Abstract
A new genus Mumulaelaps (Mesostigmata: Laelapidae: Hypoaspidini) is proposed to hold large, pale, agile, gregarious mites associated with the larvae of the endemic xylophagous sand scarab Pericoptus. Mumulaelaps ammochostos sp. n. is described and illustrated as its type species from P. truncatus (Fabricius) from Southshore, Christchurch, New Zealand. The new genus bears a novel mix of characters shared with Hypoaspis G. Canestrini, 1884 and Coleolaelaps Berlese, 1914 but differs from both by having, in both sexes, stout terminal spines al1 and pl1 on legs II–IV and bearing long or macrosetae of the dorsal series on femora and genua of legs II–IV. Both sexes have leg IV longest and leg II shortest with III never longer than I. The posterior edge of the sternal shield is deeply concave; the post anal seta is longer than the paranals; the hypostomal seta h3 clearly longer than h1 or h2. Stable isotopic (13C/12C; 15N/14N) analysis showed that the new species is not parasitic, but its trophic association with P. truncatus is unclear. It may feed on the hypopodes of Sancassania (=Caloglyphus) or nematodes associated with P. truncatus.
Introduction
Although some genera within the family Laelapidae Berlese, 1892 are free-living predators, many are associated with arthropods. Associations include paraphages, commensals, phoretics, symbionts and obligate parasites. Some of the parasitic laelapid genera inflict significant economic damage, such as the honey bee mite Varroa Oudemans, 1904. Strong & Halliday (Citation1994) reviewed the literature on Hypoaspis G. Canestrini, 1884, the most speciose laelapid genus in the assemblage of arthropod-associated Laelapidae. They reported difficulties in delineating the genus, as did Casanueva (Citation1993), and they repeated earlier recommendations for detailed descriptions ‘of a variety of species from all over the world, combined with studies of … host associations and symbiotic relationships’. Joharchi & Halliday (Citation2011) pointed to the continued instability and confusion about the status of genera within the Laelapidae. Dowling & OConnor (Citation2010) investigated the cladistic relationships between the parasitic Laelapidae, noting that the diversity of laelapid associations with arthropods remains poorly known. One approach to this lack of information is to explore the ecological associations, where possible, when describing new species—an approach used here.
Stable isotope analysis, typically 13C/12C and 15N/14N, provides an avenue for elucidating trophic interactions pertaining to animals for which direct observation is difficult such as soil-dwelling invertebrates, and parasites and their hosts. In most applications, 13C/12C indicates C source and 15N/14N indicates trophic level (Scheu Citation2002). Although stable isotope studies on soil animals are increasingly common (e.g. Maraun et al. Citation2011), corresponding studies on parasites are less so. Parasite studies have included the trophic relationships of endoparasites within the gut of herbivorous lizards (O'Grady & Dearing Citation2006), ectoparasites with their pelagic seabird hosts (Gómez-Díaz & González-Solís Citation2010) and larval stages of seabird parasites found off-host in soil (Hawke & Clark Citation2010).
The scarab beetle Pericoptus truncatus (Fabricius, 1775) is an iconic xylophagous macroinvertebrate endemic to New Zealand. It feeds on drift wood, or well below the beach surface in a narrow strip of sand between the high water mark and the base of the sand dunes (Dale Citation1963; Ratcliffe & Orozco Citation2009). Although they travel the surface at night, apparently in search of moisture or new foraging opportunities (Dale Citation1963; Ratcliffe & Orozco Citation2009), their primary feeding habitat is subsurface. Feeding by Pericoptus larvae on drift wood is therefore taking advantage of a non-marine allocthonous nutrient source, distinct from the marine sources of beachwrack now accepted as important drivers of terrestrial systems close to the sea worldwide (Polis et al. Citation2004; Mellbrand et al. Citation2011). Pericoptus presently holds five species but there are at least as many undescribed species (PM Johns, Entomologist CMNZ, October 2010 pers. comm.).
Ramsay in Brown (Citation1967) reported ‘heavy infestations of presumably ectoparasitic mites of the family Laelapidae: Hypoaspidinae … on larvae (of) … Pericoptus sp. (They) were especially numerous in folds of the skin’. In this paper, these mites are placed in a new genus that is clearly differentiated from Hypoaspis Canestrini, 1884 s.str and their probable sister genus Coleolaelaps Berlese, 1914. The successful application of stable isotope analysis to soil biology over the past decade now offers a way to include ecological information with taxonomic descriptions (Clark & Hawke Citation2011). We use stable isotope analysis to explore the C source and trophic relationship of the new mite to its host.
Methods
Larvae of P. truncatus were collected from under drift wood on Southshore beach, Christchurch in January and February 2011, or by digging into sand above the high water mark. Mites were either brushed from the scarab larvae into vials on site or the larvae were collected and removed to the laboratory. Here, some larvae were held in Petri dishes and when uncurled and extended, the distribution and numbers of mites was recorded. Other larvae were killed by freezing; mites were removed with a needle, cleared and mounted in lactic acid for temporary viewing or in Hoyers medium for permanent slides. Mites were dissected by removing the dorsal shield, legs and chelicera for better viewing of morphology. We also collected Pericoptus larvae and their attendant mites from Okains Bay, Banks Peninsula; Kaitorete Spit, Canterbury; Ōakura beach, Taranaki (western North Island) and Rabbit I. beach, Nelson (northern South Island), in February or early March 2011, to help establish the morphological characters of the new genus.
Idiosomal setal names follow Lindquist & Evans (Citation1965), and leg chaetotaxy follows Evans (Citation1963). Poroidotaxy follows Johnston & Moraza (Citation1991) and Moraza (Citation2004). All measurements were made with a stage calibrated micrometer eyepiece and are in micrometers (µm). As a measure of the spread of the data values, the sample standard deviation (SD) is given where n>2, n=sample size; brackets hold (range of data). Shield measurement lengths are in the mid-sagittal plane.
For stable isotope analysis, mites were collected from four scarab larvae alongside wood samples (c. 50 g) under which the scarab larvae were found. Three additional scarab larvae were analysed, without associated mites. Mites were too small for individual analysis, so three to seven animals were pooled from each scarab. The scarab head capsules (primarily chitin C) and the parietal fat depot (lipid C) was analysed to see whether the mites used a particular scarab tissue as C source. All material was dried for 24 h at 50 °C before analysis.
Stable isotope ratios were measured on finely ground material using a DeltaPlus (Thermo-Finnigan, Bremen, Germany) continuous flow isotope ratio mass spectrometer at the National Institute of Water and Atmospheric Research (NIWA) Stable Isotope Laboratory (Wellington, New Zealand). The isotope ratios were calculated as per mil (‰) deviation from the international limestone standard VPDB (13C/12C, reported as δ13C) and atmospheric N (δ15N) using DL leucine as secondary standard. The standard deviations of repeated analyses of secondary standard were 0.12‰ (δ13C) and 0.16‰ (δ15N). A duplicate sample of soil, prepared by us as an in-house reference material, was submitted blind to the stable isotope laboratory along with the mite and scarab material as a quality control check. Results for the two duplicates agreed within 0.04‰ (δ13C) and 0.20‰ (δ15N), and were within measurement uncertainty of the assigned values for the in-house reference material.
Initial statistical analysis of the isotope data used repeated measures one-way analysis of variance (ANOVA) for each isotope and the paired mite-scarab-wood results, but the pairing was found not to be effective. All subsequent statistical analysis used ordinary (unpaired) one-way ANOVA, and a Tukey–Kramer post hoc test.
The phylogenetic position of the new genus in the Laelapidae was investigated with PAUP 4.0 (Swofford Citation2002) by using the data matrix of 44 genera in Casanueva (Citation1993) with the corresponding 72 characters from the new genus. This outgroup data was used in the analysis.
Abbreviations: Podomeres are referred to as; fem, ge, tib, tar, for femora, genua, tibia and tarsus, respectively. Legs 1–4 are designated in Roman numerals (I–IV). Setal names are in italics. CMNZ, Canterbury Museum, Christchurch, New Zealand; ANIC, Australian National Insect Collection, Canberra, ACT 2601, Australia.
Systematics
Family Laelapidae Berlese, 1892
Subfamily Hypoaspidinae Vitzthum, 1940
Tribe Hypoaspidini Vitzthum, 1940 (sensu Casanueva Citation1993)
Mumulaelaps gen. n. Clark
DIAGNOSIS: FEMALE (Figs. ): Large, pale, agile, gregarious hypoaspidine symbionts of larval Pericoptus, a New Zealand endemic genus of Scarabaeidae: Dynastinae. Dorsum: Adults with single reduced un-notched dorsal shield with reticulate pattern and acuminate posterior with chaetotaxy variable; bearing c. 22 (12 podonotal, 10 opisthonotal) pairs of setae. Venter: Sternal shield either parallel-sided or hexagonal with anterior margin indistinct and posterior margin deeply excavated/concave. Postanal setae not distinctly shorter than paranal setae. Gnathosoma: Seta h3 longest of subcapitular series. Deutosternum with six short rows of minute teeth: Apotele 2-tined. Epistome broadly triangular, denticulate. Legs: Setae al1 and pl1 on legs II–IV as spines. Long setae (macrosetae) on femur and genua (pd1 on legs I & II and ad1 on III & IV) and the tibia of leg IV (third dorsals). Leg chaetotaxy of trochanter, femur, genua, tibia for legs I–IV; I, 6, 13, 13, 13; II, 5, 11, 11, 10; III, 5, 6, 9, 8; IV, 5, 6, 9, 10. Leg IV longest: leg I longer than II and III.
MALE: With pv1 of femur II as a stout spine: spermatodactyl simple, projecting anteriorly from the fixed digit by the length of the digit and with curved tip. Males with teeth of fixed digit proximal to pilus dentilis on raised structure. Cheliceral setae short. Cheliceral lyrifissure present. Male never with holoventral shield; it is either eroded to a sterniventral shield and an anal shield or it is represented by sternal, ventral and anal shields with an asymmetry of sternal and ventral shield margins.
Figure 1 Mumulaelaps ammochostos sp. n. Female. Dorsal shield setae; legs show location of macrosetae with other setae removed except al1 and pl1 as spines. Idiosomal setal names follow Lindquist & Evans (Citation1965) and leg setal names follow Evans (Citation1963).
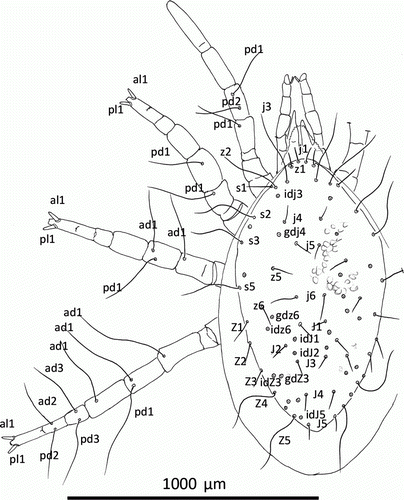
Figures 2–6 Mumulaelaps ammochostos sp. n. Female. 2, Hypostome, sternal and epigynal shield. 3, Dorsal view of fimbriate internal mala and labrum with chelicera removed. 4, Chelicerae. 5, Venter of mite showing lateral reduction in sternum and reduction in endopodal shields. 6, Lateral view of mite with peritreme and long post lateral setae. Idiosomal setal names follow Lindquist & Evans (Citation1965).
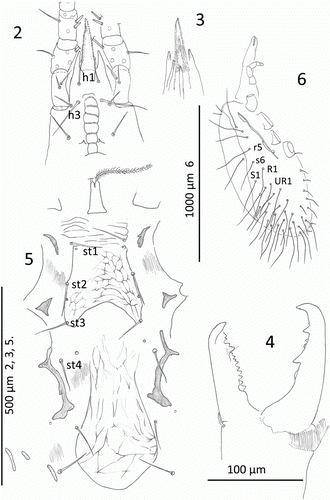
Figures 7–15 Mumulaelaps ammochostos sp. n. Ventral views of legs. 7–11, Female legs. 7, Leg I, genua and tibia. 8, Leg II, genua, tibia and tarsus. 9, Leg III, genua, tibia and tarsus. 10, Leg IV, genua, tibia and tarsus. 11, Femur II, ventral view. 12–15, Male legs. 12, Femur II, ventral view showing stout av1 as sexual dimorphism and as wider setae al1 and pl1 as flattened blunt spines. 13, Leg II. 14, Leg III. 15, Leg IV. Leg setal names follow Evans (Citation1963).
Type species: Mumulaelaps ammochostos Clark by original designation.
Remarks: The identity of the ‘host’ beetle is given first in the generic diagnosis here following Costa & Hunter (Citation1970) and Bell & Robinson (Citation2011). Costa (Citation1971) emphasised the importance of ‘host’ identity to the mite taxonomy. Mumulaelaps is clearly related to but able to be separated from Coleolaelaps (sensu Costa & Hunter Citation1970) by the following: dorsal shield without lateral notches of soft cuticle between podonotal and opisthonotal sections; these are present in the deutonymph of the new genus and retained in the adults of Coleolaelaps Berlese. The new genus bears an acuminate posterior margin to the dorsal shield; it is rounded in Coleolaelaps. Both Coleolaelaps and the new genus have a reduction in the number of dorsal shield setae as the shield in both is reduced especially post-laterally. Thus Costa (Citation1971) gave 37 pairs of dorsal shield setae for Hypoaspis s.str. and Strong & Halliday (Citation1994) gave 34–40. Costa & Hunter (Citation1970) gave a maximum of 27 pairs for Coleolaelaps (16 pairs of podonotal setae and 11 pairs of opisthonotal setae). In the new genus, the deutosternal teeth are not greatly different in number between rows and usually about 20+. The posterior row is flanked by one or two larger teeth outside the groove. Setae h3 longest of the subcapitular setae is a character shared with Hypoaspis. In Coleolaelaps, h3 is never markedly longer than other subcapitular setae. Hypoaspis bears spinous al1 and pl1, on II however Coleolaelps is without spinous al1 and pl1 on any tarsus. This character of setae al1 and pl1 on legs II–IV as spines therefore appears to be autapomorphic for the new genus. The presence of at least some of the dorsal series as macrosetae on all legs separates the new genus from Hypoaspis and Coleolaelaps. The dorsal series of leg setae display a trend to increased length in the new genus more than in Hypoaspis and Coleolaelaps. Leg I fem ad1 as a macrosetae occurs on the type species of the new genus and on specimens from Nelson and Taranaki that appear to represent a different species from the new species described below. The Nelson material also has pd1 as a macrosetae on ge I. But the specimens from Kaitorete Spit bear no macrosetae on I; very short dorsals on II & III but leg IV with fem, ge, tib and tar with macrosetae. They also have hexagonal sternal shields that are not eroded laterally yet bear the spinous al1, pl1 on II–IV. Tibia III bears eight setae in the new genus in all material examined while the type species of Coleolaelaps, C. agrestis Berlese, bears nine as 2, 1/1, 2/1, 2. Costa & Hunter (Citation1970) state that this number varies and can be eight or nine with the post-lateral number variable. The new species and all specimens from the other four locations have eight. Costa & Hunter (Citation1970) diagnosed Coleolaelaps as, ‘Legs III longer than legs I (except in C. abnormalis) or II; legs IV longest; legs I–IV without blunt spurs or spines.’ Bregetova (Citation1977) and recently Joharchi & Halliday (Citation2011) give the same relativity of leg lengths for Coleolaelaps. Both male and female mites from all five locations (see Methods section) in this study had leg IV longest and leg II shortest with III never longer than I. Seta pv1 as a stout spine is a character common to Hypoaspis and Coleolaelaps. In the males of the type species of the new genus described below, the sternal shield can be asymmetrically eroded and is usually connected to the ventral shield. No specimens collected from the other four locations and considered possible candidates for the new genus had a holoventral shield. Strong & Halliday (Citation1994) diagnose Hypoaspis as having holoventral shields. As far as we are aware, the males of Coleolaelaps are unknown perhaps because that genus is described from phoretic female mites on scarab imagos while the male mites, we hypothesise, exist in association with the scarab larvae. This view is also that of Costa & Hunter (Citation1970) and Halliday (Acarologist, CSIRO, Canberra 2011, pers. comm.).
Costa & Hunter (Citation1970) revised Coleolaelaps and left it with four species from the Palearctic and five species from the Nearctic. Species from Australia and Samoa assigned to Coleolaelaps by Womersley (Citation1954, Citation1956) were removed. Therefore, the new genus occupies a biogeographical area far removed from that of Coleolaelaps.
Phylogenetic position: Dowling & OConnor (Citation2010), using nuclear ribosomal DNA, found Coleolaelaps to be sister to Hypoaspis as did Casanueva (Citation1993). However, their unresolved ‘clade D’ also contained Varroa and Holostaspis, Laelaspis and Cosmolaelaps; all genera associated with Hymenoptera. They commented that ‘further study of arthropod associations is necessary and that these may turn out to be much more diverse than the vertebrate associations’. The new austral Mumulaelaps is added as part of that diversity.
PAUP (Swofford Citation2002) was used to investigate the phylogenetic position of the new genus. In particular, we wanted to know the most probable sister genus. We used the data matrix of Casanueva (Citation1993) that has 72 characters in 44 other genera of laelapids and adding our 72 characters for Mumulaelaps. Coding for some characters presented a problem for some of Casanueva's character states such as assigning setal presence/absence for her characters 53, 54, 65, 67–71. Much of the difficulty was to determine which seta has been lost in evolution in the lateral and dorsal series on podomeres. Some setae are neither inserted in a clearly lateral or dorsal position and determining their proximal-distal (1–3) location was far from clear. However, the topological output from PAUP consistently grouped Coleolaelaps as the sister genus of Mumulaelaps. The use of only gnathosomal characters (characters 1–19); excluded gnathosomal characters (using characters 20–72); and excluding characters 1–45 therefore using only leg chaetotactic characters, all created topology demonstrating a robust argument of Coleolaelaps being the most likely sister group with Mumulaelaps as did using all 72 characters in the analysis. Using setae on tibia III and IV as al2 or pd1 did not change the topology. Bootstrapping gave strong support (>85) to the trees.
Mumulaelaps ammochostos sp. n. Clark. Figs.
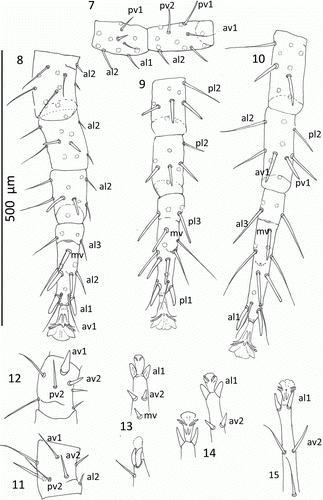
Figures 16–18 Mumulaelaps ammochostos sp. n. Male. 16, Ventral view of sterniventral shield with asymmetry and islands of striate cuticle within the shield. 17, Chelicera and spermatodactyl, axial view. 18, Chelicerae and spermatodactyl, antaxial view. Idiosomal setal names follow Lindquist & Evans (Citation1965).
FEMALE: (Figs. )
Dorsum (): Dorsal shield reduced: length 996, SD = 45, n=7; widest 553, SD = 44, n=7 at podonotum and tapering to blunt acuminate posterior: reticulate pattern throughout; no notch between podonotum and opisthosoma (). Macrosetae 200–250 long, setiform, sometimes wavy but mostly curved and confined to shield margins as j3, z2, s2, s3 and set in soft striate cuticle as r2–5, S1–6, R1–6, UR1–6 and URx1–5. Thus no r or R series on dorsal shield. Many setae lost in type series but c. 22 pairs present on dorsal shield. There are 12 pairs of podonotals in the holotype as j1, 3, 4–6, z1, 2, 5, s1–3, 5 and 10 pairs of opisthonotals as J1–5, Z1–5. The j and J series, z1 and s1, and Z1–3 are short (c. 75 except j1, c. 60), setiform and not or barely reaching the alveolus of the next posterior setae in the series. Setae j3, z2, s2, 3 long as are Z4, Z5.
Gnathosoma (): Apotele 2-tines; corniculi long, narrow reaching to at least mid-palp-femur. Internal mala and its dorsal labrum supports are long, reaching the distal end of the palp-genua and with usual fimbriate margins (). Six rows of minute deutosternal teeth the posterior row flanked on each side by short tooth on infracapitulum. Seta h3 longest; h2 shortest. Chelicerae () with 6–7 moderate teeth on fixed digit and two large retrose teeth on the movable digit with a row of small teeth to engage fixed digit teeth; pilus dentilis short (). Epistome triangulate with deticulate margin.
Venter (): Sternal and endopodal shields reduced. Sternum length 130, SD = 8, n=7; anterior margin indistinct with presternals: posterior deeply concave. Sternum straight sided with three pairs of setae and two pairs of pores set on shield margin; width at st2 148, SD = 7, n=7. Sternum reticulate as illustrated. Small endopodals between Coxa I and II; between II and III. Largest endopodal at coxa IV. Metasternal setae and pores free in striate cuticle. Genital setae on or off epigynal shield. Epigynal shield length 424, SD = 29, n=7; width 177, SD = 28, n=7 tongue-shaped with reticular pattern as illustrated. Metapodals represented by three fragments as illustrated (). Anal shield length 191, SD = 17, n=7; width 142, SD = 16, n=7. Post-anal seta a little longer than paranals. Lateral (): Peritreme with two short thin relict shield remnants and free at both the anterior and posterior end. All dorso-lateral setae in striate cuticle are macro-setae; some individuals with wavy setae, some more or less straight 250–350 long with only r2 and S2 shorter at 200. (Their appearance depends on preparation—see Costa Citation1971.) Setae s6, S1–6, R1–6 and UR1–6 in striate cuticle ().
Legs (Figs. ): Length I–IV excluding pretarsi; I, 895, SD = 59, n=7; II, 798, SD = 52, n=7; III, 864, SD = 69, n=7; IV 1258, SD = 89, n=7. Macrosetae of legs (): I, fem pd1, ge pd1: II, fem pd1 296, SD = 27, n=5; ge pd1 274, SD = 23, n=5: III, fem ad1 304, SD = 36, n=5; ge ad1 294, SD = 26, n=5; IV, fem ad1 378, SD = 63, n=5; ge ad1 416, SD = 79, n=5; tib ad1 320, SD = 27, n=5; tar ad3 and pd3 272, SD = 26, n=5. Tar II–IV armed with blunt spines as al1 and pl1 (Figs. ). Setae av2, pv2 as spines on II–IV and mv spinous on II and III. The setae md, av1 and pv1 weak, short on tarsi II–IV. Chaetotaxy for femora, genua and tibia; I, 2, 3/1, 2/3, 2; 2, 6/3, 2; 2, 3/1, 1 3/1, 2 (av1, pv1): II, 2, 3/1, 2/2, 1; 2, 3/1, 2/1, 2; 2, 2/1, 2/1, 2: III, 2, 1/1, 1/1, 0; 2(1), 2(3)/1, 2/1, 1; 2, 1/1, 2/1, 1: IV, 1, 2/0, 1/1, 1; 2, 2/1, 3/0, 1 (pl2); 2, 1/1, 3/1, 2.
MALE (Figs. ):
Dorsum: Dorsal shield length 880, SD = 49, n=7 and width 454, SD = 16, n=7. Dorsal shield shape as in female with variation in the number of setae inserted on shield.
Gnathosoma (, ): Spermatodactyl simple, projecting anteriorly from the fixed digit by the length of the digit and with curved tip. Dorsal cheliceral setae and cheliceral lyrifisure present. Pilis dentilis thick, tapering to point. The posterior teeth of the fixed digit modified on hyaline plate. Movable digit with a massive distal hook-like tooth similar to the unguis.
Venter (): Sterniventral shield length 464, SD = 58, n = 4 and width at widest behind coxa IV, 212, SD = 25, n=4 and between st3 setae 119, SD = 2.5, n=4. Genital opening at anterior of sterniventral shield. Sterniventral shield variable; it can be a continuous sterniventral, or it can be a sternal shield with a ventral shield fragment often with its lateral margins eroded. Anal shield as in female, with a rounded anterior and well separated from sterniventral or ventral shield; commonly with sternal shield tapering to rounded end level with the posterior of coxa IV and flanked posteriorly by an asymmetrical ventral shield fragment. Or as illustrated () as an intact sterniventral shield but with degenerative ‘islands’ of striate cuticle within the shield. Metapodal shield as separate small fragments ().
Legs (Figs. ): Termination of legs II–IV as illustrated. Males showing sexual dimorphism with pv1 of femur II as a stout spine () and al1 and pl1 as shorter, flatter and wider on legs II & III than in female ( and ). Leg length including coxa but excluding pretarsus, I, 782, SD = 56, n=7; II 640, SD = 45, n=7; III, 728, SD = 32, n=7; IV, 970, SD = 32, n=7.
LARVAE: Single specimen holding developing protonymph 650 long; 450 wide. Idiosoma typically laelapine: no pygidial shield. No macrosetae on idiosoma or legs. Setae al1 and pl1 setae on legs II–IV are setiform, not spinous. Leg lengths; I, 550; II 475; III, 500.
PROTONYMPH: Two specimens; Idiosoma length 750, 800; width, 525, 550. Legs: leg length including coxa but excluding pretarsus; I, 130, 140; II, 120, 120; III, 120, 120; IV 16, 170. Macrosetae present on the legs as in deutonymphs and adults: tarsal setae al1 and pl1 thickened but with attenuated tips.
DEUTONYMPH: Pale with notch between podonotum and opisthonotal shield (a character retained in adults by most Coleolaelaps species). Tarsi with less spinous terminal and ventral setae than adults. Legs: Leg length including coxa, excluding pretarsus, I, 833, SD = 96, n=3: II, 750, SD = 50, n=3; III, 833, SD = 75, n=3; IV, 1056, SD = 75, n=3.
BIOLOGY: Scarab larvae held in petri dishes allowed the distribution of the mites to be recorded as common in the cuticle folds/fossae (acarinaria) on the ventral surface between the tergites and sternites—sometimes four mites/fossa: often at the base of the legs or on the dorsal surface of the larvae in the cuticular folds posterior to the abdominal spiracles. Larger third instar larvae had more mites than second instars. Of more than 20 larvae handled from Southshore, none was without M. ammochostos sp. n. Load ranged from 7–19 mites/larvae. Mites were never observed to leave the larvae. After killing several larvae, the larval cuticle was examined for signs of piercing by the mites but none was seen. The chelicera of the mite appear to be unspecialised for haemolymph feeding.
ETYMOLOGY: Named from the first syllables of the Māori name mumutaua or mumutawa or mumuwaru for the adults (Miller Citation1984) combined with the common root, ‘laelaps’. The species name is Greek ammochostos, meaning ‘hidden in sand’.
MATERIAL COLLECTED: The type series was collected by JMC from the late instar larvae of P. truncatus. These were located under drift wood just above the strand line (mean high-water mark) at Southshore, Christchurch (South Island, New Zealand), at or within a few hundred metres of 43° 33.668′ S, 172° 42.986′ E. Holotype female, five paratype males, five paratype females, one larvae (holding a protonymph) and two deutonymphs are in CMNZ. Five paratype males and five paratype females are deposited in ANIC. Other material referred to is in the collection of JMC or CMNZ. That includes Sancassania specimens and material collected from Okains Bay, Kaitoerete Spit, Rabbit I., and Ōakura beach with their alcohol preserved ‘host’ Pericoptus larvae.
Results
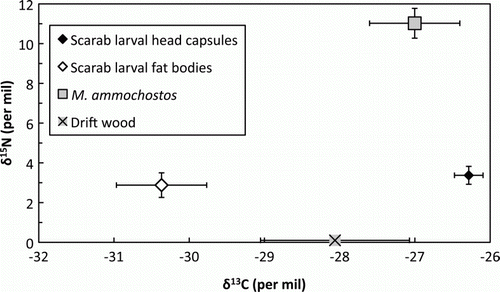
All δ13C values fell within the range expected for terrestrial C3 photosynthetic carbon, but were well dispersed (). The nitrogen content of the drift wood was too low for δ15N analysis. The one-way ANOVA showed significant variation between mite, scarab head capsule, scarab fat body and wood means for δ13C (P=0.0002). Mite δ13C was much closer to the values for scarab head capsules than scarab fat bodies, post hoc comparisons showing that scarab fat bodies were significantly lower than either scarab head capsules (95% CI 2.0–6.1‰) or mites (95% CI 1.0–5.8‰). Other comparisons were not significant. For δ15N, the one-way ANOVA was again highly significant (P<0.0001). In contrast to the δ13C results, post hoc comparisons were not significant for scarab head capsules and scarab fat bodies, the numerical results being closely comparable (). However, mite δ15N was much greater than either scarab tissue (95% CI head capsules, 5.3–10.0‰; fat bodies, 5.8–10.5‰).
Figure 19 Isotope data (mean±SEM) for Mumulaelaps ammochostos sp. n. and contextual material. Larval head capsule (n=7 samples) and fat body (n=7) data represent chitinaceous and lipid material respectively for Pericoptus truncatus. Drift wood samples (n=4) came from material supporting the P. truncatus; nitrogen content was too low for δ15N analysis, and is arbitrarily set to zero for display purposes. Mites were too small for individual analysis and so were analysed as four replicates of 3–7 pooled individuals.
Discussion
Mumulaelaps ammochostos is clearly not parasitic. Nitrogen isotopes fractionate in a broadly predictable fashion through food webs at around 3‰ in δ15N per trophic level (Post Citation2002), allowing the nature of a potentially parasitic interaction to be explored. For endoparasites, a true parasite shows a negligible increase in δ15N over its host but a commensal or mutualist is at least one trophic level higher (O'Grady & Dearing Citation2006). For ectoparasites, a true parasite will be one trophic level higher, with the exact difference in δ15N depending on both the parasite taxon and on the tissue consumed by the parasite (Gómez-Díaz & González-Solís Citation2010). At a δ15N of 8±3‰ (two to three trophic levels) higher than the scarab larvae host, M. ammochostos is non-parasitic.
Carbon isotopes show little fractionation through food webs (Post Citation2002), thus allowing identification of C source. Although many investigations of food web C flow use only bulk δ13C values, δ13C is not homogenous within an organism (Martínez del Rio et al. Citation2009). In particular, δ13C of lipids are typically well below those for other tissues (DeNiro & Epstein 1977; Post et al. Citation2007). The location of M. ammochostos δ13C between Pericoptus head capsules and fat bodies is consistent with Pericoptus as the source of C in M. ammochostos, although other terrestrial C sources cannot be excluded (see below). The abundant fat deposits found in Pericoptus larvae (Dale Citation1963) raise the possibility that M. ammochostos obtains its C from lipid rather than chitin. Our data allow us to reject this hypothesis. That is because although M. ammochostos δ13C was slightly lower than Pericoptus head capsule δ13C, values were much higher than for Pericoptus fat body material. Head capsules are high in chitin, so we conclude that if M. ammochostos C comes from Pericoptus, it is mostly from host chitin, with only a small contribution from Pericoptus lipid.
If the C for M. ammochostos is not derived from Pericoptus haemolymph or cuticular lipids, it may come from a diet of Sancassania Oudemans, 1916 (=Caloglyphus Berlese, 1923) (Acari: Astigmata: Acaridae) or nematodes. In all locations that we examined, individual Pericoptus larvae carried phoretic Sancassania deutonymphs as well as Mumulaelaps. Some scarab larvae had dozens of hypopodes present in the acarinaria situated ventrally between the abdominal sternites and tergites usually occupied by Mumulaelaps or dorsally posterior to the abdominal spiracles. Predation of the deutonymphs by Mumulaelaps would then yield a mutualistic relationship if the deutonymphs consume Pericoptus cuticle lipids or a commensal relationship if they are non-feeding. Exploring the role of deutonymphs will require an alternative approach such as molecular analysis of Mumulaelaps gut contents (King et al. Citation2008), the deutonymphs being too small for stable isotope analysis.
Other possible mutualistic interactions between Mumulaelaps and Pericoptus include predation of entomopathogenic nematodes, fungi or bacteria. Predation by mites can significantly decrease integumental penetration of scarab larvae by this class of nematodes (Karagoz et al. Citation2007). Although particular nematodes entomopathogenic to Pericoptus have not been identified, Dale (Citation1964) records Tetleyus pericopti as a parasite and c. 40 species of free-living nematodes occur in sand dunes close to the type locality of Mumulaelaps (WC Clark, emeritus prof. zool. 2010 pers. com.).
Acknowledgements
Lily and Robin Clark collected the Taranaki Pericoptus samples. Peter Johns shared his knowledge of Pericoptus. Two anonymous referees and the associate editor of this journal greatly improved the paper. The research was supported by a CPIT Academic Research Committee grant.
References
- Bell , AM and Robinson , GE . 2011 . Behavior and the dynamic genome . Science , 332 : 1161 – 1162 .
- Bregetova NG 1977 . Family Laelapidae Berlese, 1892 . In : Gilyarov MS , Bregatova NG . Key to the soil inhabiting mites . Leningrad , Nauka . 483 – 554 [in Russian] .
- Brown , JG . 1967 . Notes and records of New Zealand Scarabaeide . New Zealand Entomologist , 3 : 42 – 50 .
- Casanueva , ME . 1993 . Phylogenetic studies of the free-living and arthropod associated Laelapidae (Acari: Mesostigmata) . Gayana (Zoologia) , 57 : 21 – 46 .
- Clark , JM and Hawke , DJ . 2011 . The sub-Antarctic mite Ayersacarus: a new species from mainland New Zealand, and its isotopic ecology . New Zealand Journal of Zoology , 38 : 43 – 54 .
- Costa , M . 1971 . Mites of the genus Hypoaspis Canestrini, 1884 s. str. And related forms (Acari: Mesostigmata) associated with beetles . Bulletin of the British Museum of Natural History (zoology) , 21 : 67 – 98 .
- Costa , M and Hunter , PE . 1970 . The genus Coleolaelaps Berlese, 1914 (Acarina: Mesostigma) . Redia , 52 : 323 – 60 .
- Dale , PS . 1963 . Ecology, life history and redescription of Pericoptus truncatus (Fabricius) . Transactions of the Royal Society of New Zealand (Zoology) , 3 : 17 – 32 .
- Dale , PS . 1964 . Tetleyus pericopti n. gen. et sp., a thelastomatid nematode from the larva of Pericoptus truncatus (Fab.) (Coleoptera: Dynastinae) . New Zealand Journal of Science , 7 : 589 – 595 .
- DeNiro , MJ and Epstein , SD . 1981 . Mechanism of carbon isotope fractionation associated with lipid synthesis . Science , 197 : 261 – 263 .
- Dowling , APG and OConnor , BM . 2010 . Phylogeny of Dermanyssoidea (Acari: Parasitiformes) suggests multiple origins of parasitism . Acarologia , 50 : 113 – 129 .
- Evans , GO . 1963 . Observations on the chaetotaxy of the legs in the free-living Gamasina (Acari: Mesostigmata) . Bulletin of the British Museum (Natural History) Zoology , 10 : 275 – 303 .
- Gómez-Díaz , E and González-Solís , J . 2010 . Trophic structure in a seabird host-parasite food web: insights from stable isotope analyses . PLoS ONE , 5 : e10454
- Hawke , DJ and Clark , JM . 2010 . Isotopic signatures (13C/12C; 15N/14N) of blue penguin burrow soil invertebrates; carbon sources and trophic relationships . New Zealand Journal of Zoology , 37 : 313 – 321 .
- Joharchi , O and Halliday , RB . 2011 . New species and new records of mites of the family Laelapidae (Acari: Mesostigmata) associated with Coleoptera in Iran . Zootaxa , 2883 : 23 – 38 .
- Johnston , DE and Moraza , ML . 1991 . “ The idiosomal adenotaxy and poroidotaxy of Zerconidae (Mesostigmata: Zerconina) ” . In Modern acarology , Edited by: Dusbábek , F and Bukva , V . Vol. 2 , 349 – 356 . Prague : Academia .
- Karagoz , M , Gulcu , B , Cakmak , I , Kaya , HK and Hazir , SD . 2007 . Predation of entomopathogenic nematodes by Sancassania sp. (Acari: Acaridae) . Experimental and Applied Acarology , 43 : 85 – 95 .
- King , RA , Read , DS , Traugott , M and Symondson , WOC . 2008 . Molecular analysis of predation: a review of best practice for DNA-based approaches . Molecular Ecology , 17 : 947 – 963 .
- Lindquist , EE and Evans , GO . 1965 . Taxonomic concepts in the Ascidae, with a modified setal nomenclature for the idiosoma of the Gamasina (Acarina; Mesostigmata) . Memoirs of the Entomological Society of Canada , 47 : 1 – 64 .
- Maraun , M , Erdmann , G , Fischer , BM , Pollierer , MM , Norton , RA , Schneider , K and Scheu , SD . 2011 . Stable isotopes revisted: their use and limits for oribatid mite trophic ecology . Soil Biology & Biochemistry , 43 : 877 – 882 .
- Martínez del Rio , C , Wolf , N , Carleton , SA and Gannes , LZ . 2009 . Isotopic ecology ten years after a call for more laboratory experiments . Biological Reviews , 84 : 91 – 111 .
- Mellbrand , K , Lavery , PS , Hyndes , G and Hambäck , PA . 2011 . Linking land and sea: different pathways for marine subsidies . Ecosystems , 14 : 732 – 744 .
- Miller , D . 1984 . Common insects in New Zealand , revised ed , Wellington : AH & AW Reed .
- Moraza , ML . 2004 . The phoretic genus Neopodocinum (Oudemans, 1902) in the Iberian Peninsula (Acari: Mesostigmata: Macrochelidae) . Revista Ibérica de Aracnología , 10 : 261 – 269 .
- O'Grady , SP and Dearing , MD . 2006 . Isotopic insights into host–endosymbiont relationships in Liolaemid lizards . Oecologia , 150 : 355 – 361 .
- Polis , GA , Sánchez-Piñero , F , Stapp , PT , Anderson , WB and Rose , MD . 2004 . “ Trophic flows from water to land: marine input affects food webs of islands and coastal ecosystems worldwide ” . In Food webs at the landscape level , Edited by: Polis , GA , Power , ME and Huxel , GR . 200 – 216 . Chicago , IL : Chicago University Press .
- Post , DM . 2002 . Using stable isotopes to estimate trophic position: models, methods, and assumptions . Ecology , 83 : 703 – 718 .
- Post , DM , Layman , CA , Arrington , DA , Takimoto , G , Quattrochi , J and Montaña , CG . 2007 . Getting to the fat of the matter: models, methods, and assumptions for dealing with lipids in stable isotope analysis . Oecologia , 152 : 179 – 189 .
- Ratcliffe , BC and Orozco , J . 2009 . A review of the biology of Pericoptus truncatus (Fabr.) (Coleoptera: Scarabaeidae: Pentodontini) from New Zealand and a revised description of the third instar . Coleopterists Bulletin , 63 : 445 – 451 .
- Scheu , SD . 2002 . The soil food web: structure and perspectives . European Journal of Soil Biology , 38 : 11 – 20 .
- Strong , KL and Halliday , RB . 1994 . Three new species of Hypoaspis Canestrini (Acarina: Laelapidae) associated with large Australian cockroaches . Australian Journal of Entomology , 33 : 87 – 96 .
- Swofford DL 2002 . PAUP ver. 4.0b10: Phylogenetic analysis using parsimony (and other methods) . Sunderland , Sinauer Associates .
- Womersley , H . 1954 . On some new Acarina-Mesostigmata from Australia, New Zealand and New Guinea. Journal of the Linnean Society of London . Zoology , 42 : 505 – 599 .
- Womersley , H . 1956 . Some additions to the Acarina-Mesostigmata of Australia . Transactions of the Royal Society of South Australia , 79 : 104 – 120 .