setae, j4, z5 and r3 (humeral) each c. 100 long; r3 set in soft striate cuticle; rest of dorsal setae 70–85 long. Shield (Fig. 11) with about 22 pairs of pore-like structures, lyrifissures or glands opening as illustrated and labelled: gdz2, gdj4, gdz5, gds4, gdz6, gdZ3 and gdZ5 as seven glands and idj1, idj3, idz3, ids4, idj6, ids6, idz6, idJ1, idS2, idJ3, idS3, idJ4, idS4, idJ5 and idZ5 as fifteen poroids/lyrifissures. Poroids not always paired or bilaterally symmetrical in position. Sigilla pattern as illustrated. Peritremal shield fused posteriorly to exopodals and narrowly fused to dorsal shield only anterior to coxa I: peritreme finishing near z1.
Figures 10–12 Ayersacarus woodi sp. n. 10, Female; dorsal shield; 11, large male dorsal shield; 12, male epistome.
Gnathosoma: Palp chaetotaxy of trochanter – tibia, 2, 5, 6, 9. Apotele two tined, tines 45 and 35 long. Deutosternum with 8 rows of teeth with 10–15 teeth per row as illustrated (Fig. 9). Corniculi long, gracile. Tritosternum with rectangle base, bipartite, pilose. Epistome toothed with two central concavities (Figs. 32–34). Chelicerae fixed digit with c. 12 small distomedial teeth arranged in arc; pilus dentilus short, flagelliform. Movable digit as illustrated (Fig. 8).
Venter: Pair of weakly thickened pre-sternal shields; sternal shield 243 long (230–250), s=10, x 205 (200–230) wide, s=10; n=9, at narrowest width between coxa II bearing 3 pairs of setiform seta (st1–3) and 2 pairs of lyrifissures. Holotype sternal setae st1, setiform 87 long; st2, flattened spooned tip 77 long; st3, flattened, spooned tip 77 long. The small meta-sternal plates bear a blunt-ended seta and a pore. Epigynal shield flask shaped and angular rather than rounded posterior (Fig. 1) with mid-sagittal length from posterior of sternum 433 long (390–460), s=23, n=7; with a concave to straight posterior margin, narrowest c. 200 at level of genital setae (gv1) and widest at 294 (270–340), s=24, n=8; never more than one egg within idiosoma. Genital setae flattened as illustrated. Metapodal shields large; 276 long (260–320), s=19, n=9, and 111 wide (100–120), s=9.2, n=9 bearing 1 pilose seta and two pore-like structures. Anal shield 212 long (180–250), s=21, n=10 and 233 wide (210–280), s=23, n=10; thus wider than long bearing simple paranal setae and pilose postanal seta; with lateral apexes capturing glandular pore gv3.
Legs: Legs I and III thin; all legs with claws; pulvilli II–IV with simple paradactyls and rounded pads. Lengths of I–IV inclusive of coxa and pretarsus: I, 1211 (1018–1300), s=99, n=6; II, 1031 (1000–1200), s=74, n=7; III, 944 (850–1000), s=60, n=7; IV, 1350 (1300–1450), s=57, n=7. Many leg setae with expanded, flattened, rounded and dished tips (spooned/spathulate) as illustrated (Fig. 1). Claws on leg I reduced. Chaetotaxy: Trochanter, femur, genua, tibia and tarsus (except tarsus I) as follows: Leg I: 1,1/3,1=6; 2,2/2,3/2,2=13; 2,6/3,2=13; 2,6/3,2=13. Leg II: 1,0/3,1=5; 2,5/3,1=11; 2,5/2,2=11; 2,3/1,2/1,1=10; 3,3/2,1/1,3/2,3=18. Leg III: 1,0/3,1=5; 2,1/2,1=6; 2,2/1,2/1,1=9; 1,2/1,2/1,2=9; 3,3/2,1/1,3/2,3=18. Leg IV: 1,0/3,1=5; 1,3/1,1=6; 2,2/0,3/1,1=9; 2,2/1,2/1,2=10; 3,3/2,1/1,3/2,3=18. On Tarsus IV, pl2 inserted on a level with dorsal lyrifissure and md inserted on level with av2 and pv2 (Fig. 37).
MALE (Figs. 13–25): Smaller males lighter colour and more gracile than female; second leg inflated, bearing apophyses on femur, genua and tibia; larger males with apophyses on tarsus II and IV. Males show much variation in body size and leg apophysis development. All males with latero-central hinged spermatodactyl more than twice the length of the movable digit.
Gnathosoma: Corniculi long, gracile: lacina fringed, normal (Figs. 15, 16); long salivary stylets seen only in dissected material; 8 rows of deutosternal teeth in all 5 males examined (Fig 15). Reticulated pattern on sub-capitulum. Spermatodactyl evenly tapered and curved 170 long thus more than twice the length of movable digit, with tip reaching junction of second and third cheliceral digits when folded. Chelicerae arthroidial membrane smooth, without ornaments or brushes. Tritosternum bipartite and pilose as figured.
Dorsum (Fig. 11): Dorsal shield 1008 (970–1230) s=122, n=6; width 635 (540–750), s=90, n=6. Dorsal shield slightly constricted in width at level of seta J1. Dorsal setae as in the female.
Venter (Figs. 13–21): Holoventral shield with ten pairs of setae, 3 pairs pilose. Paranal setae are simple and postanal pilose. Pre-sternal shields weakly thickened; holoventral shield reticulated throughout; genital opening pre-sternal. Peritremal shield fused to holoventral.
Figures. 13–21 Ayersacarus woodi sp. n. Male. 13, ventral view; 14, tibia I lateral view of alveoli positions; 15, subcapitulum, ventral view; 16, chelicerae and spermatodactyl; 17, distal tarsus IV spines and pre-tarsus paradactyls; 18, pre-tarsus and distal tarsal III spines, ventral view; 19, genua [2,2/1,2/1,1], tibia [1,2/1,2/1,2] and basi-tarsus leg III, ventral view; 20, inset of leg apophyses and companion setae; 21, pre-tarsus and distal tarsus of leg II, ventral view.
![Figures. 13–21 Ayersacarus woodi sp. n. Male. 13, ventral view; 14, tibia I lateral view of alveoli positions; 15, subcapitulum, ventral view; 16, chelicerae and spermatodactyl; 17, distal tarsus IV spines and pre-tarsus paradactyls; 18, pre-tarsus and distal tarsal III spines, ventral view; 19, genua [2,2/1,2/1,1], tibia [1,2/1,2/1,2] and basi-tarsus leg III, ventral view; 20, inset of leg apophyses and companion setae; 21, pre-tarsus and distal tarsus of leg II, ventral view.](/cms/asset/d11459e1-e9ec-4ee3-91ea-022013daa900/tnzz_a_525746_o_f0003g.gif)
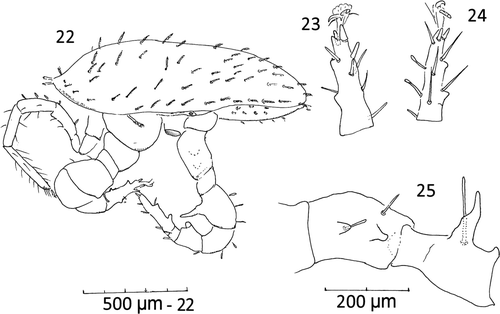
Legs: Legs I and III thin; all legs with claws, smaller on I and pulvilli II–IV with simple paradactyls and rounded pads (as for females). Many setae thickened and spooned terminally; tarsus II with av1, pv1, short spines; all other setae thickened or spooned except setiform ad1 and pd1. Leg I with small medio-distal spine/apophysis on femur; leg II with large and small apophysis on femur and one apophysis on both the genua and tibia. Leg III with apophysis on femur. Leg IV with two prominent medial apophyses on trochanter and a larger anterio-lateral thumb-like apophysis at telofemur base 150 long; two small apophyses on tibia (Figs. 13, 22). Most apophyses with companion spooned setae. Length of legs I–IV incl. pretarsus; I, 1220 (1010–1450), s=237, n=4; II, 1030 (830–1230), n=2; III, 783 (730–850), s=61, n=3; IV, 1062 (1010–1200), s=92, n=4. Larger males have 3 or 4 apophyses on the venter of tarsus II and IV (Figs. 22–25). Leg chaetotaxy as for females.
Figures 22–25 Male, large. 22, idiosomal, lateral view showing leg apophyses; (leg III and many setae removed); 23, tarsus II lateral view showing apophyses (many setae removed); 24, tarsus IV showing apophyses, medial view; 25, trochanter and femur IV ( most setae removed) medial view.
DEUTONYMPH: Yellow. Dorsal shield length 766 (700–850), s=48, n=6; width (at level of r3), 475 (450–480), s=39, n=6 with lateral incisions; shield tapering posteriorly and bearing reticulate pattern. Setae and pore/gland pattern as for female but with setae shorter. Leg chaetotaxy as for female but setae shorter and less distinctly spooned. Leg lengths I–IV; I, 896 (850–930), s=34, n=6; II, 690 (650–760), s=41, n=6; III, 671 (620–700), s=30, n=6; IV, 960 (940–990), s=21, n=6.
PROTONYMPH AND LARVA: Unknown
ETYMOLOGY: The species is named after the New Zealand Department of Conservation (DoC) Biodiversity Ranger GC Wood, longstanding manager involved with Westland petrels in Paparoa National Park.
MATERIAL COLLECTED: All mites were collected on 5 January 2010 from the floor of a vacant burrow in a Westland petrel (Procellaria westlandica) colony at Punakaiki (South Island, New Zealand; 42° 08′S, 171° 20′E) by JM Clark and DJ Hawke. Type series are slide mounted in Hoyer's gum chloral; 4 males, 12 females and 10 deutonymphs. Holotype female, paratype males (large and small) and paratype deutonymphs deposited at Canterbury Museum, Rolleston Avenue, Christchurch 8013. Paratype males, females and deutonymphs to be lodged with the Australian National Insect Collection, CSIRO, Canberra, Australia.
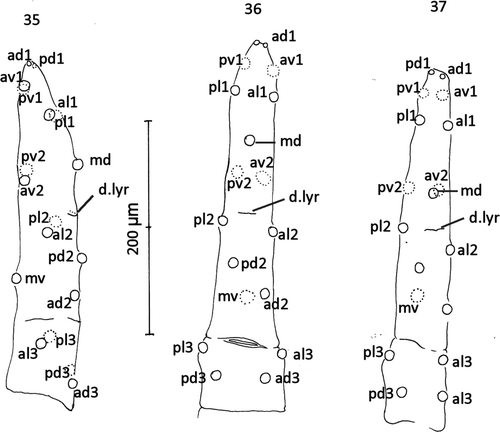
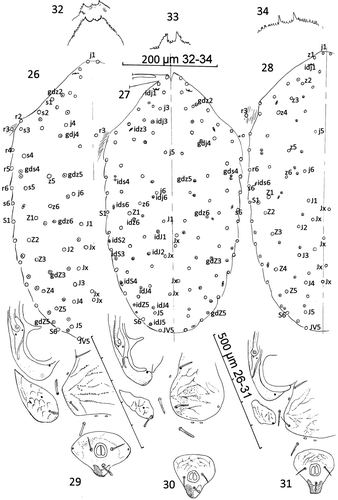
DIFFERENTIAL DIAGNOSIS (Figs. 26–34): The new species resembles A. plumapilus but differs from it in the size and shape of the epigynal shield and the much larger metapodal shield. The relative position of the individual poroids differs between A. gressitti, A. plumapilus and the new species (Figs. 26–28). In the new species, the peritremal shield is fused to the exopodals while in A. gelidus, A. gressitti, A. strandtmanni and A. tilbrooki it is not. In A. gressitti, seta md of tarsus IV is inserted about midway between av2 and al1 but at about the level of av2 and pv2 in A. plumapilus and A. woodi (Figs. 35–37). Costa (Citation1975) pointed out the characters shared by Ayersacarus, Hunteracarus and Stevacarus as more than 6 deutosternal grooves with more than 6 ridges of teeth; paradactyls on pre-tarsi II–IV; similar spermatodactly form; apophyses on femur II and lesser apophyses on genua and tibia II, and bearing 8 setae on genua III. However, the new species and the holotypes of A. gressitti and A. plumapilus have 9 setae on both genua III [2,2/1,2/1,1] and tibia III [1,2/1,2/1,2]. Therefore leg chaetotaxy (I–IV) of Ayersacarus; I, 6,13,13,13,(-). II, 5,11,11,10,18. III, 6,5,9,9,18. IV, 5,6,9,10,18.
Figures. 26–34 Ayersacarus woodi sp. n. compared with females of two sub-Antarctic species. 26, Ayersacarus woodi sp. n. dorsal shield setae (open circles) and poriods (dotted circles) compared to 27, A. plumapilus holotype and 28, A. gressitti holotype; 29, Ayersacarus woodi sp. n. post-ventral morphology; 30, A. plumapilus post-ventral morphology; 31, A. gressitti post-ventral morphology; 32, Ayersacarus woodi sp. n. epistome of two female paratypes; 33, A. plumapilus, holotype epistome; 34, A. gressitti, holotype epistome. Note: for Figs. 29–31, distances between shields not diagnostic as the shields are set in soft striate cuticle. The shield shapes and sizes are diagnostic.
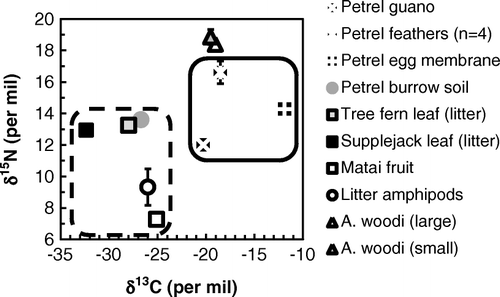
Results
The δ13C values of the A. woodi samples lay within the range of Westland petrel-derived materials, and well above the values for terrestrial materials (Fig. 38). Values were c. 1‰ higher than bulk fresh guano, and ≤1‰ lower than Westland petrel feathers. Egg membrane δ13C was 7‰ higher, but burrow soil δ13C lay within the range of other terrestrial material from the site. Ayersacarus woodi δ15N values were higher than any of the materials collected.
The pooled duplicates for the small individuals had a more negative δ13C than the large individuals, although the difference was small (0.5‰) and only just significant (t=4.707, df=2, P=0.0423). Differences in δ15N were not significant.
Figure 38 Isotope data for Ayersacarus woodi sp. n. and data for other components of the terrestrial system occupied by Westland petrels; terrestrial C sources are shown in the dashed box, and marine C sources are shown in the solid box. Isotopic values for fresh Westland petrel guano are from Hawke (Citation2005); data for terrestrial amphipods (Talitridae), matai (Prumnopitys taxifolia) fruit and Westland petrel feathers are from Hawke & Holdaway (Citation2005). Error bars (where shown) are±1SD; some are smaller than symbol size.
Discussion
Ayersacarus is not confined to the sub-Antarctic region. Its presence on both sub-Antarctic seabird islands and mainland New Zealand opens up the possibility that its range includes the many other temperate seabird breeding sites. Seabird islands around the New Zealand coast, the breeding area for Hutton's shearwater (Puffinus huttoni Matthews, 1912) in the Seaward Kaikoura Range, and the remnant mainland breeding sites for grey-faced petrel (Pterodroma macroptera gouldi Hutton 1869), sooty shearwater (Puffinus griseus Gmelin, 1789) and other procellariids all provide potential habitat for Ayersacarus.
The δ13C of a soil animal can be used to infer C source because C isotopes fractionate only slightly through a food web (Scheu Citation2002). Conversely, comparative values of δ15N indicate relative trophic level because of the broadly predictable trophic fractionation of c. 3‰ (Post Citation2002). As explained elsewhere (Hawke Citation2004; Hawke & Holdaway Citation2005), N within the Westland petrel colony is predominantly marine but soil and vegetation C are from terrestrial photosynthesis. Our results show that even burrow soil C is terrestrial, emphasising the labile nature of C from seabird discards. From its δ13C, A. woodi is clearly dependent on marine C. This contrasts with forest litter amphipods from the site, which show terrestrial levels of δ13C driven by terrestrial photosynthetic (not marine) C input.
On the basis of the materials we tested, Westland petrel guano is the most likely food source for A. woodi. The δ13C of A. woodi is slightly higher than guano, and their δ15N is 6-7‰ or two trophic levels (Post Citation2002) higher. Guano is also likely to be abundant, and so is a realistic potential food source. From an isotopic perspective, A. woodi could be consuming any life-history stage of invertebrates (arthropods or nematodes) feeding directly on the guano.
Another possibility is that A. woodi preys on petrel parasites such as fleas, ticks, lice or mites, but we were not able to directly test this hypothesis because no parasites were recovered from burrow soil. The only published study of the isotopic enrichment of petrel parasites in relation to their hosts is the study of Gómez-Díaz & González-Solís (Citation2010) involving Calonectris shearwaters. Most Calonectris parasites have δ13C similar to host feathers, while feather parasite δ15N is typically 3–4‰ higher (Gómez-Díaz & González-Solís Citation2010) as expected (Post Citation2002). Because A. woodi is itself <3‰ higher in δ15N than Westland petrel feathers, A. woodi is unlikely to depend on feather parasites. Although Westland petrel haematophages may play a role in A. woodi diet, we found none in burrow soil, and none have been reported from Westland petrels (Murray et al. Citation1990).
We conclude that Ayersacarus is not confined to the sub-Antarctic as previous taxonomic descriptions implied, and that A. woodi depends on petrel material for its nutrition. The isotopic results are consistent with predation of petrel guano-dependent invertebrates, and inconsistent with consumption of Westland petrel feather parasites. Determining whether the sub-Antarctic Ayersacarus also depend on seabird material would be most interesting, and would help in understanding the evolutionary pathway undertaken by the genus. The value of stable isotopic analysis is recommended to other taxonomists with an interest in soil biology.
Acknowledgements
We thank two anonymous referees for valuable comments that greatly improved the paper. This research was carried out under permit WC-26497-RES from the West Coast Conservancy, New Zealand Department of Conservation. Ricardo Palma (Museum of New Zealand – Te Papa Tongarewa) organised the loan of the holotypes of A. plumapilus and A. gressitti.
References
- Costa , M . 1975 . Hunteracarus womersleyi gen. n., sp. n., a laelapid mite (Acari) associated with Cephalodesmius armiger Westwood (Coleoptera: Scarabaeidae) . Journal of the Australian Entomological Society , 14 : 263 – 269 .
- Evans , GO . 1963 . Observations on the chaetotaxy of the legs in the free-living Gamasina (Acari: Mesostigmata) . Bulletin of the British Museum (Natural History) Zoology , 10 : 275 – 303 .
- Gómez-Díaz , E and González-Solís , J . 2010 . Trophic structure in a seabird host-parasite food web: insights from stable isotope analyses . PLoS ONE , 5 : e10454
- Hawke , DJ . 2004 . Maximum possible age of a petrel breeding colony near Punakaiki (South Island, New Zealand) from radiocarbon and stable isotope analysis of soil . Journal of the Royal Society of New Zealand , 34 : 1 – 7 .
- Hawke , DJ . 2005 . Soil P in a forested seabird colony: inventories, parent material contributions, and N:P stoichiometry . Australian Journal of Soil Research , 43 : 957 – 962 .
- Hawke , DJ and Holdaway , RN . 2005 . Avian assimilation and dispersal of carbon and nitrogen brought ashore by breeding Westland petrels Procellaria westlandica: a stable isotope study . Journal of Zoology , 266 : 419 – 426 .
- Heather , BD and Robertson , HA . 2005 . The field guide to the birds of New Zealand , Auckland : Viking .
- Hunter , PE . 1964a . Insects of Campbell Island. Mesostigmata: Laelaptidae . Pacific Insects Monograph , 7 : 121 – 128 .
- Hunter , PE . 1964b . Laelaptid mites from Auckland and Macquarie Islands (Acarina: Laelaptidae) . Pacific Insects Monograph (Supplement) , 7 : 630 – 641 .
- Hunter , PE . 1967 . Mesostigmata: Rhodacaridae, Laelapidae (mesostigmatic mites). In: Gressitt JL ed. Entomology in Antarctica . Antarctic Research Series , 10 : 35 – 39 .
- Johnston , DE and Moraza , ML . 1991 . “ The idiosomal adenotaxy and poroidotaxy of Zerconidae (Mesostigmata: Zerconina) ” . In Modern acarology , Edited by: Dusbábek , F and Bukva , V . Vol. 2 , 349 – 356 . Prague : Academia .
- Karg , W . 1978 . To the knowledge of the genera Macrocheles Latreille, 1829 and Leptolaelaps Berlese, 1918 (Acarina: Parasitiformes). Zoologische Jahrbucher, Abteilung für Systematik . Geographie und Biologie der Tiere , 105 : 360 – 367 .
- Karg , W . 1983 . Systematic investigation of the predatory mite family Leptolaelapidae Karg (Acarina: Parasitiformes). Zoologische Jahrbucher, Abteilung für Systematik . Geographie und Biologie der Tiere , 110 : 377 – 396 .
- Karg , W . 1997 . Die raubmilbenfamilie Leptolaelapidae Karg (Acarina, Parasitiformes) . Acarologia , 38 : 207 – 218 .
- Lindquist , EE and Evans , GO . 1965 . Taxonomic concepts in the Ascidae, with a modified setal nomenclature for the idiosomal of the Gamasina (Acarina Mesostigmata) . Memoirs of the Entomological Society of Canada , 47 : 1 – 64 .
- Lindquist , EE , Krantz , GW and Walter , DE . 2009 . “ Order Mesostigmata ” . In A manual of acarology , Edited by: Krantz , GW and Walter , DE . 124 – 232 . Lubbock : Texas Tech University Press .
- Moraza , ML . 2004 . The phoretic genus Neopodocinum (Oudemans, 1902) in the Iberian Peninsula (Acari: Mesostigmata: Macrochelidae) . Revista Ibérica de Aracnología , 10 : 261 – 269 .
- Murray , MD , Palma , RL and Pilgrim , RLC . 1990 . “ Ectoparasites of Australian, New Zealand and Antarctic birds ” . In Handbook of Australian, New Zealand and Antarctic birds. Volume 1. Ratites to ducks , Edited by: Marchant , S and Higgins , PJ . 1365 – 1374 . Melbourne : Oxford University Press .
- Polis , GA , Sánchez-Piñero , F , Stapp , PT , Anderson , WB and Rose , MD . 2004 . “ Trophic flows from water to land: marine input affects food webs of islands and coastal ecosystems worldwide ” . In Food webs at the landscape level , Edited by: Polis , GA , Power , ME and Huxel , GR . 200 – 216 . Chicago : Chicago University Press .
- Pollierer , MM , Langel , R , Scheu , S and Maraun , M . 2009 . Compartmentalization of the soil animal food web as indicated by dual analysis of stable isotope ratios (15N/14N and 13C/12C) . Soil Biology and Biochemistry , 41 : 1221 – 1226 .
- Post , DM . 2002 . Using stable isotopes to estimate trophic position: models, methods, and assumptions . Ecology , 83 : 703 – 718 .
- Scheu , S . 2002 . The soil food web: structure and perspectives . European Journal of Soil Biology , 38 : 11 – 20 .
- Spain , AV and Luxton , M . 1971 . Catalog and bibliography of the Acari of the New Zealand subregion . Pacific Insect Monographs , 25 : 179 – 226 .