Abstract
The hihi is an endangered New Zealand species and its survival depends on the health of translocated populations. Low nestling survival was detected at Zealandia–Karori Sanctuary (KS) during the 2008–09 breeding season with 34% of eggs surviving to fledge. Samples obtained from live and dead hihi nestlings showed that several disease syndromes contributed to nestling deaths. Cause of death was diagnosed by necropsy and histopathology for 25 nestlings. Mortality was highest (60%) in nestlings ≤7 days old and was associated with seasonally low minimum daily temperatures (<11°C). Deaths in this age group were most commonly attributed to poor pulmonary aeration and starvation. Ventriculitis was associated with mortality in eight 6–19-day-old nestlings with no indication that weather was implicated. The remaining deaths were from a variety of unrelated events or causes. Candida albicans, Aspergillus sp. and an unidentified haemoparasite were found among the nestling population. There was no evidence of coccidia or other intestinal parasite infections. In contrast to translocated populations at other sites, nesting material recovered from nest boxes contained low densities of tropical fowl mites (Ornithonyssus bursa). To improve survival for nestlings up to 7 days old, extra food provisioning and nest box weather protection during inclement weather is suggested.
Introduction
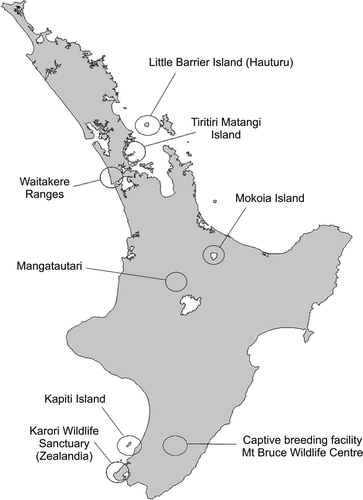
Extended isolation has resulted in the flora and fauna of New Zealand consisting of a small albeit distinctive number of species and relicts that no longer exist elsewhere. Recent phylogenetic studies of the endangered hihi (stitchbird Notiomystis cincta) placed this passerine into a newly formed endemic family, the Notiomystidae (Driskell et al. Citation2007; Ewen et al. Citation2006). Formerly widespread across the North Island and some adjacent offshore islands, hihi disappeared from the mainland following European colonisation (Oliver Citation1955). Habitat modification and destruction, introduced predators and infectious disease (Angehr Citation1984; Alley et al. Citation1999) probably combined to restrict hihi to a single remnant population on Hauturu (Little Barrier Island; Oliver Citation1955) of ≤2000 individuals (Taylor et al. Citation2005). Hihi recovery efforts have focused on translocations. Currently all reintroduced populations () depend on management to remain viable and are constantly monitored (Taylor et al. Citation2005). The number of hihi in the translocated populations may number ≤500; estimates ranging from 20 hihi in the Waitakere Ranges up to c. 200 on Kapiti Island and Tiritiri Matangi Island. Food availability is the only factor experimentally tested and proven to affect population growth in hihi (Castro et al. Citation2003; Armstrong et al. Citation2007). Ecological factors, such as forest type and maturity, climate, number and types of competitor species, that limit the long-term survival of the reintroduced populations may vary for each site (Armstrong et al. Citation2002).
Parasites and pathogens cause morbidity and mortality in hihi at all translocation sites (Taylor et al. Citation2005). Nest mite infestations limited the Mokoia population during the breeding season (Armstrong et al. Citation2002) and nestlings on Tiritiri Matangi Island are subject to ongoing management strategies to control nest mite numbers (Taylor et al. Citation2005). Coccidiosis causes sub-clinical infections that may compromise the immune system when stress factors are introduced, and has been a cause of deaths in captive hihi (Pauli Citation2000; Twentyman Citation2001). Aspergillosis causes disease at all sites but a high incidence on Mokoia Island that increased adult mortality (Alley et al. Citation1999) meant all surviving birds were removed in 2002 (Armstrong et al. Citation2007). An outbreak of Salmonella Typhimurium DT195, a human strain not previously described in New Zealand wildlife, was estimated to have killed c. 26% of the adult hihi in a short, acute outbreak (Ewen et al. Citation2007). Fortunately, this outbreak was self-limiting (Ewen et al. Citation2007), although it gave an indication of how infectious disease may decimate a naive population. Disease consequences may be greater in young birds than in adults and, because high mortality in nestlings affects recruitment, disease outbreaks may directly affect population viability (Friend & Franson Citation1999).
The aim of this study was to investigate the diseases that impact on nestling survival in a reintroduced hihi population.
Materials and methods
Monitoring and sampling
This study was undertaken during the hihi breeding season from December 2008 to March 2009 at Zealandia–Karori Sanctuary (KS) (41°17′S, 174°44′E), a mainland island sanctuary situated in suburban Wellington. Management of this recently reintroduced population includes colour banding all individuals for monitoring survival and breeding success, as well as provision and maintenance of nest boxes and supplementary food (Empson & Booth Citation2009).
Hihi rear several clutches per year (Castro et al. Citation1996, Citation2003) and nestlings fledge 28–30 days after hatching (Higgins et al. Citation2001). As part of management, nest boxes were checked every 2–3 days except during periods of change such as before hatching and fledging when checks were done daily. In this investigation, dead nestlings and samples from live nestlings were collected and screened for the possible presence of disease causing processes. It was necessary to limit sample collection from live nestlings in order to cause minimal stress and disruption. We therefore sampled nestlings at 5–9-day intervals from hatching until banding at approximately 20 days old. The sampling schedule included the collection of faecal and oral swabs together with examination of chicks and nests for ectoparasites at each sampling occasion; and a single blood sample and nest lining sample at the time of banding.
To ensure that comparisons could be made between live and dead nestlings, similar swab samples from dead nestlings were collected. Immediately following collection of the microbiology samples, the carcasses were preserved in formalin, and while this restricted the information regarding infectious pathogens present, the tissues were well preserved for histopathology.
Daily temperature from the Kelburn weather station was provided by New Zealand National Institute for Water and Atmospheric Research (NIWA). Daily rainfall for the Karori Reservoir was provided by the Resource Investigations Department of the Greater Wellington Region.
Sample collection was approved by Massey University Animal Ethics Committee protocol 08/96 and Department of Conservation Low Impact Research Permit WE/346/RES.
Histopathology
Dead featherless nestlings were fixed whole in 10% buffered formalin and feathered chicks were incised down the ventral midline to expose the visceral organs before fixation. Dead birds were submitted to the New Zealand Wildlife Health Centre (NZWHC) at Massey University, Palmerston North, for necropsy. Following fixation, neonates and very small nestlings were cut in 3–5 mm transverse sections along the length of the body, so that the whole bird was processed. For larger birds, four to five 3–5-mm cross-sections were obtained along the length of the body. Sections were routinely processed and embedded in paraffin blocks from which 5-µm sections were cut and stained using haematoxylin and eosin (H&E). Following microscopic examination, further sections were cut to access tissues or lesions deeper in the blocks, or to confirm the presence of bacteria or fungi using Gram–Twort (Twort Citation1924) or Young's fungal (Young Citation1969) stains.
Microbiology
Faecal samples from live nestlings were collected directly into specimen containers or into plastic bags, Oral and faecal and/or cloacal swabs from live and dead nestlings were placed into Amies transport medium (Copan Venturi TransystemTM, Amies Agar Gel Medium without Charcoal, Fort Richard Laboratories, Auckland, NZ). Samples were held at 4°C during transportation and until testing. Samples were cultured using routine methods for bacterial and fungal isolation (Quinn et al. Citation1994). Columbia 5% Sheep Blood agar (CBA; Fort Richard Laboratories, Auckland, NZ) was inoculated and incubated aerobically at 35°C for 3 days. Sabouraud's Dextrose agar with antibiotics (SDA; Fort Richard Laboratories, Auckland, NZ) was incubated aerobically at 25°C for 10 days to screen for Candida spp. and Aspergillus spp. Colonies of interest were subcultured onto SDA and CBA for purity and preliminary identification by colonial morphology and Gram stain morphology. Diagnostic tests including germ tube production (Quinn et al. Citation1994) and specific growth characteristics on BBLTM CHROMagarTM Candida agar (Fort Richard, New Zealand) (Hazen & Howell Citation2003) were undertaken where necessary.
Haematology
Blood samples (≤75 µl) were collected by venipuncture of the brachial vein, stored on ice for transportation and analysed within 8 h of collection. Blood films were prepared using the slide drag technique (Fudge Citation2000) and air dried at the time of collection. Blood parameters were assessed using standard haematological techniques (Fudge Citation2000).
Parasitology
Nestlings were examined for the presence of mites, with particular attention to areas around the vent or cloaca and eyes, under the bill and wings, between the toes and on the ventral surface of the abdomen (Powlesland Citation1976). Nest boxes were checked for the presence of mites by rustling nest material (Powlesland Citation1976) and visual examination. Nest lining samples, as well as entire nests, at the conclusion of each nesting attempt, were sealed in plastic containers, and frozen to preserve mites for later identification. Nest-lining samples and nests were thawed, dismantled and examined for the presence of nest mites. Mites were preserved in 95% ethanol and submitted to Dr Allen Heath, AgResearch Wallaceville, Upper Hutt, NZ.
Faecal flotation and examination of faeces for coccidia, capillaria and other helminth eggs was performed using standard techniques (Fudge Citation2000) and a modified centrifugal method using saturated zinc sulphate solution (Hendrix Citation1997) for trematodes.
Where indicated, molecular techniques were used to test for the presence of avian haemoparasites. DNA extraction from blood samples and paraffin-embedded tissues was carried out using the Qiagen DNeasyTM blood and tissue kit (Qiagen, Valencia, CA, USA) (Alley et al. Citation2008). The DNA obtained was amplified using multiple cycle polymerase chain reactions (PCR) in a nested assay, to screen for the presence of Leucocytozoon spp. alone, and for Plasmodium spp. and Haemoproteus spp. in combination (Hellgren & Waldenstrom Citation2004). The products of PCR were separated by electrophoresis on an agarose gel containing ethidium bromide and visualised on a transilluminator under UV light (Hellgren & Waldenstrom Citation2004).
Statistical analyses
Kendall correlations were used to examine the relationship between the categorical variables: cause of death, nestling age, temperature and clutch order. Packed cell volume (PCV) data were normally distributed (Shapiro–Wilkinson test W=0.948, P-value = 0.27) and Pearson correlations were used to determine the relationship between PCV values and both nestling age and weight at the time of sampling. T-test was used to look at differences in PCV values between clutches two and three. Tests were deemed significant if P≤0.05.
Results
Eighty-two eggs hatched and 50 chicks fledged during the 2008–09 breeding season at KS. Thirty-two hihi nestlings died during the study period and 25 (78%) were recovered for necropsy examination; 14 from the first clutch, nine from the second and two from the third. The 22% of nestlings not recovered have been excluded from this study because the cause of death is unknown. The 2008–09 breeding season outcomes were poor compared with previous years at this site (34% of eggs survived to fledge versus 52% in 2005–06 and 58% in 2006–07) (Empson & Booth Citation2009) because of increased hatching failure rates and nestling deaths affecting the first clutch ().
Table 1 Productivity for 2008–09 hihi breeding season at Zealandia–Karori Sanctuary.
Nestling age and mortality
Primary diagnoses have been grouped into four major categories: starvation, unknown with poor lung aeration, ventriculitis (inflammation of the gizzard) and other, which includes trauma, sporadic infectious diseases and congenital malformations (). Nestling age was significantly correlated with cause of death (Kendall correlation coefficient (KCC) =0.102; P=0.049; A) with younger nestlings more prone to die of starvation and poor pulmonary aeration than older nestlings. Sixty per cent of the mortality events occurred in nestlings <7 days old.
Figure 2 Correlation between age at death for 25 hihi nestlings that died at Zealandia– Karori Sanctuary during the 2008–09 breeding season. A, COD, cause of death. 1, starvation; 2, unknown with poor lung aeration; 3, ventriculitis; 4, other (see for details). B, Minimum average temperature on the day of, or immediately preceding, death. C, Clutch order.
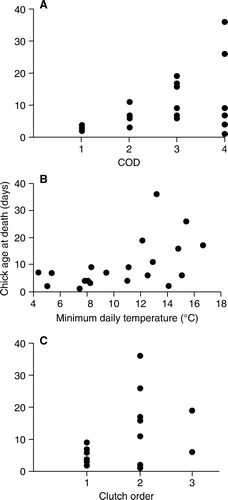
Table 2 Causes of mortality and pathology in hihi nestlings at Zealandia–Karori Sanctuary.
Young nestlings ≤10 days old were also more prone to die following daily minimum temperatures between 4 and 11°C (KCC = 0.165; P=0.006; B). Intermittent unseasonal temperatures below 11°C between 26 October and 31 December affected nestlings up to 7 days old at six nest boxes during the first and second clutches. During this period, hypothermia was associated with the deaths of 11 nestlings; five with poor lung aeration, four from starvation, and one each from necrotising hepatopathy and unknown cause.
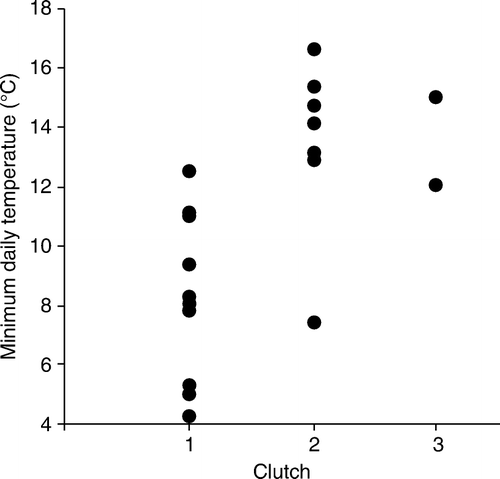
There was a significant correlation between clutch order and age at death (KCC = 0.146; P=0.026; C). First-clutch nestlings all died when ≤10 days old while nestlings of all ages died from the second and third clutches. First-clutch nestlings were particularly affected by starvation and poor lung aeration that was associated with low temperatures (). In contrast, starvation and poor pulmonary aeration were each associated with the death of a single nestling in the second clutch and none from the third.
Figure 3 Correlation of minimum daily temperature at the time of death and clutch order for 25 hihi nestlings that died at Zealandia–Karori Sanctuary during the 2008–09 breeding season.
Other causes of death in nestlings included ventriculitis and trauma; however, these deaths were not associated with specific weather events and/or occurred in older nestlings (B). Ventriculitis lesions were found in 32% of the nestlings examined, and in three of the four affected, was associated with penetration lesions caused by remnants of insects in their diet (bee or wasp sting shafts) (Rippon et al. in press). Deaths in this category affected 6–19-day-old nestlings at a single nest from each of the three clutch orders. Mortality for the remaining 28% of nestlings was related to a variety of causes (; A). In nestlings less than 7 days old, there was one case of necrotising hepatopathy, one death was from a congenital abnormality (cranial meningiocoele) and cause of death for the remaining nestling was undetermined. Two older nestlings died at or after fledging and these deaths were not associated with low temperatures (B); one 26-day-old chick showed lesions consistent with haemoparasitism while the other succumbed to aspergillosis at 36 days of age.
Pathology
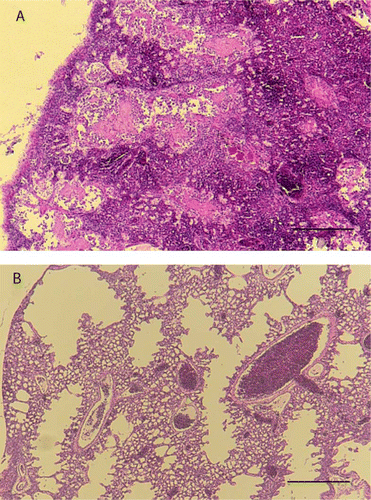
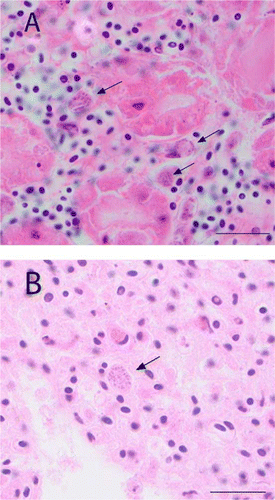
Starvation was diagnosed in nestlings with little or no fat reserves in subcutaneous tissues, in conjunction with no, or very little, ingesta present in the gastrointestinal tract; where yolk sacs were found, these were very small. Chicks with poor lung aeration and no other findings were placed in a category of ‘unknown with poor lung aeration’. These chicks had evidence of total or partial airway collapse (bronchi and parabronchi) indicating that the chick was not breathing adequately at the time of death. Congestion and haemorrhage was frequently observed associated with poorly inflated airways and the presence of proteinaceous fluid (A) within airways. Occasionally fibrin, sloughed epithelial cells, macrophages and small amounts of aspirated debris were found in conjunction with the proteinaceous fluid. In one case, there was trauma associated with the fluid accumulation, but in most cases there was no evidence of underlying systemic disease or trauma.
Figure 4 Photomicrographs of nestling lung tissue A, Tissue from chick 42499 showing poor lung aeration, congestion and accumulation of proteinaceous fluid in the airways. B, Tissue from chick 42843 with ventriculitis and showing good lung aeration. (Bars = 400 µm) (H&E stain).
Histopathological lesions consistent with a bacteraemia were seen in seven nestlings. These consisted of multifocal lesions of ac ute inflammatory necrosis involving skeletal muscle, myocardium, liver, spleen and occasionally the kidney. A few heterophils, which were sometimes necrotic, surrounded clumps of Gram-negative bacteria present within blood vessels, endothelial cells or necrotic myocytes. All these cases showed evidence of traumatic ventriculitis associated with insect sting penetration through the gizzard or intestine wall (Rippon et al. in press).
In the nestling that died of renal failure and nephrosis, protozoal organisms were present in hepatocytes, kidney cells, and liver and lung endothelial cells () and these resembled Plasmodium spp., the causative organism of avian malaria. Mycotic bronchiectasis and pneumonia caused the death of one nestling. The nature of the lesions produced and the morphology of the fungal hyphae were consistent with those seen previously in cases of aspergillosis in adult hihi (Alley et al. Citation1999).
Figure 5 Photomicrographs of tissue sections from chick 86437 showing evidence of haemoparasite infection. The protozoa-like organisms can be seen as granular bodies (arrows) resembling merozoites that distend the cytoplasm. A, Enlarged kidney endothelial cells (bar = 20 µm). B, Lung endothelial cells (bar = 25 µm). (H&E stain).
Haematology
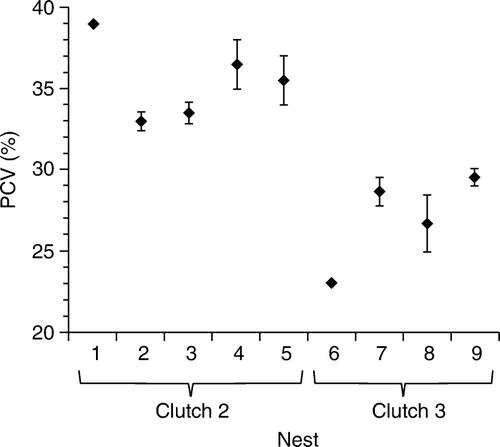
The PCV results for the 23 blood samples collected from 18–26-day-old nestlings at nine nest sites ranged from 23% to 39%, with mean PCV of 31% (95% confidence interval 28.8–32.9). There was no evidence of correlation between age and PCV (Pearson Correlation = 0.188; P=0.391), or between nestling weight and PCV (Pearson Correlation=−0.249; P=0.251). The PCV was significantly higher (PCV > 30%) in the second-clutch nestlings when compared with third-clutch nestlings (PCV < 30%; ; one-sample two tailed t-test: t=30.73, df = 22; P<0.0001).
Figure 6 Mean values for nestling packed cell volume (PCV,%) in hihi nests at Zealandia–Karori Sanctuary. Numbers indicate individual nests (1 = 129, 2 = 21, 3 = 123, 4 = 138, 5 = 39, 6 = 32, 7 = 26, 8 = 30, 9 = 123a). Number of chicks per nest from left to right = 1, 3, 4, 2, 2, 3, 3, 3, 2. Error bars indicate range of results at each nest site.
It was not possible to draw firm conclusions following microscopic examination of the blood films. Red cell appearance was essentially normal, with increased numbers of polychromatic red blood cells indicating active haemopoiesis (Samour Citation2006). Platelets were plentiful and morphologically normal and white cells were morphologically normal (Samour Citation2006). All samples were collected immediately prior to fledging, and with one known exception, all the birds tested fledged. Variations were noted in the differential counts but the lack of clinical information prevented diagnostic interpretation of the changes. Haemoparasites were not seen in any of the blood films.
Microbiology
Aerobic culture of gastrointestinal swabs from 59 nestlings (34 live nestlings at 11 nest boxes and 25 dead nestlings at 12 nest boxes) showed the oral flora comprised predominantly Gram-positive organisms that included various Staphylococcus spp., Streptococcus spp. and Corynebacteria. Faecal and cloacal flora comprised Gram-positive cocci, mainly faecal Streptococci, and varying numbers of Gram-negative bacilli, predominantly coliforms. Swabs taken from dead birds showed increased numbers (colony forming units) of coliforms present at both sites demonstrating that these opportunists are capable of rapid post-mortem proliferation. Although yeasts were commonly isolated from the gastrointestinal swabs of live and dead nestlings, Candida albicans was isolated from nestlings at four nest boxes only (Rippon et al. Citation2010). Candida albicans was associated with ventriculitis in the nestlings that died, but eight chicks colonised with C. albicans survived to fledge. Aspergillus spp., primarily a respiratory pathogen, was not isolated from the gastrointestinal swabs collected.
Parasitology
Some nestlings from two nest boxes only were found to carry up to five mites per nestling. Mites were not seen during visual inspections of nest boxes and were not detected by ruffling the nest lining material. From the 38 nesting attempts, 18 nests (a single nest from the first clutch; 10 of 12 second-clutch nests; and seven of eight third-clutch nests) were examined for ectoparasites. Ornithonyssus bursa was found in 12 of 14 nest lining samples (0–31/sample) and in 14 of the 18 nests collected (0–c. 150 mites/nest). An unidentified feather mite, Hemialges sp., was found in seven of the nests (0–18 mites/nest/sample). A single nest contained one free-living mite and one feather louse, of the order Phthiraptera. Two of the nests examined showed no evidence of ectoparasites; four nestlings successfully fledged from the first, while eggs failed to hatch in the second.
Haemoparasites (Plasmodium spp., Haemoproteus spp. and Leucocytozoon spp.) were not detected by PCR analysis or blood film examination. Faecal samples from 31 live nestlings aged 5–23 days tested negative for intestinal parasites. There was no evidence of coccidia infection in any of the dead nestlings examined.
Discussion
Mortality events
Nestling age was significantly associated with specific mortality events, as 60% of nestling mortality occurred within 7 days of hatching. Deaths in this group were mainly related to starvation or associated with poor pulmonary aeration, both correlated with daily minimum temperatures ≤11°C. Poorly developed anatomical and physiological thermoregulation in altricial nestlings means they are vulnerable to external temperature fluctuations (Dawson et al. Citation2005) and may succumb to hyper-or hypothermia (Mertens Citation1977). Signs of thermoregulation in altricial birds are first seen at 4–6 days old and nestlings up to 7 days old may suffer hypothermia when the parents are away from the nest for prolonged periods (Mertens Citation1977; Pereyra & Morton Citation2001). The range of temperature tolerance is determined by the thermal properties of the nest as well as some biological and life history traits (Mertens Citation1977). Dawson et al. (Citation2005) showed experimentally that in cool temperate zones increasing the nest temperature by approximately 5°C was positively correlated with nestling survival, increased body mass and faster growth rates in tree swallows (Tachycineta bicolor). Therefore, the low daily minimum temperatures at KS that are associated with nestling deaths may also affect growth rates but more detailed studies will be required to test this hypothesis.
Nestlings are thought to be more likely to succumb to hyper-than hypothermia (Mertens Citation1977). On Tiritiri Matangi Island, Low & Part (Citation2009) consistently found that hihi nestlings were affected by temperature-dependent mortality caused by high maximum temperatures later in the breeding season. There is a large temperature gradient from the northern tip (subtropical) of the North Island of New Zealand to the southern tip (temperate rain forest) and it is possible that hihi were adapted to the particular thermal conditions in the areas where they originated. The hihi population at KS comes from Tiritiri Matangi Island founders, and our results together with those of Low & Part (Citation2009), suggest that minimum and maximum temperatures outside the 12–30°C range adversely impact on young nestlings. Thermal tolerance may therefore be an important factor limiting nestling survival in translocated hihi populations.
The association between mortality in young nestlings, hypothermia and sta rvation seen in the present study can be easily understood because greater energy will be required and utilised for thermoregulation by poorly feathered young chicks of low body mass. Also observed, however, was an association between low environmental temperatures and poor aeration of the lungs with collapse or accumulation of fluid and fibrin in pulmonary airways. Because the lungs of birds are tubular and more rigid than those of mammals, neonatal respiratory distress syndrome related to lack of surfactant (surface tension reducing lipoprotein) is not recognised as a major cause of neonatal death as it is in mammals. Nevertheless, surfactant secreted from epithelial cells located in atrial walls, air sacs and parabronchi of birds needs to spread into air capillaries in order to maintain expansion and air flow (Bernhard et al. Citation2001). The slowing of respiration during hypothermia could therefore be a factor that inhibits the spread of surfactant in nestling lungs resulting in the poor aeration and fluid accumulation observed in the present study.
Nestlings aged 6–19 days from all three clutch orders died from sequelae relating to ventriculitis lesions (Rippon et al. in press) but no evidence was found that these deaths were temperature-related. Similarly, deaths that were attributed to infectious diseases such as aspergillosis (36-day-old) and an unidentified avian haemoparasite (26-day-old) did not appear to be correlated with minimum temperature.
Aspergillosis is known to cause deaths in adult hihi at all current locations although the impact of this disease on each of the translocated populations has not been quantified. Aspergillosis manifests as low-grade chronic respiratory infections through to a severe, acute cause of mortality that may be related to stress and other causes of immunosuppression (Alley et al. Citation1999). A detailed study of aspergillosis in the Mokoia Island hihi population showed that high mortality in adult birds (Alley et al. Citation1999) was limiting the population (Armstrong et al. Citation2007). Environmental spore counts on the island and two mainland sites revealed increasing levels of Aspergillus fumigatus spores with increasing habitat modification (Perrott Citation2001). Since KS is a mainland site which suffered high modification it is likely that the A. fumigatus spore count will also be high but further work will be required to confirm this.
Parasites
Recent research is finding Plasmodium spp. present in increasing number of native and introduced avian species, some of which may be endemic to the native birds (Castro et al. unpubl. data; Tompkins et al. unpubl. data). There are concerns that avian malaria may qualify as an emerging disease in this country (Derraik et al. Citation2008). Disease screening of hihi for translocations includes PCR analysis for blood parasites and Plasmodium sp., has been detected in some blood samples collected from hihi on Tiritiri Matangi Island (Makan Citation2009). In our study, both PCR and microscopic blood film examination failed to detect haemoparasites in the nestling from KS in which the parasites were seen in histological sections. Alley et al. (Citation2008) report similar negative findings following an outbreak of avian malaria in a small captive population of mohua (Mohoua ochrocephala). Although PCR tests for malaria show high specificity, they have relatively low sensitivity and may not detect 20–40% of low-grade infections (Jarvi et al. Citation2002). Similarly, microscopic examination of blood films for malarial parasites is generally only useful in acute phases of infection when parasites are released into the peripheral circulation in large numbers (Jarvi et al. Citation2002). In naive populations, disease manifestations may not follow the normal infectious process (Pierce Citation2000). For example, penguins exposed to avian malaria may die from the effects of Plasmodium schizonts in tissue phase only, with no detectable presence in the erythrocytes (Pierce Citation2000). If this is also the case with hihi, it may be difficult to detect and monitor the impacts of this disease on hihi populations and further investigations will be necessary to identify any haemoparasites that are present.
Nest ectoparasites mostly affect the growth and survival of nestlings, although some such as O. bursa (tropical fowl mite) that require a blood meal every 4–6 weeks, live more-or-less permanently on the avian host and have the potential to affect all life stages (Powlesland Citation1978). Mites are introduced to nests by adult birds and when not feeding burrow deep into the nest substrate (Powlesland Citation1978). In a study of starlings in nest boxes, Powlesland (Citation1978) found that once chicks fledge the nest, mites move to the top of the nest box in order to re-colonise fledglings or adults to ensure mite survival through to the next breeding season. Mite infestations vary within the population; nests are seldom colonised by mites prior to eggs hatching and some nests may remain free of mites, while others become heavily infested (Powlesland Citation1978). The predominant ectoparasites O. bursa (nest mites) and Hemialges spp. (feather mites) found in the nests at KS were consistent with the findings from hihi nests at other sites (Higgins et al. Citation2001). Nest mite numbers at Mokoia Island and Tiritiri Matangi Island have been actively managed to reduce nestling mortality caused by these parasites (Armstrong et al. Citation2002). In contrast to KS, nests and nestlings on Mokoia Island and Tiritiri Matangi Island were visibly infested and nestlings have been known to leave the nest box prior to fledging to escape from biting nest mites (Armstrong et al. Citation2002). High mite burdens have the potential to reduce the haemoglobin and PCV in affected species. However, frequent handling of the nestlings and frequent nest checks performed throughout the course of this study detected few mites in the nests and therefore there was no evidence that mite burdens at KS impacted on the PCV results obtained. The relatively low numbers of nest mites at KS compared with other sites may be attributed to the lower ambient temperatures that slow the population growth rates of ectoparasites (A. Heath 2010 pers. comm.).
Health parameters in live nestlings
There was no correlation between nestling weight and PCV at KS, suggesting that for hihi, PCV is not correlated to body condition. PCV has been used by some authors as an indicator of fitness (Cuervo et al. Citation2007; Morrison et al. Citation2009) but the evidence linking PCV values and body condition or fitness in birds is contradictory. Partial cross-fostering studies indicate that although the relationship is weak, PCV is related to body condition in barn swallow nestlings (Hirundo rustica) (Cuervo et al. Citation2007) but it is not related to body condition in tree swallow nestlings (Morrison et al. Citation2009).
There are very few studies providing data on PCV values for birds, but normal adult PCV values are >39% for many pet bird species (data derived mainly from parrots: California Avian Laboratory, USA; Fudge Citation2000). Although paediatric guidelines for avian species are lacking the data from nestlings suggests that lower values may be expected in nestlings up to 3–6 months of age (Fudge Citation2000). Clinically, normal kori bustard (Ardeotis kori) chicks (n = 16) had a mean PCV value of 23±7% at 1 month of age rising to plateau at 39.9±9% at 5 months of age before increasing to 47±9% at 12–15 months of age (Samour Citation2006). There is little haematological data pertaining to hihi and, where species specific information is not available, conclusions are limited because comparisons of data across species may not be relevant. Nevertheless, the PCV results for the 23 blood samples collected from hihi nestlings aged 18–26 days ranged from 23% to 39%, with mean PCV of 31±2% and are consistent with those of Samour (Citation2006). The nestlings sampled were in a narrow age range (18–26 days) and our results showed no correlation between PCV and the age of the nestlings.
Partial cross-fostering studies of barn swallow (Cuervo et al. Citation2007) and tree swallow (Morrison et al. Citation2009) nestlings showed that the nest in which the chicks were raised influenced the PCV result but that the nest of origin did not. This indicates that PCV in these birds is not heritable and suggests that parental care and environmental conditions have a significant effect on PCV values (Cuervo et al. Citation2007; Morrison et al. Citation2009). In this study, a significant correlation was found between PCV values and clutch order. PCV values for nestlings from the third clutch were lower than the PCV values obtained for second-clutch nestlings. With few exceptions, when hihi lose their first clutch early and lay replacement clutches, the second and third clutches occur within a narrow period shared by a number of breeding birds at a given site. Under these circumstances, factors that could influence PCV could affect a number of nests at the same time. Environmental factors which affect provisioning by the parent birds; the presence of ectoparasites, coccidia or haemoparasite infection, microclimate conditions at the nest, and the number of nestlings present could all affect PCV values and account for the findings in this study.
Conclusions
This study has identified a number of factors that impact adversely on the nestling population at KS. The implications of ventriculitis, caused by insect remnant penetration of the gastrointestinal tract (Rippon et al. 2010 unpubl. data), and the presence of C. albicans as a colonising organism that has the potential to cause disease in nestlings (Rippon et al. Citation2010), have been discussed in separate papers. However, the major influence on nestling survival over the study period was temperature and it is likely that thermoregulation in nestlings is not sufficiently developed to compensate for temperature drops below 11°C. This may be alleviated when the habitat is mature enough to provide natural nesting cavities, as it is likely that the nest boxes provided for breeding purposes lack the same degree of insulation. Further work would be necessary to confirm these findings, and the use of temperature data-loggers to measure the temperature within the nest box microclimate might be one way to achieve this. Low temperatures may also affect numbers of insect prey, thereby reducing the available food supply. In the short term, it may be possible to develop management strategies to counteract the effects of low temperatures; extra provisioning during inclement weather may reduce the amount of time parent birds spend away from the nest and weather protection or insulation might be incorporated into the nest box design.
Although infectious pathogens were present in the nestling population, they appeared to have minimal impact on survival, causing mortality in relatively small percentages of the population during the study period. This may be somewhat misleading as fledgling survival and recruitment into the population was unknown at the time of writing. However, there is always potential for infectious disease processes to increase in prevalence and virulence, and since this population is still in the establishment phase, ongoing surveillance is important to detect such changes before major mortality events occur.
Acknowledgements
Thanks to Raewyn Empson, Matu Booth, the staff and volunteers of Zealandia–Karori Sanctuary for collecting and submitting samples and data used in this study. Many thanks to Allen Heath and staff at AgResearch Ltd, Wallaceville for identifying and quantifying the ectoparasites found in nesting material. Thanks to Jane Kerridge of Medlab Wanganui who provided the facilities for much of the laboratory work and to Doug Hopcroft and staff at Manawatu Microscopy and Imaging Centre. We acknowledge the outstanding technical help from IVAB's staff including Elaine Booker, Evelyn Lupton and Nicola Wallace, and the members of the Ecology Group including Cleland Wallace, Tracy Harris, Sharon Tozer and Paul Barrett who were always willing to help. Thanks to Alasdair Noble for statistical advice. Particular mention is worthy of the tireless efforts and input from Ellen Schöner, and for assistance in the field from Monica Awasthy. Scholarship grants from the New Zealand Wildlife Health Centre Trust Research Fund, Graduate Women Manawatu Charitable Trust and J.P. Skipworth (Ecology) Fund were used to fund some of the work undertaken.
References
- Alley , MR , Castro , I and Hunter , JEB . 1999 . Aspergillosis in hihi (Notiomystis cincta) on Mokoia Island . New Zealand Veterinary Journal , 47 : 88 – 91 .
- Alley , MR , Fairley , R , Martin , D , Howe , L and Atkinson , T . 2008 . An outbreak of avian malaria in captive yellowheads/mohua (Mohoua ochrocephala) . New Zealand Veterinary Journal , 56 : 247 – 251 .
- Angehr , GR . 1984 . Ecology and behaviour of the stitchbird with recommendations for management and future research , Wellington : Department of Internal Affairs .
- Armstrong , DP , Castro , I and Griffiths , R . 2007 . Using adaptive management to determine requirements of re-introduced populations: the case of the New Zealand hihi . Journal of Applied Ecology , 44 : 953 – 962 .
- Armstrong , DP , Davidson , RS , Dimond , WJ , Perrott , JK , Castro , I , Ewen , JG , Griffiths , R and Taylor , J . 2002 . Population dynamics of reintroduced forest birds on New Zealand islands . Journal of Biogeography , 29 : 609 – 621 .
- Bernhard , W , Gebert , A , Vieten , G , Rau , GA , Hohlfeld , JM , Postle , AD and Freihorst , J . 2001 . Pulmonary surfactant in birds: coping with surface tension in a tubular lung. American Journal of Physiology—Regulatory . Integrative and Comparative Physiology , 281 : 327 – 337 .
- Castro , I , Brunton , DH , Mason , KM , Ebert , B and Griffiths , R . 2003 . Life history traits and food supplementation affect productivity in a translocated population of the endangered hihi (stitchbird, Notiomystis cincta) . Biological Conservation , 114 : 271 – 280 .
- Castro , I , Minot , EO , Fordham , RA and Birkhead , TR. 1996 . Polygyandry, face-to-face copulation and sperm competition in the hihi Notiomystis cincta (Aves: Meliphagidae) . Ibis , 138 : 765 – 771 .
- Cuervo , JJ , Moller , AP and De Lope , F . 2007 . Haematocrit is weakly related to condition in nestling barn swallows Hirundo rustica . Ibis , 149 : 128 – 134 .
- Dawson , RD , Lawrie , CC and O'Brien , EL . 2005 . The importance of microclimate variation in determining size, growth and survival of avian offspring: experimental evidence from a cavity nesting passerine . Oecologia , 144 : 499 – 507 .
- Derraik , JGB , Tompkins , DM , Alley , MR , Holder , P and Atkinson , T . 2008 . Epidemiology of an avian malaria outbreak in a native bird species (Mohoua ochrocephala) in New Zealand . Journal of the Royal Society of New Zealand , 38 : 237 – 242 .
- Driskell , A , Christidis , L , Gill , BJ , Boles , WE , Barker , FK and Longmore , NW . 2007 . A new endemic family of New Zealand passerine birds: adding heat to a biodiversity hotspot . Australian Journal of Zoology , 55 : 73 – 78 .
- Empson R , Booth M 2009 . Zealandia–Karori Sanctuary Report to Hihi Recovery Group Meeting April 2009. Unpublished .
- Ewen , JG , Flux , I and Ericson , PGP . 2006 . Systematic affinities of two enigmatic New Zealand passerines of high conservation priority, the hihi or stitchbird Notiomystis cincta and the kokako Callaeas cinerea . Molecular Phylogenetics and Evolution , 40 : 281 – 284 .
- Ewen , JG , Thorogood , R , Nicol , C , Armstrong , DP and Alley , M . 2007 . Salmonella Typhimurium in Hihi, New Zealand . Emerging Infectious Diseases , 13 : 788
- Friend , M and Franson , JC . 1999 . Field Manual of Wildlife Diseases: General Field Procedures and Diseases of Birds , Washington, DC : US Geological Survey .
- Fudge , AM . 2000 . Laboratory Medicine: Avian and Exotic Pets , Philadelphia, PA : W. B. Saunders Company .
- Hazen , KC and Howell , SA . 2003 . “ Candida, Cryptococcus and other yeasts of medical importance ” . In Manual of clinical microbiology , 8th edition , Edited by: Murray , PR , Baron , EJ , Jorgensen , JH , Pfaller , MA and Yolken , RH . 1693 – 1711 . Washington, DC : American Society for Microbiology Press .
- Hellgren , O and Waldenstrom , J . 2004 . A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium and Haemoproteus from avian blood . Journal of Parasitology , 90 : 797 – 802 .
- Hendrix , C . 1997 . “ Chapter 6. Internal Parasites ” . In Laboratory procedures for veterinary technicians , Edited by: Pratt , P . 307 – 384 . St Louis, MO : Mosby Year Book .
- Higgins , PJ , Peter , JM and Steele , WK . 2001 . “ Meliphagidae: Notiomystis cincta Stitchbird ” . In Handbook of Australian, New Zealand and Antarctic birds, Volume 5: Tyrant-flycatchers to chats , Edited by: Higgins , PJ , Peter , JM and Steele , WK . 954 – 966 . Melbourne : Oxford University Press .
- Jarvi , SI , Schultz , JJ and Atkinson , CT . 2002 . PCR diagnostics underestimate the prevalence of avian malaria (Plasmodium relictum) in experimentally-infected passerines . Journal of Parasitology , 88 : 153 – 158 .
- Low , M and Part , T . 2009 . Patterns of mortality for each life history stage in a population of the endangered New Zealand stitchbird . Journal of Animal Ecology , 78 : 761 – 771 .
- Makan , T . 2009 . A critique of disease-screening requirements for community-managed species translocations: a case study of hihi (Notiomystis cincta) , Auckland : Waitakere Branch Royal Forest and Bird Society of New Zealand Inc .
- Mertens , JAL . 1977 . Thermal conditions for successful breeding in great tits (Parus major L.) I. Relation of growth and development of temperature regulation in nestling great tits . Oecologia , 28 : 1 – 29 .
- Morrison , ES , Ardia , DR and Clotfelter , ED . 2009 . Cross-fostering reveals sources of variation in innate immunity and haematocrit in nestling tree swallows Tachycineta bicolor . Journal of Avian Biology , 40 : 573 – 578 .
- Oliver , WRB. 1955 . Stitchbird. Hihi. New Zealand Birds , 510 – 511 . Wellington : A.H. & A.W. Reed .
- Pauli JM. Diseases of NZ native birds: disease encountered in captive breeding of the stitchbird and shore plover at the National Wildlife Centre Mt Bruce ; 2000 ; Veterinary Continuing Education . Massey University Palmerston North . Pp. 105 – 113 .
- Pereyra , ME and Morton , ML . 2001 . Nestling growth and thermoregulatory development in subalpine dusky flycatchers . The Auk , 118 : 116 – 136 .
- Perrott JK 2001 . The ecology of Aspergillus fumigatus and implications for wildlife conservation in modified environments . PhD , Massey University , Palmerston North .
- Pierce , MA . 2000 . “ Hematozoa ” . In Avian medicine , Edited by: Samour , J . 245 – 252 . London : Mosby .
- Powlesland RG 1976 . A study of the relationship between the starling Sturnus vulgaris and the haematophagus mite Ornithonyssus bursa . MSc , Massey University , Palmerston North .
- Powlesland , RG . 1978 . Behaviour of the haematophagous mite Ornithonyssus bursa in starling nest boxes in New Zealand . New Zealand Journal of Zoology , 5 : 395 – 399 .
- Quinn , PJ , Carter , ME , Markey , BK and Carter , GR . 1994 . Bacterial pathogens: microscopy, culture and identification. Clinical veterinary microbiology , 21 – 66 . London : Wolfe .
- Rippon , RJ , Alley , MR and Castro , I . 2010 . Candida albicans infection in free-living populations of hihi(stitchbird;Notiomystis cincta) . New Zealand Veterinary Journal , 58 : 299 – 306 .
- Samour , J . 2006 . “ Diagnostic value of haematology ” . In Clinical avian medicine , Edited by: Harrison , GJ and Lightfoot , TL . 587 – 609 . Palm Beach, FL : Spix .
- Taylor , S , Castro , I and Griffiths , R . 2005 . Hihi/Stitchbird (Notiomystis cincta) Recovery Plan 2004–2009 , Wellington : Department of Conservation .
- Twentyman , CM . 2001 . A study of coccidial parasites in the hihi (Notiomystis cincta) , Palmerston North : Master of Veterinary Science, Massey University .
- Twort , FW . 1924 . An improved neutral red, light green double stain for staining animals, parasites, micro-organisms and tissues . Journal of State Medicine , 32 : 351
- Young , BJ . 1969 . Staining of fungal hyphae in tissue sections . Journal of Medical Laboratory Technology , 25 : 342 – 346 .