Abstract
We used a non-destructive method to identify important definitive host species in the complex life cycles for two groups of digenetic trematodes (Microphallus sp. and Notocotylus spp.). Specifically, we exposed experimental populations of the intermediate snail host Potamopyrgus antipodarum to faeces collected from New Zealand waterfowl. We found that most infections were produced when the snails were exposed to faeces from Mallard (Anas platyrhynchos), Grey Duck (A. superciliosa), Mallard–Grey duck hybrids, or New Zealand Scaup (Aythya novaeseelandiae). In addition, the parasite communities clustered into three distinct groups in multivariate space: (1) control populations and snail populations that were exposed to faeces collected from Paradise Shelduck Tadoma varigata and Black Swan (Cygnus atratus), (2) snail populations that developed mostly Microphallus sp. infections and that were exposed to scaup, Mallard, or Grey Duck faeces, and (3) snail populations exposed to scaup faeces, which developed mostly Notocotylus spp. infections. These results suggest that Mallard, Grey Duck, and scaup are functionally relevant to the life cycles of both Microphallus sp. and Notocotylus spp.
Introduction
Most studies of parasite abundance and community structure focus on surveys in either the definitive host (e.g., Bush Citation1986; Janovy Citation1997; Davis Citation2006) or the intermediate hosts (e.g., Kuris & Lafferty Citation1994; Jokela & Lively Citation1995) alone, thereby ignoring processes that take place during transmission between the final and intermediate hosts in complex life cycles. The abundance and diversity of larval trematodes infecting intermediate molluscan hosts, however, has been shown to depend on the distribution of the definitive hosts (e.g., Smith Citation2001; Kube et al. Citation2002; Whitney et al. 2007; Byers et al. Citation2008) and on infectivity and resistance between the individual trematode species and their intermediate hosts (Lively Citation1989; Davies et al. Citation2001; Webster & Davies 2001; Lively, et al. Citation2004; King et al. Citation2009). Thus, the mere observation of a parasite in a (definitive) or intermediate host does not measure the importance of that host in the life cycle. A more direct measure is the ability of a host to transmit the parasite to the next stage in a complex life cycle. In general, transmission from definitive to intermediate host depends on: (1) the abundance of parasites within the definitive host, (2) the rate of parasite propagule release from the definitive host, (3) the timing and location of parasite propagule release from the definitive host, such that there is exposure of propagules to the next host in the life cycle, and finally (4) the infectivity of those propagules to the next host in the life cycle. For confidence in the importance of a definitive host species, the combined effects of parasite abundance, propagule release, exposure, and infectivity should be observed, and this is the approach we take here by exposing experimental populations of the New Zealand snail, Potamopyrgus antipodarum, to faeces from different waterfowl species.
Potamopyrgus antipodarum is the first intermediate host for up to 14 species of trematodes across New Zealand, where parasite communities have been particularly well-studied in Lake Alexandrina, South Island (Jokela & Lively Citation1995). In this lake, the most common trematode infection of P. antipodarum and the subject of study for >20 years is an unnamed digenetic trematode species, Microphallus sp. (Lively Citation1987; Dybdahl & Lively Citation1998; Lively & Dybdahl Citation2000; Lively et al. Citation2004). Although unnamed to species level, population genetic data suggest that Microphallus sp. is clearly a single species widely distributed across New Zealand (Dybdahl & Lively Citation1996; Lively et al. Citation2004). Adult Microphallus sp. are sexually reproducing hermaphrodites and live in the intestines of waterfowl, where eggs are laid and shed into the environment upon defecation of the bird host. Upon leaving the bird host, eggs are ingested by snails and hatch into miracidia, which penetrate the gut wall and migrate to the gonads. In the gonads, asexual reproduction produces cercariae, which sterilizes the snail. The cercariae become metacercariae and remain in the gonads until eaten by a bird to complete the life cycle.
The second most common trematode at Lake Alexandrina is from the genus Notocotylus (N. gippyensis or N. tadornae, Bisset Citation1977; Jokela & Lively Citation1995; Jokela et al. Citation1999). Adult Notocotylus spp. live in the bursa Fabricus (an immune organ of juvenile birds) and cloaca of waterfowl, where eggs are shed and expelled from the host in faeces and can infect P. antipodarum (Bisset, Citation1977). Once in the snail, Notocotylus spp. then develop and shed cercariae, which form durable metacercariae that attach to the outside of the snail's shell or plants. Once these metacercariae are ingested by waterfowl, the life cycle is completed (Bisset Citation1977; Jokela & Lively Citation1995). Other trematode species use fish as the definitive hosts and are rare in Lake Alexandrina compared to Microphallus sp. (Jokela & Lively Citation1995).
In the present study, we exposed snails to faecal material collected from different waterfowl species in order to identify final hosts that are important in the life cycle of the trematode species in Lake Alexandrina. Identifying the waterfowl host species is important because their movement will determine gene flow of trematodes within and between lakes, and potentially determine patterns of genetic structure of the trematode populations. In addition, determination of the definitive host identity is the first step towards future detailed studies. Finally, our non-destructive method of sampling can allow ethical identification of parasite communities in definitive hosts that may be of conservation concern.
Materials and methods
Field collections
We collected faeces of individual ducks and swans in January 2002 along the entire western shore of Lake Alexandrina. For Mallard (Anas platyrhynchos), Grey Duck (A. superciliosa), and New Zealand Scaup (Aythya novaeseelandiae), the species identity of the faeces was determined by watching individual birds leave a roost site after our approach. Because the scaup often roosted on large rocks and were visible from a distance, whereas mallards and grey ducks often roosted in the grass, we were more successful collecting scaup faeces from identified individuals. Thus, the frequency of our samples used does not reflect the relative abundance of waterfowl species at our study area. Paradise Shelduck (Tadorna variegata) and Black Swan (Cygnus atratus) faeces were collected as above, except that species identity of the faeces was determined by size when multi-species flocks were observed in the immediate area. Swan faeces are much larger than shelduck faeces. Mallards, Grey Duck, and their hybrids were not distinguished in analyses below; hereafter we refer to these as Mallard–Grey Duck. After collection, the faeces were stored on ice and transported to the laboratory at the Edward Percival Field Station. Potamopyrgus antipodarum were collected from the ‘West Bay’ site at Lake Alexandrina (43.949° S, 170.441° E; Jokela & Lively Citation1995) by sweeping a net through vegetation at the shoreline. Because these are wild-caught snails, some natural infections were expected.
Laboratory methods
Each faecal sample was placed in a 2 L plastic container filled with artificial pond water to remove soluble waste such as uric acid. The water was carefully decanted and replaced several times a day for two days, and then 120 snails were randomly selected from the field-collected population and added to each container. These snails were allowed to feed on the faeces in the container for 14 days before the contents were washed out. The snails were then maintained in these containers by replacing the water and adding Spirulina algae every other day. Control snail populations were established to estimate background infection rates of the field-collected population by adding 120 snails to each of three containers without faeces on the same day that the experimental snail populations were established. The snails were then transported under permit to Indiana University and maintained in a similar fashion, except that water was replaced once a week.
Approximately 120 days after exposing the snails to the faeces, the snails were inspected for trematode cercariae or metacercariae infections by dissecting each snail under a microscope. In past work, 90–100 days post-infection is sufficient for most Microphallus sp. infections to be visually detected. Infection status and, if an infection was present, species of trematode was recorded following Winterbourn (Citation1974). If a parasite was not identifiable, it was classified as unknown. This was rare except for one faeces treatment from a shelduck.
Statistical analysis
For statistical analysis, parasite species were classified into one of six groups based on taxonomy (Winterbourn Citation1974), life-cycles, and commonness: Microphallus sp., Notocotylus spp. (N. gippyensis or N. tadornae), Eel parasites (Stegodexamene and Telogaster), Furcocercariae, Xiphidocercariae, and Unknown. The prevalence of infection for the surviving snails in each container was calculated for each group. These averages were then used as independent data points for the analyses below.
We described the community structure of all resulting experimental trematode communities. We first used Spearmen's correlation coefficient to ask if infection prevalence of one parasite species was correlated to other parasite species. Because this resulted in a large number of correlations (15) Bonferroni's correction was used to determine statistical significance. We calculated correlations across all host sources combined and for scaup only. Scaup were the only species with a relatively large sample size (13 experimental snail populations).
We used k-means cluster analysis to identify groups of snail populations that developed similar parasite communities across host sources. K-means cluster analysis is a descriptive procedure that requires the user to specify the number of clusters. When we specified four clusters, we found that one of the clusters contained only the unidentified parasites from a shelduck faeces treatment. Thus, we removed this sample from the analysis and performed a cluster analysis where we specified three groups. We then used multivariate analysis of variance to test for a difference in trematode communities across groups identified from the cluster analysis and validated the results using a randomisation test. Finally, we used canonical discriminate function analysis to plot the groups in canonical variate space and describe how each group differed in parasite composition (Green & Vascotto Citation1978). All analysis was performed in R (R Development Core Team Citation2009).
Results
We found and identified faeces from four Mallard–Grey Duck, 13 scaup, six shelduck, and five Black Swans. When exposed to wild snails, Mallard–Grey Duck and scaup faeces produced the most infections. Shelduck and swan produced trematode communities in the exposed snails that were similar to the control snail populations that were not exposed to faeces (). Across all definitive host species, Microphallus sp. was most common, followed by Notocotylus spp. (). Other parasites were rare except for an unidentified species in one shelduck faeces treatment. Based on an observation of a double infection in one individual snail, this unidentified species may have been an early sporocyst stage of Xiphidocercariae, a trematode with an unknown life cycle (Winterbourn Citation1974; Jokela & Lively Citation1995).
Table 1 Mean infection (%) for each experimental snail population by parasite group and definitive host source. Parasite group codes are: Microphallus sp. (Mi), Notocotylus spp. (No), Telogaster and Stegodexamene (Eel), Furcocercariae (Fu), Xiphidocercariae (Xi), and Unknown (UNK). The number of individual faeces sampled is shown in parentheses, and ‘Total’ is the sum of the prevalence across all parasite groups. Mallard–Grey Duck refers to Mallards, Grey Ducks, and their hybrids.
Correlations between parasite species over all definitive host species were generally weak, and none were statistically significant with Bonferroni's correction (P<0.003, ). Without Bonferroni's correction, however, there were weak correlations between the Eel and Notocotylus spp. groups and between the Unknown and Eel groups. When the correlations between trematode species were calculated for only the scaup definitive hosts, there were stronger correlations but none were significant with Bonferroni's correction (). Without Bonferroni's correction, there were strong positive correlations between the Notocotylus spp., Eel, and Unknown groups. Because the Eel parasites could not have come from the faeces and double infections and eel parasite were very rare, positive correlations with these parasites probably represent random variation due to small samples and Type I error.
Table 2 Spearman's correlation between each species of trematode parasite found in experimental snails exposed to waterfowl faeces collected from Lake Alexandrina, South Island. Asterisk (*) indicated a significant correlation at P<0.05. No correlation was significant after Bonferoni's correction. Correlations above the diagonal are across all definitive host species (n=31), and correlations below the diagonal are within scaup only (n=13).
The three distinct community types identified by the cluster analysis differed substantially in trematode species composition (MANOVA, F=67.028.1, P<<0.001; randomisation test, P<<0.001). When we used canonical discriminant analysis to describe the variation between the clusters, we found that the two canonical functions clearly distinguished each group in multivariate space (). The first function explained 81% of the between-group variation and correlated strongly and negatively (−0.94) with the prevalence of Notocotylus spp. in the experimental snails (). Other trematode groups, except Furcocercariae (Fu), were also negatively correlated with this function (). The second canonical function explained 19% of the between-group variation and was highly correlated (0.99) to the prevalence of Microphallus sp. (). These results indicate that there are three types of waterfowl faeces among those we sampled: (1) those that contain few infective trematodes; (2) those that contain infective Notocotylus spp. and few other species, and; (3) those that contain infective Microphallus sp. and little else (). Of these groups, the first contained all of the control snail populations as well as all snail populations exposed to swan or shelduck faeces (). The second group, infected with Notocotylus spp., was composed entirely of faeces collected from scaup, and the third group, infected with Microphallus sp., included snail populations exposed to scaup or Mallard–Grey Duck faeces ().
Fig. 1 Plot of canonical discriminate scores for each parasite community. Groups were defined from a k-means cluster analysis where we specified three groups and excluded one community that produced a large number of unknown parasites (see text). Symbol shape refers to the host species’ faeces and shading indicates group membership. Function 1 is strongly and negatively correlated (-0.94) with Notocotylus spp., and function 2 is strongly correlated (0.99) with Microphallus sp.
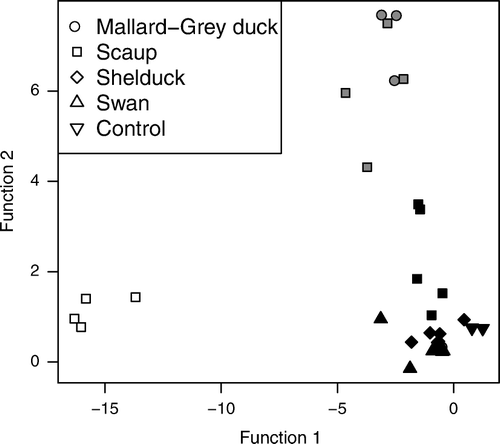
Table 3 Results from the canonical discriminate function analysis. Shown are the correlations between discriminating variables and the first two canonical discriminate functions.
Discussion
The traditional approach to understanding the distribution of macroparasites among definitive host species is to examine faeces for macroparasite eggs or to use destructive sampling of the definitive host to examine tissues for the presence of parasites (Esch et al. Citation1990; Clayton & Moore Citation1997). While either method can give a good sample of the presence of macroparasites or their propagules, the ecological relevance of between-host processes, such as variation in infectivity or susceptibility, that might be important in particular locations remains unknown. Because we know from past work that infectivity varies greatly for Microphallus sp. when exposed to different snail host genotypes or populations (Lively Citation1987; Lively et al. Citation2004; King et al. Citation2009), the between-host transmission process is at least or even more relevant in this system than simple counts of macroparasite abundance in individual hosts. Thus, we took a different approach to destructive sampling and direct counts of adult trematodes in the definitive host or eggs in faeces. By exposing the faeces of different waterfowl individuals to the intermediate host population of interest to us, we could directly determine the joint effects of macroparasite abundance and infectivity. Although this does not given us a mechanistic understanding of the infection process between final and intermediate host, it does allow us to determine the definitive hosts that are likely to be important to trematodes infecting P. antipodarum.
We found that Mallard–Grey Duck (Mallards, Grey Ducks, and their hybrids) and scaup are likely important in the life cycles of the two most common trematodes, Microphallus sp. and Notocotylus spp., at Lake Alexandrina, South Island. While our limited sampling cannot definitively exclude the possibility that other species might also serve as definitive hosts, we failed to find any evident of their importance in the samples we obtained. In addition, Mallard–Grey Duck and scaup might vary in their propensity to deposit parasite propagules into the snail population, and we know little about this other than that scaup tend to spend more time in deeper water than do Mallard–Grey Ducks (pers. obser.). Lake Alexandrina has been the focus of a long-term study on trematode communities in the intermediate snail host and, especially, on host-parasite coevolution, yet little is known about which of approximately five waterfowl species present at this lake are important for trematode life cycles. Mallard–Grey duck and scaup have a large component of aquatic invertebrates in their diet and spend a large amount of time at the shallow margins of the lake (pers. obs.), which promotes acquiring trematodes and cycling trematode eggs back into the snail population. Shelduck and swan, however, did not produce infections of either common trematode in our samples. These species are largely herbivorous and spend some time foraging on land, both habits that do not promote infections of the trematodes we studied. Bisset (Citation1977), however, found many infections of Notocotylus spp. in shelduck, so these host species deserve additional sampling. Documenting the importance of Mallard–Grey Duck and scaup for the life cycles of the two common trematodes will allow for future detailed observational or experimental studies involving these definitive hosts, including additional sampling of shelduck and swan. It could be that swan and shelduck are important in other seasons or that faeces collected from terrestrial roost sites did not reflect trematode abundance, perhaps due to propagules release that occurs when the definitive host is over water.
Although we did not directly observe communities of adult trematodes within the definitive hosts, our data is consistent with the general observation of aggregated distributions of parasites across many systems (Shaw & Dobson Citation1995). While there are many mechanisms that can produce such aggregated distributions, our data show that some scaup faeces produced relatively more Notocotylus spp. infections than Microphallus sp. infections; whereas, most Mallard–Grey Duck faeces produced mainly Microphallus sp. (; ). Thus, while both parasite species are aggregated, the hosts tend to differ in which trematode species they produce from their faeces. This suggested individual hosts vary in either (1) their exposure to and species identity of ingested trematodes; (2) the fate of ingested trematode propagules (including competitive interactions between trematode species); (3) the propensity of trematode species to produce eggs, or; (4) the infectivity of these trematode eggs. All of these mechanisms can produce the aggregated distribution and without further study, such as destructive sampling of the duck hosts or intensive dose-response experiments (Osnas & Lively Citation2004), we cannot distinguish between these mechanisms. However, from the snail's point of view and that of the life cycle of the parasites at our study lake, the joint variation of all mechanisms are the important effect, and our results show that Mallard–Grey Duck and scaup are important hosts and that these individual hosts vary in the snail parasite community that they produce.
In summary, much of the spatial structure in the community of larval trematodes at Lake Alexandrina, New Zealand (Jokela & Lively Citation1995), could be due to distribution and community composition of definitive host species, as has been found in other systems (Smith Citation2001; Kube et al. Citation2002; Whitney et al. Citation2007; Byers et al. Citation2008). Because Microphallus sp. and Notocotylus spp. trematodes are prominent in infections caused by Mallard–Grey Duck and scaup faeces, distributions within and between lakes of these host species will be the primary factors determining the prevalence of these trematodes and of coevolutionary patterns between the trematodes and the intermediate host. However, other host species could be important at other lakes or other times at Lake Alexandrina. Additional sampling and experimental work would need to be completed for a more detailed understanding of important definitive host species and the infection process across hosts.
Acknowledgements
J Jokela, M Dybdahl, and two anonymous reviewers provided important comments during manuscript preparation. Research was funded by grants from the Center for the Integrative Study of Animal Behavior, Bloomington, IN, and from NSF grant DEB-9904840 to CM Lively.
Additional information
Notes on contributors
EE Osnas
Department of Ecology and Evolutionary Biology, Princeton University, Princeton, USAReferences
- Bisset , SA . 1977 . Notocotylus tadornae n-sp and Notocotylus gippyensis (Beverley-Burton, 1958) (Trematoda Notocotylidae) from waterfowl in New Zealand—morphology, life-history and systematic relations . Journal of Helminthology , 51 : 365 – 372 .
- Bush , AO . 1986 . Intestinal helminths of lesser scaup ducks: patterns of association . Canadian Journal of Zoology , 64 : 132 – 141 .
- Byers , JE , Blakeslee , AMH , Linder , E , Cooper , AB and Maguire , TJ . 2008 . Controls of spatial variation in the prevalence of trematode parasites infecting a marine snail . Ecology , 89 : 439 – 451 .
- Clayton DH , Moore J . 1997 . Host-parasite evolution: general principles and avian models . Oxford, , UK , Oxford University Press .
- Davis , NE . 2006 . A survey of waterfowl for echinostomes and schistosomes from Lake Wanaka and the Waitaki River watershed, New Zealand . Journal of Helminthology , 80 : 33 – 40 .
- Davies , CM , Webster , JP and Woolhouse , MEJ . 2001 . Trade-offs in the evolution of virulence in an indirectly transmitted macroparasite . Proceedings of the Royal Society of London Series B-Biological Sciences , 268 : 251 – 257 .
- Dybdahl , MF and Lively , CM . 1996 . The geography of coevolution: comparative population structures for a snail and its trematode parasite . Evolution , 50 : 2264 – 2275 .
- Dybdahl , MF and Lively , CM . 1998 . Host-parasite coevolution: evidence for rare advantage and time-lagged selection in a natural population . Evolution , 52 : 1057 – 1066 .
- Esch G , Bush A , Aho J . 1990 . Parasite communities: patterns and processes . New York , Chapman and Hall .
- Green , RH and Vascotto , GL . 1978 . A method for the analysis of environmental factors controlling patterns of species composition in aquatic communities . Water Research , 12 : 583 – 590 .
- Janovy J 1997 . Protozoa, helminths, and arthropods of birds Clayton , DH , Moore , J ., Host-parasite evolution: general principles and avian models . Oxford , Oxford University Press 303 – 337 .
- Jokela , J and Lively , CM . 1995 . Spatial variation in infection by digenetic trematodes in a population of fresh-water snails (Potamopyrgus antipodarum) . Oecologia , 103 : 509 – 517 .
- Jokela , J , Lively , CM , Taskinen , J and Peters , AD . 1999 . Effect of starvation on parasite-induced mortality in a freshwater snail (Potamopyrgus antipodarum) . Oecologia , 119 : 320 – 325 .
- King , KC , Delph , LF , Jokela , J and Lively , CM . 2009 . The geographic mosaic of sex and the Red Queen . Current Biology , 19 : 1438 – 1441 .
- Kube , J , Kube , S and Dierschke , V . 2002 . Spatial and temporal variations in the trematode component community of the mudsnail Hydrobia ventrosa in relation to the occurrence of waterfowl as definitive hosts . Journal of Parasitology , 88 : 1075 – 1086 .
- Kuris , AM and Lafferty , KD . 1994 . Community Structure—Larval Trematodes in Snail Hosts . Annual Review of Ecology and Systematics , 25 : 189 – 217 .
- Lively , CM . 1987 . Evidence from a New-Zealand snail for the maintenance of sex by parasitism . Nature , 328 : 519 – 521 .
- Lively , CM . 1989 . Adaptation by a parasitic trematode to local populations of its snail host . Evolution , 43 : 1663 – 1671 .
- Lively , CM and Dybdahl , MF . 2000 . Parasite adaptation to locally common host genotypes . Nature , 405 : 679 – 681 .
- Lively , CM , Dybdahl , MF , Jokela , J , Osnas , EE and Delph , LF . 2004 . Host sex and local adaptation by parasites in a snail-trematode interaction . American Naturalist , 164 : S6 – S18 .
- Osnas , EE and Lively , CM . 2004 . Parasite dose, prevalence of infection and local adaptation in a host-parasite system . Parasitology , 128 : 223 – 228 .
- R Development Core Team 2009 . R: A language and environment for statistical computing . R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, http://www.R-project.org .
- Shaw , DJ and Dobson , AP . 1995 . Patterns of macroparasite abundance and aggregation in wildlife populations: a quantitive review . Parasitology , 111 : S111 – S133 .
- Smith , NF . 2001 . Spatial heterogeneity in recruitment of larval trematodes to snail intermediate hosts . Oecologia , 127 : 115 – 122 .
- Webster , JP and Davies , CM . 2001 . Coevolution and compatibility in the snail-schistosome system . Parasitology , 123 : S41 – S56 .
- Whitney , KL , Hechinger , RF , Kuris , AM and Lafferty , KD . 2007 . Endangered light-footed clapper rail affects parasite community structure in coastal wetlands . Ecological Applications , 17 : 1694 – 1702 .
- Winterbourn , MJ . 1974 . Larval Trematoda parasitizing the New Zealand species of Potamopyrgus (Gastropoda: Hydrobiidae) . Mauri Ora , 2 : 17 – 30 .