Abstract
Temperate species of sea cucumber found on rocky subtidal reefs often have a patchy distribution. If rocky reef complexity is important to the temperate sea cucumber Australostichopus mollis then this should be correlated with relative differences in their abundance along the coast. These temperate subtidal rocky reefs present a variety of possible habitat types that can be patchy in their distribution along the coast. Subtidal surveys were conducted along a 2.5-km stretch of coast and counts of A. mollis recorded along with changes in the composition of a number of physical features of reef structure. Abundance of A. mollis decreased from 1 individual/2 m2 in headland outcrops to 1 individual/20 m2 and 1 individual/100 m2 for coastal areas and inner bays respectively. This was associated with a decrease in percentage composition of large boulders, which were between 0.5 and 1 m along their longest diameter and were part of a predominantly rock and boulder substrate. Where sea cucumber abundance decreased for identified location types there was also a corresponding decrease in kelp cover and an increase in open rock faces of a ridged or corrugated structure. Physical features of temperate reef structure, their contribution to overall reef complexity, and location along the coast, are determining factors mediating the distribution and abundance of A. mollis.
Introduction
Limited quantitative information exists on the role of rocky reef structure in determining patterns of distribution and abundance of the New Zealand aspidochirote deposit feeding sea cucumber Australostichopus mollis (Hutton, 1872) (Holothuriidae) (Sewell Citation1990; Mladenov & Campbell Citation1996; Shears & Babcock Citation2007), which was formally known as Stichopus mollis (Moraes et al. Citation2004). Within these subtidal rocky reefs, topographic features exposed and acted on by erosion can be used to describe the relationship between a reef and inhabitants such as sea cucumbers. Previously, temperate reef systems have been classified according to community composition of flora and fauna, depth and exposure gradients (Ayling Citation1978; Choat & Schiel Citation1982; Taylor Citation1998). Classification of this topographic complexity by recording changes in the proportional composition of physical features should relate directly to the often patchy distribution and abundance of sea cucumbers such as A. mollis (Sewell Citation1990; Shears and Babcock Citation2007).
Other studies have related the distribution and abundance of sea cucumbers to physical features of reef structure (McEuen Citation1988; Mladenov & Campbell Citation1996; Zhou & Shirley Citation1996). In Milford Sound (South Island of New Zealand), abundance estimates of A. mollis were an average of 1 individual/10 m2 (Mladenov & Campbell Citation1996). These areas consisted of sheltered plunging deep rock faces with limited topographic complexity. Parastichopus californicus (Stimpson, 1857) has also been found at densities of 1 individual/5 m2 on nearly vertical rock walls (Zhou & Shirley Citation1996). Shell rubble and small rocks contained more P. californicus than habitats consisting of mud, sand and debris (Zhou & Shirley Citation1996). Small-scale variation in reef composition has also been related to the patchy distribution and abundance of Holothuria leucospilota (Brandt, 1835) (Dzeroski & Drumm Citation2003). Holothuria leucospilota was found to be more abundant in areas of rubble and boulders. Changes in the proportioning of substrate composition over small scales may have a significant influence on sea cucumber distribution and abundance.
Changes in distribution and abundance may reflect differences in movement patterns over time in relation to feeding behaviour. This has been observed for P. californicus, Holothuria mexicana (Ludwig, 1875) and Apostichopus parvimensis (Clark, 1913), and Holothuria scabra (Jaeger, 1933), respectively (Hammond Citation1982a; Silva et al. Citation1986; Purcell & Kirby Citation2006; Cieciel et al. Citation2009). Once a sea cucumber encounters a nutrient enriched area, they may discriminate between it and others. This has been observed in species such as Isostichopus badionotus (Selenka, 1867), H. mexicana, Holothuria arienicola (Semper, 1868), Holothuria gresiea (Selenka, 1867) and Actinopyga agassizi (Selenka, 1867) (Hammond Citation1982a, Citationb). Sediment patch selectivity may influence small-scale variation in distribution and abundance, but it is a contentious issue. Stichopus chloronotus (Brandt, 1835) and Stichopus horrens (Selenka, 1867) have been found to select sediment patches with a higher content of microalgae (Uthicke & Karez Citation1999). However, once in such an area there was no selective uptake of nutrient rich particles. Other studies have found significant increases in organic content in the gut of aspidochirote sea cucumbers because of organically selective feeding (Webb et al., Citation1977; Haukson Citation1979; Moriarty Citation1982; Roberts & Bryce Citation1982; Paltzat et al. Citation2008).
On rocky subtidal kelp-covered reefs, any resulting patchy distribution of sea cucumbers in relation to sediment nutrient composition and movement may be incidental, and more related to living macroalgae (kelp) cover and substrate composition being able to provide shelter. The degree of water movement and depth related distribution may also limit movement and behaviour in relation to feeding and thus distribution and abundance, irrespective of sediment nutrient content or its composition. For I. badionotus, occupation of substrate types was found to have more to do with shelter than food availability (Sloan & von Bodungen Citation1986). Distribution of I. badionotus was related to shelter from turbulent areas rather than depth (Sloan & von Bodungen 1986). Furthermore, total organic content (TOC) was not correlated with abundance, as substrate that had the lowest TOC was preferred. In another study, the high density of P. californicus on rock walls was also not related to food availability (Zhou & Shirley Citation1996). High densities of P. californicus at particular depth ranges was related to the amount of rock wall substrate present (Zhou & Shirley Citation1996). Depth related distribution may be a secondary consequence of the relationship between available shelter and habitat complexity and its influence on abundance in an area.
Irrespective of differences in feeding behaviour, deposit-feeding species utilise plant carbon as a major source of nutrition, in particular living diatoms and microalgae biomass (Hammond Citation1983; Uthicke & Karez Citation1999). However, the contribution of bacterial biomass and meiofauna to ingested carbon in sea cucumbers may be limited (Yingst Citation1976; Hammond Citation1983), although contrary evidence is presented for Holothuria tubulosa (Gmelin) and Holothuria theeli (Amon & Herndi Citation1991; Sonnenholzer Citation2003). Current evidence suggests that for detritovore feeding in sea cucumbers plant carbon may not be available without a bacterial vector (Wing et al. Citation2008; Slater & Carton Citation2010).
For the sea cucumber Australostichopus mollis, distribution occurs across a range of habitats around New Zealand (Mladenov & Campbell Citation1996; Shears & Babcock Citation2007). A. mollis can be found on coarse sand and mud in sheltered regions from low tide to 1530 m depth in Milford Sound, Southern-Western New Zealand (Dawbin Citation1949). It is also found in large numbers in the subtidal zone, low tide to 12 m depth, on sheltered and exposed rocky reefs and coastal areas of north eastern New Zealand (Sewell Citation1990; Sewell & Bergquist Citation1990; Archer Citation1996). Australostichopus mollis is typically 13–25 cm in length and often has a uni-modal size-frequency distribution. The ecology of A. mollis is little understood and limited to a few publications (Dawbin Citation1949; Sewell Citation1990; Archer Citation1996; Mladenov & Campbell Citation1996, Raj Citation1998). Its production, biology and feeding ecology has been studied in detail (Slater & Carton Citation2007; Morgan Citation2008, Citation2009; Stenton-Dozey & Heath Citation2009; Slater et al. Citation2010). In the present study, the relationship between distribution and abundance and physical features of rocky subtidal reef habitat complexity is described for a 2.5-km stretch of coastline. If rocky reef complexity is important to A. mollis, then this should be correlated with changes in distribution and abundance along the coast.
Materials and Methods
Surveying and data collection
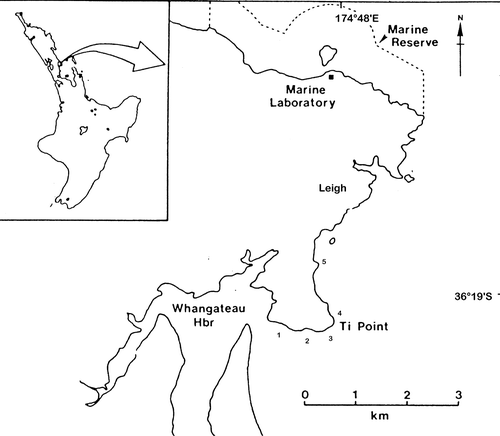
Diver transects were conducted over a period of three months from outside the entrance to Whangateau harbour (1), north-eastern New Zealand (36°19.24′S, 174°47.00′E) along the coast to Ti Point headland (2 and 3) (36°19.24′S, 174°48.04′E), around Ti Point headland (4) and Mathesons Bay (5) (36°18.31′S, 174°47.75′E) (). Covering an area of approximately 2.5 km, for each of groups 1–5 at a distance of about 50 m apart, a single line transect (50 m) was laid out from the edge of the coast approximately 1 m below Mean Low Water Spring (MLWS) to the deepest part of the rocky reef. In a similar manner subsequent transects were conducted. A total of 22 transects were completed and data for 247 quadrats recorded.
Figure 1 Map showing location of transect sites. 1, Whangateau harbour entrance (3 transects); 2, Torkington Bay (6 transects); 3 and 4, either side of Ti Point headland (3 transects and 5 transects respectively); 5, Mathesons Bay (5 transects). Modified from Sewell (1990). Dotted area represents boundary of Leigh Marine reserve.
A gridded rope quadrat (4×2 m; 8 m2) attached to plastic poles at either end was laid out and flipped horizontally end over end at 4-m intervals along the single line transect until the edge of the reef was reached at its deepest part. The rope grid was divided into 18 rope rectangles approximately 0.44 m2. For each quadrat, the grid was used to record the percentage composition of reef structure characteristics as well as the numbers of sea cucumbers (Dzeroski & Drumm Citation2003). Characteristics of reef structure were recorded as substrate type, boulder size class and depth, gradient and kelp cover. Depth (D) was recorded in metres and gradient (G) as an estimate of slope in degrees. Kelp cover (canopy C) within the rope quadrat grid was estimated as percentage of total cover. In each quadrat, the numbers of sea cucumbers were counted. In areas with high kelp cover and/or topographic complexity, a thorough search was conducted. However, it is likely numbers in these areas were underestimated.
The five substrate types identified and recorded as percentage composition of each quadrat were flat rock (FR), ridged rock (R), corrugated rock (C), rock and boulder (RB) and sediment (S_A). FR consisted of a rock face descending with little or no surface complexity, whereas R or C rock was either descending rock with ridges or valleys or undulating rock parallel to the coastline. RB consisted of a descending rock face of various rocks and boulders, and S_A was a continuous descending slope of particulate matter. Within each quadrat, boulder size classes were recorded in relative proportions as small (S < 20 cm), medium (M = 20–50 cm), large (L = 50–100 cm) and very large (VL > 100 cm) along their longest diameter.
Data analysis
All data was formatted and analysed using SPSS16 (SPSS UK Ltd, Surrey). Percentage composition of reef variables listed previously and counts of sea cucumbers were converted to proportions between 0 and 1 and density per m2respectively, log transformed and standardised. Next, habitat variables (independent variable) were used to determine if there was any clustering of transects by location type along the coast for density of sea cucumbers (dependent variable). For successive iterations of possible groupings that gave significant results, the most plausible number of possible groupings is then set by the user a priori based on knowledge of the area surveyed. In this case, area types were predetermined as headland outcrops, coastal areas and inner bays. This was done using two-step cluster analysis, which identifies mean sea cucumber density for all quadrats within each transect, groups them by location and then compares them with the overall mean density of sea cucumbers across the identified location types.
Concordance between reef structure and subjective classification of reef composition assigned to each survey quadrat was investigated. Multidimensional scaling (MDS) was used to create proximity transformations in a Bray–Curtis dissimilarity matrix to determine relatedness among reef structure variables. Coordinates were created for the first and second dimension for all variables simultaneously. Stress and fit was measured using Tuckers coefficient of congruence. A distance dendrogram was plotted from the resulting agglomeration schedule using the centroid method. Residual plots and normal probability plots showed the reef structure data as being homogenous and distributed normally.
Hierarchical analysis compiled from reef complexity parameters for the coast showed important attributes of recorded reef composition (Ruitton et al. Citation2000). Linkage was based on Euclidian distances and principal component analysis used to det ect the importance of these habitat variables and their contribution to variation in recorded reef composition. Possible relationships between log transformed habitat variables (independent variable) and density of sea cucumbers (dependent variable) for principal components were then tested using multiple regressions. Statistical differences in composition among habitat variables identified as significant and associated with changes in sea cucumber density between the three identified location types were sought with analysis of variance (ANOVA) and the Student–Newman–Keuls (SNK) test.
Results
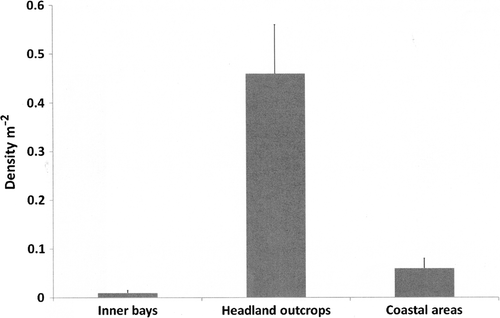
Clustering of density of sea cucumbers by transects around the coast was divided into three groupings, these being inner bays, headland outcrops and coastal areas for cluster one, two and three respectively (). By composition, clusters accounted for 42.5, 31.6 and 25.9% respectively. Density of sea cucumbers varied between the three groupings. The density of sea cucumbers for headland outcrops was significantly higher than both inner bays and coastal areas (0.45±0.10 m−2; P<0.01). For headland outcrops, counts decreased from 1 individual/2 m2 to 1 individual/20 m2 and 1 individual/100 m2 for coastal areas and inner bays respectively.
Figure 2 Mean density m−2 of Australostichopus mollis for transect cluster groupings by location type along a 2.5-km stretch of coast using two-step cluster analysis (mean±SEM).
Relatedness among habitat variables indicated that RB substrate, VL and L boulder size classes, kelp cover (canopy C) and to a certain extent depth, gradient, R and C reef structures were important (Tuckers coefficient of congruence 0.996). Consequently, other variables were not used for PCA. The first two axes of PCA across all habitat variables simultaneously explained 38.6% of total variability, with PCA3 making up another 12.4% (). Two further axes provided limited information of explicative value. Eigen values for habitat assemblages in the first two PCs affecting sea cucumber density (dependent variable) were mainly explained by the opposition between presence of RB substrate, VL and L boulder size classes and the absence of R, C and FR reef structure in relation to depth, gradient and canopy (kelp) cover, which appeared in subsequent axes.
Table 1 The first five principal components, their Eigen value, and the weighting of each habitat composition variable (independent variable) for density in m−2 of the sea cucumber Australostichopus mollis (dependent variable).
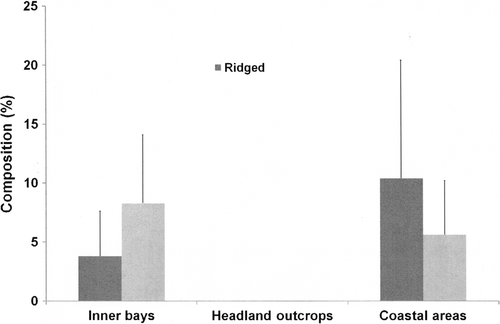
For PC1, VL boulder, R, C and RB substrate were associated with the density of sea cucumbers (df = 4, F=9; P<0.01). Of these, both RB substrate and VL rocks were significant (P<0.01). In PC2, gradient, VL and L were associated with the density of sea cucumbers (df = 3, F=8.3, P<0.01). Of these, L boulders were significant (P<0.01). For PC3, 4 and 5, depth, R, canopy (kelp cover) and FR were associated with the density of sea cucumbers (df = 5, F=7.3, P<0.01). Of these, R, kelp (canopy C) and FR were significant (P<0.01). This was related to the absence of the other defining reef structures where R, C and FR bottom types became predominant (). A complete absence of R and C habitat types existed in headland outcrop areas where high densities of A. mollis occurred, and although present in the other two location types along the coast, it was variable.
Figure 3 Percentage composition of ridged (R) and corrugated (C) reef structure across the three identified location type clusters along the coast as being significantly associated with the absence of Australostichopus mollis (mean±SEM).
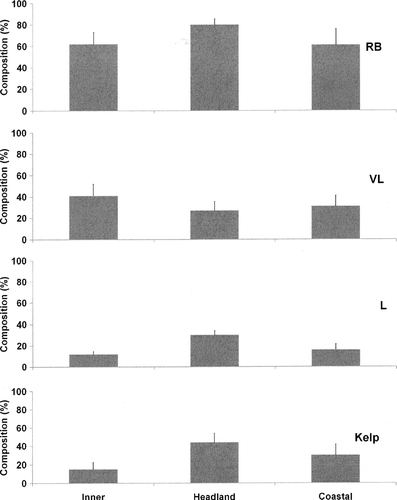
Across the three location types, there was significantly less RB substrate for inner bays (P<0.01; ). However, there was no difference in the contribution of VL boulder size class to reef composition across the three location types (P>0.01). Despite this, for L boulders (between 0.5 and 1 m along the longest diameter), significantly more occurred in headland outcrops than coastal areas or inner bays (P<0.01). Lastly, there was significantly less kelp cover in inner bays compared with headland outcrops or coastal areas (P<0.01). Abundance of sea cucumbers in headland outcrop areas were highest where high proportions of large boulders (30±4%) contributed to predominantly RB substrate (80±5.5%) and where overlying kelp (canopy C) (44±10%) existed in greater proportions compared with inner bays and coastal areas (mean±SEM).
Figure 4 Percentage composition of habitat variables identified as significantly associated with changes in abundance of Australostichopus mollis for each identified location type (mean±SE). L, large (0.5–1 m); VL, very large rocks (>1 m) along longest diameter; RB, rock and boulder substrate (mean±SEM).
Discussion
Changes in the distribution and abundance of A. mollis were associated with reef structure across three location type classifications along the coast. Locations consisted of headland outcrop areas where abundance of A. mollis was high, to coastal areas and inner bays where less occurred. Abundance of A. mollis was also highest in areas previously examined (Sewell Citation1990). These areas consisted of boulders and cobbles mixed with areas of the algae Caropphyllum flexuosum and some Ecklonia radiata (Sewell Citation1990). Similar observations have been made for P. californicus in Barlow Cove, South east Alaska Bay, where densities decreased from 104 individuals/ha in the outer cove to 71 and 21 individuals/ha in the middle and inner cove respectively (Zhou & Shirley Citation1996). Across these locations, the proportion of rock wall, rock substrate, shell and debris decreased towards the inner cove. Even for H. leucospilota, changes in distribution and abundance were associated with four types of site in addition to differences in the composition of habitat (Dzeroski & Drumm Citation2003). In the present study the proportion of large boulders (between 0.5 and 1 m along their longest diameter) contributing to RB substrate decreased across the three location types and was associated with relative changes in abundance of sea cucumbers.
The proportion of kelp cover was also variable across the three location types identified and less kelp cover was found in inner bays. However, within these locations the density of sea cucumbers on substrate amongst kelp was inconsistent and not well correlated. This may be related to changes in underlying substrate complexity beneath the kelp providing variable amounts of shelter. For example, dendrochirotid sea cucumbers have been found to occupy rocky habitats and passes between channel islands in areas of high water movement (McEuen Citation1988). Psolus chitonoides (Clark, 1901) has also been found in high current areas that are topographically complex and have lots of water movement (Cameron & Fankboner Citation1989). Alternatively, the aspidochirote sea cucumber P. californicus has been found in topographically less complex rocky intertidal and subtidal bays and pilings away from strong wave action (McEuen & Chia Citation1991). In the present study outside of inner bays, the proportional changes in sea cucumber abundance relative to percent cover of kelp appeared influenced by the degree of underlying substrate complexity. It may not be the presence of kelp itself, but changes in the underlying topographic structure in providing shelter within a location that determined changes in abundance of sea cucumbers with distribution amongst kelp.
More sea cucumbers were found in deeper areas (over 4 m) than in shallower areas. In deeper locations in headland outcrops, 1 individual/5.0 m2 was found, decreasing to 1 individual/12.5 m2 in shallow coastal areas in less than 4 m of water. In a previous study, although P. californicus was found to occupy sheltered rocky intertidal and subtidal areas, it was not known how abundance varied with depth (Cameron & Fankboner Citation1989). However, P. californicus was found in higher densities above 60 m on rock walls and between 100 and 150 m depth (Zhou & Shirley Citation1996). This was linked to the depth distribution of rock wall rather than any specific association with water depth. In the present study, deeper coastal areas and headland outcrops with increased kelp cover can be topographically less complex with varying substrate types. Consequently, in these areas any increase in abundance of sea cucumbers may be related to the amount of shelter present rather than depth. Depth appeared secondary to the importance of underlying topographic complexity and its interaction with kelp cover, a key determinant of sea cucumber distribution and abundance.
Available shelter may also be used as a refuge from predation. Predation has been observed previously on A. mollis by the sea star Luidia maculata (Müller & Troschel, 1842) (Sewell Citation1990). No evisceration in response to predation was observed and in one instance, the individual survived predation and crawled off under rocks. Altho ugh 76 types of predator species have been reported for sea cucumbers, and seastars are cited most often (Francour Citation1997), there is little evidence to suggest that predation would have a significant impact on the distribution and abundance of A. mollis. Toxicity of the animal is suggested to be an effective defence against generalist predators, except specialist predators where the only escape is by shedding a piece of body wall (Francour Citation1997). ‘These escape behaviours may be a factor in providing apparent size refuge for from predators’ (Francour Citation1997). Shelter as a refuge from predation may be coincidental rather than a determining factor mediating distribution and abundance of A. mollis.
Previous studies on other sea cucumbers have also shown that topographic complexity appears not to be a limiting factor in affecting movement and orientation, nor is the absence of food necessarily an incentive to leave an area. For instance, it is common for sea cucumbers to travel up to 4 m/day (Hammond Citation1982b; Silva et al. Citation1986). Parastichopus californicus departed an 8-m radius sample site within 8 days (Silva et al. Citation1986). For H. scabra, which occupies sea grass beds, movement was 1.3 m over a 16-h period at night (Purcell & Kirby Citation2006). It was also found that the random orientation in P. calfornicus, appeared to be associated with an evenly distributed and renewable food source. In extreme cases, sites that are a repository for detritus have been shown to minimise movement in P. californicus (Silva et al. Citation1986). Similarly, for A. mollis limitations on movement are also not likely to be associated with the degree of topographic complexity or with the lack of presence of carbon sources for ingestion.
In the present study although food availability was not quantified, feeding could be random with continuous movement in an environment where food sources are limited or seasonally abundant. For example, seasonally rather than moving to try and locate a new food source, P. californicus was found to stop growing until conditions improved (Silva et al. Citation1986). This ceasing of growth may also be linked to the association of habitat variables with entrapment of appropriate food sources and distribution and abundance in H. theeli (Sonnenholzer Citation2003). Equally, A. mollis also exhibits seasonal growth and during the autumn and winter month's population mortality rate is high and body weight decreases (Sewell Citation1990; Slater & Jeffs Citation2010). This may reflect a possible link between habitat and random foraging, rather than actively seeking a food source over large areas. This may be linked with the seasonal availability of phytoplankton and production of associated benthic diatoms and bacterial biomass (Moriarty Citation1982; Wing et al. Citation2008; Slater & Carton Citation2010). The seasonal presence of a living benthic food source and its association with available habitat is more likely to affect the abundance of A. mollis than its distribution. Some sea cucumbers may ‘starve’ themselves to death.
In conclusion, variation in identified reef habitat structure that was closely associated with the presence of sea cucumbers was attributed to the three identified location types. Within and between these, ideal sea cucumber habitat was characterised by a high proportion of large sized boulders contributing to predominantly RB substrate and the degree of underlying substrate complexity beneath decreasing kelp cover. Typically, where this contribution decreased the numbers of sea cucumbers decreased proportionately.
Acknowledgements
I thank R. Babcock and M. Sewell for their assistance and mentoring during research. I am grateful to L. Hack for assistance with diving and surveying. Staff at the University of Auckland Leigh Marine Laboratory provided assistance with equipment and facilities. Thanks to two anonymous reviewers for comments on the manuscript.
References
- Amon , RMW and Herndi , GJ . 1991 . Deposit feeding and sediment. I. Interrelationship between Holothuria tubulosa (Holothuroida, Echinodermata) and the sediment microbial community . Marine Ecology , 12 : 163 – 174 .
- Archer JE 1996 . Aspects of the reproductive and larval biology and ecology, of the temperate holothurian Stichopus mollis (Hutton) . School of Biological Sciences. Auckland, University of Auckland. Masters degree thesis .
- Ayling AM 1978 . Cape Rodney to Okakari Point marine reserve survey . Leigh Marine Laboratory Bulletin 1. Leigh Marine Laboratory, Auckland .
- Cameron , JL and Fankboner , PV . 1989 . Reproductive biology of the commercial sea cucumber Parastichopus californicus (Stimpson) (Echinodermata: Holothuroidea). II. Observations on the ecology of development, recruitment, and the juvenile life stage . Journal of Experimental Marine Biology and Ecology , 127 : 43 – 67 .
- Choat , JH and Schiel , DR . 1982 . Patterns of distribution and abundance of large brown algae and invertebrate herbivores in subtidal regions of northern New Zealand . Journal of Experimental Marine Biology and Ecology , 60 : 129 – 162 .
- Cieciel , K , Pyper , BJ and Eckert , GL . 2009 . Tag retention and effects of tagging on movement of the Giant Red Sea cucumber Parastichopus californicus . North American Journal of Fisheries Management , 29 : 288 – 294 .
- Dawbin , WH . 1949 . Auto-evisceration and the regeneration of viscera in the holothurian Stichopus mollis (Hutton) . Transactions of the Royal Society of New Zealand , 77 : 497 – 523 .
- Dzeroski , S and Drumm , D . 2003 . Using regression trees to identify the habitat preference of the sea cucumber Holothuria leucospilota on Raotonga, Cook Islands . Ecological Modelling , 170 : 219 – 226 .
- Francour , P . 1997 . Predation on holothurians: a literature review . Invertebrate Biology , 116 : 52 – 60 .
- Hammond , LS . 1982a . Patterns of feeding and activity in deposit-feeding holothurians and echinoids (Echinodermata) from a shallow back-reef lagoon, Discovery Bay, Jamaica, West Indies . Bulletin of Marine Science , 32 : 549 – 571 .
- Hammond , LS . 1982b . Analysis of Grain-Size selection by deposit-feeding holothurians and echinoids (Echinodermata) from a Shallow Reef lagoon, Discovery Bay, Jamaica . Marine Ecology Progress Series , 8 : 25 – 36 .
- Hammond , LS . 1983 . Nutrition of deposit-feeding holothuroids and echinoids (Echinodermata) from a shallow reef lagoon, Discovery Bay, Jamaica . Marine Ecology Progress Series , 10 : 297 – 305 .
- Haukson , E . 1979 . Feeding biology of Stichopus tremulus, a deposit feeding holothurian . Sarsia , 64 : 155 – 159 .
- McEuen , FS . 1988 . Spawning behaviours of northeast Pacific sea cucumbers (Holothuroidea: Echinodermata) . Marine Biology , 98 : 565 – 585 .
- McEuen , FS and Chia , F-S . 1991 . Development and metamorphosis of two psolid sea cucumbers, Psolus chitnoides and Psolidium bullatum, with a review of reproductive patterns in the family Psolidae (Holothuroidea: Echinodermata) . Marine Biology , 109 : 267 – 279 .
- Mladenov PV Campbell A Resource evaluation of the sea cucumber, Stichopus mollis, in the environmentally sensitive Fiordland region of New Zealand Proceedings of the ninth international echinoderm conference. San Francisco, CA; A.A. Balkema, Rotterdam, Brookfield 9 1996 481 487
- Moraes , G , Norhcote , PC , Kalinin , VI , Avilov , SA , Silchenko , AS , Dmitrenok , PS , Stonik , VA and Levin , VS . 2004 . Structure of the major triterpene glycoside from the sea cucumber Stichopus mollis and evidence to reclassify this species into the new genus Australostichopus . Biochemical Systematics and Ecology , 32 : 637 – 650 .
- Morgan , AD . 2008 . The effect of food availability on phenotypic plasticity in larvae of the temperate sea cucumber Australostichopus mollis . Journal of Experimental Marine Biology and Ecology , 363 : 89 – 95 .
- Morgan , AD . 2009 . Assessment of egg and larval quality during hatchery production of the temperate sea cucumber Australostichopus mollis . Journal of the World Aquaculture Society , 40 : 629 – 642 .
- Moriarty , DJW . 1982 . Feeding of Holothuria atra and Stichopus chloronotus on bacteria, organic carbon and organic nitrogen in sediments of the Great Barrier Reef . Australian Journal of Marine and Freshwater Research , 33 : 255 – 263 .
- Paltzat , DL , Pearce , CM , Barnes , PA and McKinley , RS . 2008 . Growth and production of California sea cucumbers (Parastichopus californicus Stimpson) co-cultured with suspended Pacific oysters (Crassostrea gigas Thunberg) . Aquaculture , 275 : 124 – 137 .
- Purcell , SW and Kirby , DS . 2006 . Restocking the sea cucumber Holothuria scabra: Sizing no-take zones through individual-based movement modelling . Fisheries Research , 80 : 53 – 61 .
- Raj L 1998 University of Otago Dunedin . Reproductive biology and the use of photo identification to study growth in Stichopus mollis (Echinodermata: Holothuroidea) in Doubtful Sounds, Fiordland, New Zealand . MSc thesis .
- Roberts , D and Bryce , C . 1982 . Further observations on tentacular feeding mechanisms in holothurians . Journal of Experimental Marine Biology and Ecology , 59 : 151 – 163 .
- Ruitton , SR , Francour , P and Boudouresque , CF . 2000 . Relationships between algae, benthic herbivorous invertebrates and fishes in rocky sublittoral communities of a temperate sea (Mediterranean) . Estuarine, Coastal and Shelf Science , 50 : 217 – 230 .
- Sewell , MA . 1990 . Aspects of the Ecology of Stichopus mollis (Echinodermata: Holothuroidea) in Northeastern New Zealand . New Zealand Journal of Marine and Freshwater Research , 24 : 97 – 104 .
- Sewell , MA and Bergquist , PR . 1990 . Variability in the reproductive cycle of Stichopus mollis (Echinodermata: Holothuroidea) . Invertebrate Reproduction and Development , 17 : 1 – 8 .
- Shears NT , Babcock RC 2007 . Quantitative description of mainland New Zealand's shallow subtidal reef communities . Science for Conservation. 280. Science and Technical Publishing. Department of Conservation .
- Silva , J , Cameron , JL and Fankboner , PV . 1986 . Movement and orientation patterns in the commercial sea cucumber Parastichopus californicus (Stimpson) (Holothuroidea: Aspidochirotida) . Marine Behaviour and Physiology , 12 : 133 – 147 .
- Slater , MJ and Carton , AG . 2007 . Survivorship and growth of the sea cucumber Australostichopus (Stichopus) mollis (Hutton 1872) in polyculture trials with green-lipped mussel farms . Aquaculture , 272 : 389 – 398 .
- Slater , MJ and Carton , AG . 2010 . Sea cucumber habitat differentiation and site retention as determined by intraspecific stable isotope variation . Aquaculture Research , 41 : 695 – 702 .
- Slater , MJ and Jeffs , A . 2010 . Do benthic sediment characteristics explain the distribution of juveniles of the deposit-feeding sea cucumber Australostichopus mollis? . Journal of Sea Research. , 64 : 241 – 249 .
- Slater , MJ , Carton , AG and Jeffs , AG . 2010 . Highly localised distribution patterns of juvenile sea cucumber Australostichopus mollis . New Zealand Journal of Marine and Freshwater Research , 44 : 201 – 216 .
- Sloan , NA and von Bodungen , B . 1986 . Distribution and feeding of the sea cucumber Isotichopus badionotus in relation to shelter and sediment criteria of the Bermuda Platform . Marine Ecology Progress Series , 2 : 257 – 264 .
- Sonnenholzer , J . 2003 . Seasonal variation in the food composition of Holothuria theeli (Holothuroidea: Aspidochirotida) with observations on density and distribution patterns at the central coast of Ecuador . Bulletin of Marine Science , 73 : 527 – 543 .
- Stenton-Dozey , J and Heath , P . 2009 . A first for New Zealand: culturing our endemic sea cucumber for overseas markets . Water and Atmosphere , 17 : 20 – 21 .
- Taylor , RB . 1998 . Density, biomass and productivity of animals in four subtidal rocky reef habitats: the importance of small mobile invertebrates . Marine Ecology Progress Series , 172 : 37 – 51 .
- Uthicke , S and Karez , R . 1999 . Sediment patch selectivity in tropical sea cucumbers (Holothuroidea: Aspidochirotida) analysed with multiple choice experiments . Journal of Experimental Marine Biology and Ecology , 236 : 69 – 87 .
- Webb KL , D'Elia CF , Dupaul WD 1977 Third International Coral Reef Symposium Miami, FL . Biomass and nutrition flux measurements on Holothuria atra populations on windward reef flats at Enewetak, Marshall Islands . In : Taylor DL . Pp. 410 – 441 .
- Wing , SR , McLeod , RJ , Clark , KL and Frew , RD . 2008 . Plasticity in the diet of two echinoderm species across an ecotone: microbial recycling of forest litter and bottom-up forcing of population structure . Marine Ecology Progress Series , 360 : 115 – 123 .
- Yingst , JY . 1976 . The utilisation of organic matter in shallow marine sediments by an epibenthic deposit-feeding holothurian . Journal of Experimental Marine Biology and Ecology , 23 : 55 – 69 .
- Zhou , S and Shirley , TC . 1996 . Habitat and depth distribution of the red sea cucumber Parastichopus californicus in a Southeast Alaska Bay . Alaska Fishery Research Bulletin , 3 : 123 – 131 .