Abstract
Individuals of Nysius huttoni White, a ground-dwelling hemipteran, develop one of three wing forms. Field studies were carried out from 1995 to 1998 to investigate the proportion of each wing form; adult populations consisted of 94.1% macropters, 5.5% sub-brachypters and 0.4% brachypters. Paired mating adults were collected for two years in 1995–1996 and 1996–1997 to investigate which wing forms were mating with which, and the proportions of the wing form combinations among the mating pairs. Theoretically, there are nine possible mating combinations among three wing forms. However, only six mating combinations were found in the field. Four mating combinations, M×M, ♀M×♂Sb, ♂M×♀Sb and Sb×Sb occurred in both years; and two mating combinations, ♂M×♀B and ♂Sb×♀B were found only in 1996–1997. The other three mating combinations, ♀M×♂B, ♀Sb×♂B and B×B were not found in either year. M×M was the predominant mating combination with an average percentage of 81.2%. The effects of temperature and photoperiod on wing development of this species were investigated at five constant temperatures (15, 20, 25, 30 and 35±0.5°C), one ambient (laboratory room conditions) temperature and three photoperiods (16-h, 12-h, and 8-h of light) in the laboratory. The results indicate that both low and high temperatures, and short day-length at lower temperature, tend to accelerate the production of sub-brachypters and brachypters, while high temperature under long photoperiod appears to favour the production of macropters. This ability to produce macropters possibly benefits this bug by enabling rapid dispersal of adults during occasional very dry summers (which may kill preferred weedy hosts) to temporary alternative crop hosts in Canterbury and Central Otago.
Introduction
Wing polymorphism, a common phenomenon among insects (Harrison Citation1980), occurs in many insects, especially species of Heteroptera (e.g., Fujisaki Citation1989; Sakashita et al. Citation1995; Sasaki et al. Citation2002). Wing polymorphism plays an important role in the life cycle of many insects and is usually viewed as an example of dispersal polymorphism (Harrison Citation1980; Roff Citation1986a; Zera & Denno Citation1997). Within a species, individuals with fully developed wings (macropters) are generally capable of flight, while those either with reduced wings (brachypters, sometimes called ‘micropters’) or without wings lack flight capability (Zera & Denno Citation1997).
A striking feature of the New Zealand insect fauna is that wing reduction is common and results in secondary flightlessness. Examples are known in New Zealand species of Phasmatodea, Acrididae, Dermaptera, Blattodea, Plecoptera, Lepidoptera, Diptera, Hymenoptera, Coleoptera and in members of several families of Hemiptera, despite fully winged forms being the normal condition in these insect orders (Watt Citation1975). The modification of wings is a fairly common phenomenon in the Lygaeidae and occurs frequently in many, if not most, other families of Hemiptera (Slater Citation1975). Malipatil (Citation1977) noted that the New Zealand ground-living lygaeid fauna is composed almost entirely of members of the tribe Targaremini and that 95% of the species are flightless, with coleoptery (hardening of wings) being the predominant condition. Malipatil also observed high proportions of flightlessness in several other orders of New Zealand insects.
Nysius huttoni, which is widely distributed in New Zealand, is a polyphagous species (Eyles & Ashlock Citation1969) that feeds on over 40 host plants (Myers Citation1921, Citation1926; Gurr Citation1952, Citation1957; Woodward Citation1954; Eyles Citation1965a, Citationb; Farrell & Stufkens Citation1993; Wei Citation2001). N. huttoni is a sun-loving insect living close to the ground in areas where there are bare patches of ground between the plants allowing the sun to strike through to the ground, such as gravel car parks, roadsides, waste areas of land, weedy headlands surrounding crops and run-down lawn or pasture (Gurr Citation1957; Eyles Citation1965a, Citationb; Farrell & Stufkens Citation1993), and in its natural habitat in stony riverbeds with areas of gravel and sand between the plants (Eyles & Malipatil Citation2010).
During very dry summers, particularly in the arable cropping areas of Canterbury, New Zealand (and to some extent Central Otago), when the preferred weedy host plants dry up and die, adults of N. huttoni move onto alternative temporary hosts, and may become an economically important pest on maturing wheat (Triticum aestivum L.) crops and on seedling and establishing crucifer crops (Gurr Citation1957; Eyles Citation1965a; Swallow & Cressey Citation1987; Farrell & Stufkens Citation1993; Eyles & Malipatil Citation2010). In dry summers, development of more of the macropterous adult form will enable many more individuals to quickly disperse by flying to such crops, not only ensuring that greater numbers of this bug survive, but potentially enabling this species to inflict greater economic loss on any unprepared growers.
N. huttoni has three generations per year in Canterbury (Wei Citation2008a). Both sexes of this species show wing polymorphism of macropters, sub-brachypters and brachypters (see ‘Materials and methods’ for definitions), and it appears to be unique amongst the Orsillidae in having three wing-forms in both sexes (Eyles Citation1960). However, there are few quantitative field data on the occurrence of these wing forms in N. huttoni. Although some environmental factors such as temperature, photoperiod, food quality and population density have been reported to be responsible for the determination of wing forms in many species (Harrison Citation1980), apart from Eyles (Citation1960), little attention has been devoted to the factors affecting wing development in N. huttoni. The objectives of this study were (1) to survey the proportion of each wing form in a field population; (2) to survey the proportions of the wing form combinations among the mating pairs in a field population; and (3) to determine whether temperature and photoperiod affect wing polymorphism.
Materials and methods
All experiments were carried out at the Department of Zoology, University of Canterbury, located in Christchurch, South Island, New Zealand. Insects were collected from a piece of wasteland in Hornby, south of Christchurch, New Zealand, as described by Wei (Citation2008a). This site of about one hectare, was a dry and sparsely vegetated sandy habitat dominated by barley grass (Hordeum marinum Huds.), perennial ryegrass (Lolium perenne L.), and vulpia hairgrass (Vulpia megalura Rydb.), but with many known host plants including shepherd's purse (Capsella bursa-pastoris (L.) Medik), twin cress (Coronopus didymus (L.) Sm.), broom (Cytisus scoparius (L.) Link.), wireweed (Polygonum aviculare L.), sheep's sorrel (Rumex acetosella L.), sand spurrey (Spergularia rubra (L.) J. Presl & C. Presl), and white clover (Trifolium repens L.). This is a typical habitat for N. huttoni, and the area supports a resident population.
Proportion of each wing form in the field population
As no data have been reported in previous papers showing the proportions of various wing forms in natural populations of N. huttoni, adults were collected from the field to determine the percentage occurrence of each wing form. Sampling was carried out from 1995 to 1998 at 10-day intervals on the 5th, 15th, and 25th of each month starting from September of the year in which the overwintered adults emerged (except 1995 when the field study began in the fourth week of December) and ending in April or May of the next year when adults of the third generation disappeared for overwintering. Collections were made using a domestic vacuum cleaner from five 0.25 m2 (0.5×0.5 m) quadrats randomly placed on the ground. Collected adults were examined under a binocular microscope and graded into three wing forms according to the criteria of Eyles (Citation1960). These forms are: (1) Macropterous (M), in which the wings extend beyond the apex of the abdomen; (2) Sub-brachypterous (Sb), in which the posterior tips of the wings are level with, or scarcely exceed, the apex of the abdomen; and (3) Brachypterous (B), in which the posterior of the wings does not reach the posterior of the abdomen.
Proportions of the wing form combinations among mating pairs in the field population
Theoretically, each wing form can be involved in nine possible mating combinations among the three wing forms. However, the proportion of each combination in the field population is unknown. To investigate this, when mating was at its peak (Wei Citation2008a), paired adults in copula were collected from the field between 11.00 am and 3.00 pm by guiding them, with tweezers, into small glass tubes, one pair per tube. They were examined under a binocular microscope using the three wing length criteria described above. Collections were made in 1995–1996 and 1996–1997.
Effect of temperature and photoperiod on the development of wings
Experimental insects and rearing
Fifth instar nymphs were collected from the field individually into small glass tubes. They were brought to the laboratory and reared together within plastic containers (17×17×8 cm) with host plant shepherd's purse available as food each day at 25°C until emergence as adults. Rearing experiments were started from the first clutch of eggs laid by these adults. As in Wei (Citation2008b) newly hatched first instar nymphs of N. huttoni were transferred to small glass vials (2 cm diameter, 5.5 cm high) on the same day that the eggs hatched. The nymphs were then reared singly on shepherd's purse. All specimens were held in incubators 74×42×67 cm, within which relative humidity was 65–75%. The rearing experiments ended after all nymphs completed their development into adults. Wing forms of the newly emerged adults were checked under the microscope, 48 hours after emergence, against the three wing length criteria described above, and the numbers of each form were recorded.
Temperature and photoperiod
The effect of temperature on wing form was studied at five constant temperatures (15, 20, 25, 30, and 35±0.5°C) and one photoperiod (12-h of light). The effect of photoperiod on wing form was studied at three photoperiods (16-h, 12-h, and 8-h of light) and two constant temperatures (20 and 27.5°C±0.5°C). Additionally, another group of nymphs was reared under ambient temperature, photoperiod and relative humidity, by placing the glass vials on the laboratory bench (instead of in incubators). Maximum and minimum laboratory room temperatures were recorded daily. The ambient temperature is referred to by its average temperature (mean 20.3°C, range 12.5–29.5°C).
All rearing experiments were conducted from November 1995 to August 1997.
Statistical analysis
A chi-squared test (χ2 test) was used to examine the difference in proportions of the three wing forms collected in field populations, and the difference between observed and expected percentages of nine wing form mating combinations among mating pairs collected from the field. The sex ratios of the three wing forms collected from the field and produced at different temperatures and photoperiods were calculated and expressed as ♀:♂=1:x according to the proportion of females to males among the total adult population. Departures from an expected 1:1 (♀:♂) ratio were examined with the χ2 test.
Results
Proportion of each wing form in the field population
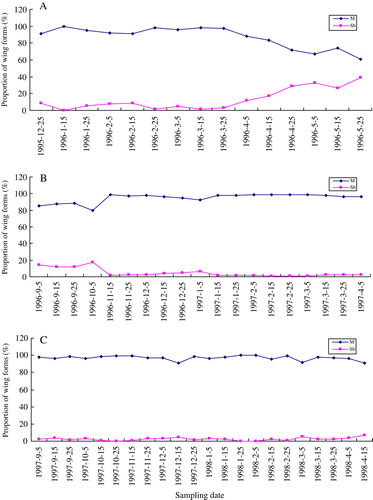
shows the proportion of each wing form in adults collected from a field population during three years from 1995–1998. In total, 14,245 adults were taken in 56 samples and examined during the three years. There were significantly large differences in proportions of the three wing forms (P<0.001, χ2 test), with the M-form being predominant (91.5–97.0% in the 3 years). Representation of the Sb-form ranged from 2.3% in 1997–1998 to 8.5% in 1995–1996, while the B-form was the rarest, not exceeding 1% of the population and ranging from 0% in 1995–1996 to 0.7% in 1997–1998.
Table 1 The proportion of each wing form of N. huttoni adults collected from the field in three years, 1995–1998.
Incidence of the M-form was greater in males than females with the sex ratio being 1:1.13 (P<0.01, χ2 test) for all three years combined (). The proportion of females was greater than that of males for the Sb-form with the sex ratio being 1:0.73 (P<0.01) for all three years combined. Differences between females and males in proportions of the B-forms were not significant in the three years (P>0.05).
The wing form did not show obvious seasonal changes (). The Sb-form, however, started increasing in proportion from the beginning of April 1996 (A) and was still high at the beginning of spring next season (1996 B).
Figure 1 The proportions of macropterous (M) and sub-brachypterous (Sb) forms of N. huttoni adults collected from the field over three years: A, 1995–1996. B, 1996–1997. C, 1997–1998. In 1995 no collections were made between the beginning of September and the fourth week in December so data for overwintered adults and some first generation adults are absent. The last sampling date varies among years in response to variation in the date that the adults of the third generation disappeared for overwintering. The brachypterous form is not shown because of its low proportion (see ).
Proportions of the wing form combinations among mating pairs in the field population
Numbers and percentages of mating pairs among different wing forms collected during 1995–1996 and 1996–1997 are given in . A total of 1414 pairs of adults in copula were collected and examined during the two years. As mentioned above, there are theoretically nine mating combinations among the three wing forms, however, only six mating combinations were found in the field. Four mating combinations, M×M, ♀M×♂Sb, ♂M×♀Sb and Sb×Sb occurred in both years; and two mating combinations, ♂M×♀B and ♂Sb×♀B were found only in 1996–1997. The other three mating combinations, ♀M×♂B, ♀Sb×♂B and B×B were not found in either year.
Table 2 The proportion of different wing form combinations found in copula in the field during 1995–1996 and 1996–1997.
M×M was the predominant mating combination in the field, ranging from 76.3% in 1995–1996 to 83.9% in 1996–1997 (). The average percentage was 81.2% when total data from the two years were summed. The next most common mating combination was ♂M×♀Sb with 10.6% in 1996–1997 and 19.6% in 1995–1996, an average of 13.8% for the two years combined. Proportions of the other four mating combinations, ♀M×♂Sb, Sb×Sb, ♂M×♀B and ♂Sb×♀B were very low and their overall percentages were 1.4–3.0% (mean 2.4%), 1.4–2.8% (mean 1.9%), 0.0–1.0% (mean 0.6%), and 0.0–0.1% (mean 0.1%), respectively.
The proportions of females and males of the three wing forms in 1995–1996 and 1996–1997 () were used to calculate the expected proportion for each of the nine mating combinations. The observed proportion of each mating combination (summed for the two year classes) was then compared with the expected proportion (). No significant difference was detected (P>0.05, χ2 test), indicating that no assortative mating occurred in the natural population.
Table 3 The observed and expected proportions of mating pairs collected from the field during 1995–1996 and 1996–1997.
Effect of temperature on the development of wings
shows the number and percentage of each wing form produced at different temperatures under a 12 h light:12 h dark photoperiod. At low temperature (15oC), no M-form was produced, but both Sb- and B-forms occurred. The proportions of these two forms were 47.4% and 52.6%, respectively. As the temperature increased towards 25oC, the proportion of the B-form decreased to zero at 25oC, at which the proportion of the M-form was 78.7%. The proportion of the B-form increased again with further increases in temperature. The proportion of the M-form was highest in the 25oC treatment group, but there was a reduction in the proportion of the M-form at temperatures higher or lower than 25oC. At ambient temperature (A-T), the proportions of three wing forms of N. huttoni were the same as those at 25oC, suggesting that an ambient temperature is favourable for producing the M-form.
Table 4 Effect of temperature on wing development of N. huttoni in the laboratory.
Effect of photoperiod on the development of wings
The wing forms produced under three different photoperiods at 20 and 27.5oC are given in . At 20oC under a 16-h photoperiod, no B-form occurred, the proportion of the Sb-form was very low (2.8%), and 97.2% of the population were of the M-form. Under a 12-h photoperiod, the proportion of the B-form was 8.6% and with further shortening of the photoperiod to 8 hours, it increased slightly more to 10%. The proportion of the Sb-form increased as well under 12-h and 8-h photoperiods to 21% and 15%, respectively. Correspondingly, the proportions of the M-form decreased to 70.4% and 75.0%. Thus, at 20oC, a shorter photoperiod resulted in an increase in the B-form. A similar tendency was observed in the proportion of the Sb-form between 16-h and 12-h photoperiods but not between 12-h and 8-h photoperiods.
Table 5 Effect of photoperiod on wing development of N. huttoni in the laboratory.
Also, it can be seen that the differences in percentages of both the Sb- and B-forms at 20oC between the 16-h (Sb-form 2.8% and B-form 0%) and 12-h photoperiods (Sb-form 21% and B-form 8.6%) were larger than those between the 12-h and 8-h photoperiods (only 6% difference in the Sb-form and 1.4% in the B-form). This may indicate that the effect of photoperiod on reduction in wing length becomes weaker as day length shortens below 12 hours.
At 27.5oC, the response of N. huttoni to photoperiod in terms of wing length was not as apparent as at 20oC. The proportion of the M-form was very high at all three photoperiods tested, especially the proportions at 12-h (100%) and 8-h (96.7%), indicating that both temperature and photoperiod commonly exert an effect on development of wings. Under an 8-h photoperiod, 2.2% of the Sb-form and 1.1% of the B-form occurred, but these percentages were lower than those under the same photoperiods at 20oC. However, under the 16-h photoperiod, the B-form comprised 3.8% of individuals.
Discussion
Proportions of wing forms in the field population
Proportions of the three wing forms of N. huttoni adults differed greatly in field populations. The M-form predominated, forming about 94% of the population when the three years of data were summed, whereas the Sb-form comprised 5.5%, and the B-form did not exceeded 1% of the population. In the field population, there was a higher proportion of males than females for the M-form, while the proportion of the Sb-form was higher in females than males (P<0.01, χ2 test); but the sex ratio of the B-form was similar (P>0.05). Wei's (2008b) study of sex ratio in N. huttoni, also found an excess of males in some field samples. Although wing form was not monitored in that work, because the Sb-form is now found to have more females, that result can now be attributed to a preponderance of M-form males. The incidence of the male-biased M-form found in N. huttoni is consistent with the results observed in Microvelia douglasi, Heteroptera: Veliidae, (Muraji et al. Citation1989) and Limnoporus canaliculatus, Heteroptera: Gerridae, (Zera et al. Citation1983). Conversely, higher proportions of macropterous forms in females than in males were found in the oriental chinch bug, Cavelerius saccharivorus Okajima, Heteroptera: Lygaeidae, (Fujisaki Citation1989) and the waterstrider, Aquarius najas, Heteroptera: Gerridae, (Ahlroth et. al Citation1999). In contrast to these, however, in Pyrrhocoris apterus (Heteroptera: Pyrrhocoridae) there were no significant differences between the proportions of macropterous males and females (Socha Citation2001). Tanaka & Wolda (Citation1987) reported that the incidence of short-winged forms of Jadera aeola (Heteroptera: Rhopalidae) was greater in females than in males which is different from the result of the B-form recorded in N. huttoni. The ecological significance of these differences is unknown.
Many insects have been reported to display seasonal changes in wing dimorphism or polymorphism, such as Dimorphopterus japonicus, Heteroptera: Lygaeidae, (Sasaki et al. Citation2003), Blissus insularis, Heteroptera: Lygaeidae, (Cherry Citation2001), C. saccharivorus (Fujisaki Citation1989, Citation1993) and Pyrrhocoris sibiricus, Heteroptera: Pyrrhocoridae, (Sakashita et. al Citation1998). In the present study, however, the results show that there is no consistent seasonal trend in wing changes in N. huttoni (). The proportions of the M- and Sb-forms, however, in third-generation adults gradually decreased and increased respectively from the beginning of April 1996 towards winter (A). During early spring of the next season the proportions of the M- and Sb-forms were lower and higher respectively than in any other season (B). This trend was not evident before or after the winter of 1997 or before the winter of 1998 (B, C) and may have been associated with the period of occurrence of third-generation adults. This is because N. huttoni overwinters in the adult stage and adults collected in the spring had completed development under lower temperature and short-decreasing photoperiod which resulted in the reduction of the wings. This trend was not found in other years in which the proportions of wing forms seem to be stable throughout seasons. Wei (Citation2008a) reported that the third-generation adults of N. huttoni occurred about 35 and 45 days later in 1996 (mid-March) than in 1997 (mid-February) and 1998 (very early February), respectively. This may explain the occurrence of more Sb-forms in 1996, due to lower temperature and shorter photoperiod (as discussed below), and to differences in weather between years.
Proportions of the wing form combinations among mating pairs in the field population
In this study of mating combinations among the three wing forms (), all nine possible mating combinations mentioned above were not found in natural populations. Occurrence and proportion of each mating combination among the three wing forms depends on the proportion and sex ratio of each wing form. The highest proportion (81.2%) of mating combination occurs between ♀M- and ♂M-forms and this is a consequence of very high proportions of M-form individuals (91.5–97.0%, ) in the field.
The proportion of the Sb-form is about 5.5% in natural populations. shows the five mating permutations which involve the Sb-form. In M×Sb, the proportion of ♂M×♀Sb (10.6–19.6%, mean 13.8% for two years) was higher than that of ♀M×♂Sb (1.4–3.0%, mean 2.4%) (P<0.05, χ2 test). This would be expected to follow from the higher numbers of males than females in the M-form and of females than males in the Sb-form ().
The proportion of the B-form is the lowest (0.4%) among the three wing forms (). In the field, 10 mating B-form adults were collected (only in 1996–1997) and all of them were females (). Nine of them were found to mate with ♂M and one mated with ♂Sb. However, no mating ♂B were found. The proportions of the two mating combinations involving the ♀B-form were either very low (♂M×♀B: 1.0% and ♂Sb×♀B: 0.1% both only in 1996–1997) or did not occur (in 1995–1996). The three possible mating combinations involving the ♂B-form (♀M×♂B, ♀Sb×♂B and B×B) were not found in both years. This indicates that in the B-form, some females can obtain opportunity to mate with ♂M due to its high proportion in the field, or with ♂Sb although that mating probability is very low, whereas the males can not obtain any mating opportunity.
In many cases, wing polymorphism has been found to be genetically determined (Roff Citation1986b; Slobreck Citation1986). However, the genetic basis of wing-form determination in N. huttoni is not yet understood. My results revealed the number of mating combinations among the three wing forms, and the percentage of each combination of N. huttoni in a natural population for the first time. Detailed experiments using different cross combinations among wing forms will be necessary to obtain a more detailed understanding of genetic control of wing forms in this species.
Effect of temperature and photoperiod on wing forms
It is of interest that Eyles (Citation1960) rearing (from N. huttoni eggs) 5 nymphs per tube on Coronopus didymus in a greenhouse at Palmerston North under ‘normal’ temperatures (minimum 6.7–13.9°C, maximum 27.8–34.4°C) obtained 90 adults of which 17.8% were macropters, 67.8% sub-brachypters and 14.4% brachypters. There were twice as many macropterous males as females, but in the brachypters there were twice as many females as males. The sub-brachypterous form was also the most common form in field samples. At ‘low’ temperatures (minimum 9.4–15.6°C, maximum 17–21°C) under a Dutch barn, and starting with field-collected fourth instar nymphs, he obtained only nine adults, of which 44.4% were sub-brachypters and 55.6% were brachypters. In my study, the percentages of the Sb- and B-forms at low temperature were similar. As Eyles’ maximum glasshouse temperatures were high, it is possible that a reduction in the proportion of the M-form developing at high temperatures as found in my study, may explain (or partly explain) the low percentage of the M-form obtained by Eyles.
In P. apterus Honek (Citation1976a) found that the proportion of macropters was higher at intermediate temperatures (25–27oC) than at lower (about 21oC) and higher (over 30oC) temperatures. The present study showed a similar effect of temperature in N. huttoni.
The present study indicates that both low and high temperatures, and short day-length at lower temperature, tend to accelerate the production of the Sb- and B-forms. In the field the rise in frequencies of the Sb-form towards autumn (1995–1996) exemplifies this; while high temperature under long photoperiod appears to favour the production of the M-form. Similar results have been reported in D. japonicus (Sasaki et al. Citation2002), C. saccharivorus (Fujisaki Citation1989, Citation2000) and Leptopterna dolobrata, Heteroptera: Miridae, (Braune Citation1983). However, more macropters of P. sibiricus were produced at higher temperature under short photoperiod rather than long photoperiod conditions (Sakashita et al. Citation1995).
It is essential to consider environmental control of wing-form determination in the context of the life cycle of N. huttoni, which has three generations each year (Wei Citation2008a). The first and second generations as well as a partial third generation occur mainly between spring and summer when high temperature and long day length predominate. Under high temperature and long photoperiod conditions, macropters are likely to be more adaptive than sub-brachypters and brachypters because the animals can adjust their body temperatures by flight, or can migrate to more favourable habitats when the weather is hotter than they prefer, or if host plants become desiccated. More sub-brachypters are likely to appear in the third generation when temperatures became lower in autumn and a shortening photoperiod prevails (A). This is also likely to be adaptive because lower temperatures do not stimulate flight (Wei Citation2001). A similar finding was reported for C. saccharivorus in which the lower temperature and short daylength in autumn season led to production of more brachypters (Fujisaki Citation1993).
It has been reported that crowding of nymphs influences wing length in many insects and usually stimulates the appearance of macropters (in nature this would enable some individuals to fly to a less crowded area). For example, in the oriental chinch bug, C. saccharivorus (Fujisaki Citation1989), D. japonicus (Sasaki et al. Citation2002), B. insularis (Cherry Citation2001) and Leptopterna dolobrata (Braune Citation1983), more macropters are produced under conditions of higher density, while lower density resulted in more brachypters. As pointed out by Honek (Citation1976b), it is necessary from an ecological point of view to evaluate whether the varying proportions of wing forms found in natural populations are consistent with experimental results obtained under laboratory conditions. My surveys showed that the M-form of N. huttoni was the predominant form in the field where it made up about 94.1% of the study population. This value was higher than the 64.0–78.7% of M-forms obtained by rearing at various temperatures in the laboratory (), but it was close to the results obtained from most of the different photoperiods at which percentages of the M-form was over 95% (). However, all nymphs were reared separately (one vial contained one nymph) in my studies until the adults emerged, so crowding could not have affected wing length in the laboratory. Also, food has been reported to affect wing forms in some species (Novotny Citation1994). In this study, however, as N. huttoni was reared on a single food, shepherd's purse, food could not be a factor responsible for the wing length variations.
Eyles & Malipatil (Citation2010) review the pest-status of N. huttoni and the newly introduced N. caledoniae (in Australia previously known as N. clevelandensis) from Australia. Both are only minor economic pests. For example, Swallow & Cressey (Citation1987), report that over the previous 50 years, attacks by N. huttoni on maturing wheat crops in Canterbury happened only every 10 years or so following spring droughts. Farrell & Stufkens (Citation1993) found that some adults of the wheat bug migrate from weeds to colonise new habitats such as fathen (Chenopodium album L.) and wheat, concluding that its success may be linked with flexibility in habitat use, and that this occasional agricultural pest is difficult to control because it moves to affected crops from fallow land when preferred host plants dry out. This paper shows that N. huttoni exhibits wing polymorphism in response to seasonal temperature and photoperiod changes that affect the dispersal ability of these bugs. These wing polymorphisms may alter the extent of the bugs’ adverse effects on economically important crops.
Acknowledgements
This paper is part of a PhD thesis, presented at the University of Canterbury, New Zealand. I am most grateful to Mr Peter Johns and Professor Mike Winterbourn for their patient guidance in my research. My thanks to AC Eyles and to two anonymous journal referees, for helpful suggestions to the manuscript. This work was, in part, supported by a University of Canterbury Doctoral Scholarship, grant number 5075/2/61.
References
- Ahlroth , P , Alatalo , RV , Hyvärinen , E and Suhonen , J . 1999 . Geographical variation in wing polymorphism of the waterstrider Aquarius najas (Heteroptera, Gerridae) . Journal of Evolutionary Biology , 12 : 156 – 160 .
- Braune , HJ . 1983 . The influence of environmental factors on wing polymorphism in females of Leptopterna dolobrata (Heteroptera, Miridae) . Oecologia , 60 : 340 – 347 .
- Cherry , R . 2001 . Seasonal wing polymorphism in southern chinch bugs (Hemiptera: Lygaeidae) . Florida Entomologist , 84 : 737 – 739 .
- Eyles , AC . 1960 . Variation in the adult and immature stages of Nysius huttoni White (Heteroptera: Lygaeidae) with a note on the validity of the genus Brachynysius Usinger . Transactions of the Royal Entomological Society of London , 112 : 53 – 72 .
- Eyles , AC . 1965a . Damage to cultivated cruciferae by Nysius huttoni White (Heteroptera: Lygaeidae) . New Zealand Journal of Agricultural Research , 8 : 363 – 366 .
- Eyles , AC . 1965b . Notes on the ecology of Nysius huttoni White (Heteroptera: Lygaeidae) . New Zealand Journal of Science , 8 : 494 – 502 .
- Eyles , AC and Ashlock , PD . 1969 . The genus Nysius in New Zealand (Heteroptera: Lygaeidae) . New Zealand Journal of Science , 12 : 713 – 727 .
- Eyles , AC and Malipatil , MB . 2010 . Nysius caledoniae Distant, 1920 (Hemiptera: Heteroptera: Orsillidae) a recent introduction into New Zealand, and keys to the species of Nysius, and genera of Orsillidae, in New Zealand . Zootaxa , 2484 : 45 – 52 .
- Farrell , JA and Stufkens , MW . 1993 . Phenology, diapause, and overwintering of the wheat bug, Nysius huttoni (Hemiptera: Lygaeidae), in Canterbury, New Zealand . New Zealand Journal of Crop and Horticultural Science , 21 : 123 – 131 .
- Fujisaki , K . 1989 . Wing form determination and sensitivity of stages to environmental factors in the oriental chinch bug, Cavelerius saccharivorus Okajima (Heteroptera: Lygaeidae) . Applied Entomology and Zoology , 24 : 287 – 294 .
- Fujisaki , K . 1993 . Wing reduction in the autumn generation of the oriental chinch bug, Cavelerius saccharivorus Okajima (Heteroptera: Lygaeidae) . Applied Entomology and Zoology , 28 : 112 – 115 .
- Fujisaki , K . 2000 . Seasonal adaptations in subtropical insects: wing polymorphism and egg diapause in the oriental chinch bug, Cavelerius saccharivorus Okajima (Heteroptera: Lygaeidae) . Entomological Science , 3 : 177 – 186 .
- Gurr , L . 1952 . Notes on Nysius huttoni F. B. White, a pest of wheat in New Zealand . New Zealand Science Review , 10 : 108 – 109 .
- Gurr , L . 1957 . Observations on the distribution, life history, and economic importance of Nysius huttoni (Lygaeidae: Hemiptera) . New Zealand Journal of Science and Technology , 38 : 710 – 714 .
- Harrison , RG . 1980 . Dispersal polymorphisms in insects . Annual Review of Ecology and Systematics , 11 : 95 – 118 .
- Honek , A . 1976a . Factors influencing the wing polymorphism in Pyrrhocoris apterus (Heteroptera, Pyrrhocoridae). Zoologische Jahrbücher Abteilung für Systematik . kologie und Geographie der Tiere , 103 : 1 – 22 .
- Honek , A . 1976b . The regulation of wing polymorphism in natural populations of Pyrrhocoris apterus (Heteroptera, Pyrrhocoridae). Zoologische Jahrbücher Abteilung für Systematik . kologie und Geographie der Tiere , 103 : 547 – 570 .
- Malipatil , MB . 1977 . Distribution, origin and speciation, wing development, and host-plant relationships of New Zealand Targaremini (Hemiptera: Lygaeidae) . New Zealand Journal of Zoology , 4 : 369 – 381 .
- Muraji , M , Miura , T and Nakasuji , F . 1989 . Phenological studies on the wing dimorphism of a semi-quatic bug, Microvelia douglasi (Heteroptera: Veliidae) . Researches on Population Ecology , 31 : 129 – 138 .
- Myers , JG . 1921 . Insect pests of lucerne and clover. Observations in the Marlborough seed-growing area . New Zealand Journal of Agriculture , 23 : 156 – 162 .
- Myers , JG . 1926 . Biological notes on New Zealand Heteroptera . Transactions and Proceedings of New Zealand Institute , 56 : 449 – 511 .
- Novotny , V . 1994 . Relation between temporal persistence of host plants and wing length in leafhoppers (Hemiptera: Auchenorrhyncha) . Ecological Entomology , 19 : 168 – 176 .
- Roff , DA . 1986a . The evolution of wing polymorphism in insects . Evolution , 40 : 1009 – 1020 .
- Roff , DA . 1986b . The genetic basis of wing dimorphism in the sand cricket, Gryllus firmus and its relevance to the evolution of wing dimorphisms in insects . Heredity , 57 : 221 – 231 .
- Sakashita , T , Fujisaki , K and Nakasuji , F . 1995 . Environmental factors affecting wing length variation of a stink bug, Pyrrhocoris sibiricus (Heteroptera: Pyrrhocoridae) . Applied Entomology and Zoology , 30 : 303 – 308 .
- Sakashita , T , Nakasuji , F and Fujisaki , K . 1998 . Seasonal variation in wing polymorphism of the pyrrhocorid bug, Pyrrhocoris sibiricus (Heteroptera: Pyrrhocoridae) . Applied Entomology and Zoology , 33 : 243 – 246 .
- Sasaki , R , Nakasuji , F and Fujisaki , K . 2002 . Environmental factors determining wing form in the lygaeid bug, Dimorphopterus japonicus (Heteroptera: Lygaeidae) . Applied Entomology and Zoology , 37 : 329 – 333 .
- Sasaki , R , Nakasuji , F and Fujisaki , K . 2003 . Seasonal changes in wing dimorphism of the lygaeid bug Dimorphopterus japonicus (Heteroptera: Lygaeidae) in relation to environmental factors . Entomological Science , 6 : 63 – 70 .
- Slater , JA . 1975 . On the biology and zoogeography of Australian Lygaeidae (Hemiptera: Heteroptera) with special reference to the southwest fauna . Journal of the Australian Entomological Society , 14 : 47 – 64 .
- Slobreck , C . 1986 . Wing and flight muscle polymorphism in a lygaeid bug, Horvathiolus gibbicollis: determinants and life history consequences . Ecological Entomology , 11 : 435 – 444 .
- Socha , R . 2001 . Latitudinal gradient in response of wing polymorphism to photoperiod in a flightless bug, Pyrrhocoris apterus (Heteroptera: Pyrrhocoridae) . European Journal of Entomology , 98 : 167 – 169 .
- Swallow , WH and Cressey , PJ . 1987 . Wheat-bug damage in New Zealand wheats. 3. An historical overview . New Zealand Journal of Agricultural Research , 30 : 341 – 344 .
- Tanaka , S and Wolda , H . 1987 . Seasonal wing length dimorphism in a tropical seed bug: ecological significance of the short-winged form . Oecologia , 73 : 559 – 565 .
- Watt , JC . 1975 . “ The terrestrial insects ” . In Biogeography and ecology in New Zealand , Edited by: Kuschel , G . 507 – 533 . Junk : the Hague .
- Wei YJ 2001 . Nysius huttoni (Hemiptera: Lygaeidae): life history and some aspects of its biology and ecology in relation to wing development and flight . Unpublished Ph.D. thesis. University of Canterbury, New Zealand
- Wei , YJ . 2008a . Studies of life history and some aspects of field biology and ecology of Nysius huttoni White (Hemiptera: Lygaeidae) . Journal of the Royal Society of New Zealand , 38 : 149 – 162 .
- Wei , YJ . 2008b . Sex ratio of Nysius huttoni White (Hemiptera: Lygaeidae) in field and laboratory populations . New Zealand Journal of Zoology , 35 : 19 – 28 .
- Woodward , TE . 1954 . New records and descriptions of Hemiptera-Heteroptera from the Three King Islands . Records of the Auckland Institute and Museum , 4 : 215 – 233 .
- Zera , AJ , Innes , DJ and Saks , ME . 1983 . Genetic and environmental determinants of wing polymorphism in the waterstrider Limnoporus canaliculatus . Evolution , 37 : 513 – 522 .
- Zera , AJ and Denno , RF . 1997 . Physiology and ecology of dispersal polymorphism in insects . Annual Review of Entomology , 42 : 207 – 230 .