Abstract
We investigated the effects of invasion by introduced grey willow (Salix cinerea) on beetle communities within four wetland vegetation types: native vegetation, native vegetation following grey willow removal, native vegetation undergoing grey willow invasion and dense grey willow-dominated vegetation. In total, 1505 beetles from 90 species were collected using modified Malaise traps. Native wetland vegetation had significantly lower beetle species richness than willow-dominated vegetation and was dominated by herbivores, whereas detritivores characterised willow-dominated vegetation. Beetle abundance was highest in the willow-dominated vegetation and mostly comprised detritivores. In contrast, beetle abundance was lowest in native wetland vegetation, but had even proportions of herbivores and detritivores. Native wetland vegetation had a high proportion of native beetles present. As grey willows invaded, introduced beetles became more common. The beetle community composition differed significantly between grey willow-dominated vegetation and native wetland vegetation. These compositional differences were mainly due to the increasing complexity of vegetation structure following grey willow invasion. The beetle communities within restored native wetland vegetation were most similar to those within the native wetland vegetation. From a conservation perspective, these results are encouraging and suggest that, although grey willows dramatically alter the composition of beetle communities present, these communities can be restored to a beetle fauna that is similar to those found within native wetland through the removal of the willows.
Introduction
Habitat invasion by introduced plants is a widespread phenomenon and has become a significant threat to global biodiversity (Houlahan & Findlay Citation2004). Wetlands are renowned for their susceptibility to invasions because they function as landscape sinks that accumulate debris, sediments water, and nutrients, all of which facilitate invasions by creating canopy gaps or accelerating the growth of opportunistic plant species (Zedler & Kercher Citation2004). Woody plant invaders, such as Melaleuca quinquenervia in North America (Serbesoff-King Citation2003) and Tamarix ramosissima in Australia (Zedler & Kercher Citation2004), are now among the worst invaders of wetlands. Such invasions by introduced plants may have important consequences for energy and material exchange between terrestrial and aquatic ecosystems (Vitousek Citation1990; Ewel et al. Citation2001), particularly if they differ structurally or functionally from native vegetation. The replacement of native vegetation by an aggressive invader directly modifies habitat, alters inputs into the wetland and may indirectly change community structure, biodiversity and ecological processes (Zedler & Rea Citation1998). In addition, altered habitat structure and resources in the wetland may affect higher-order consumers, such as invertebrates and birds. For example, invasion by Tamarix spp. forming extensive monocultures has resulted in reduced bird diversity and diminished use of the habitat by mule deer, beaver and woodrats (Cox Citation1999).
In New Zealand, willows (Salix spp.) are major invaders of riparian zones and wetlands (Lester et al. Citation1994; Clarkson Citation2002). Originally from Europe and Asia, willows were planted along rivers and streams to stabilise banks and provide shelter for livestock (Glova & Sagar Citation1994). The ability of many willows to reproduce vegetatively from fragile branches or twigs that break and take root is probably an important factor in the invasiveness of these species. The spread of grey willow (Salix cinerea), in particular, has been further aided by the prolific production of lightweight, wind-dispersed seed. Willows within the riparian zone are known to affect aquatic invertebrates (Glova & Sagar Citation1994; Lester et al. Citation1994; Schulze & Walker Citation1997; Read & Barmuta Citation1999), terrestrial arthropods (Greenwood et al. Citation2004) and fish (Glova & Sagar Citation1994). For example, Lester et al. (Citation1994) observed that stream reaches flanked by S. fragilis supported relatively few macroinvertebrates, but Glova & Sagar (Citation1994) found a more diverse benthic invertebrate fauna and a relatively high biomass of brown trout (Salmo trutta) in streams with willow-dominated riparian vegetation.
The impacts of willows on the invertebrate fauna within wetlands have yet to be quantified. Wetlands dominated by native plant species provide habitat and resources for many native insects (Watts & Patrick Citation2001; Watts Citation2009) but it is unknown whether invaded areas provide similar habitat and resources. A major threat to wetlands in the Waikato region is invasion from introduced woody species. Grey willow (S. cinerea) is the most common and widespread willow species in Waikato wetlands and potentially the most troublesome species (Clarkson Citation2002). In this study, we examined whether invasion by grey willow had changed the abundance, diversity and composition of the terrestrial beetle community in plots with varying densities and invasion stages of grey willow. These plots were compared with plots where grey willow was removed and native wetland species were re-establishing, and with plots dominated by native wetland species.
Methods
Study area
The study was conducted at Whangamarino, Toreparu and two Horsham Downs peat lakes (Lake Kaituna and Lake Tunawhakapeka) within the Waikato, North Island, New Zealand (). The wetlands were located within 80 km of each other at altitudes of less than 50 m above sea level, had similar climatic conditions and were surrounded by pasture. Four vegetation types were chosen for study: (1) native wetland vegetation, (2) restored native wetland vegetation, (3) native wetland vegetation undergoing grey willow invasion, and (4) dense, grey willow-dominated vegetation. The number of plots for each site and vegetation type is given in . Not all vegetation types occurred within each wetland but all plots in the same vegetation type were matched as closely as possible for similar plant species composition as the vegetation was relatively homogenous. Two circular sampling plots (10 m in diameter) were spaced at least 100 m apart within the centre of each vegetation type at each of the study wetlands. The willow-dominated vegetation at Whangamarino, Toreparu and Lake Tunawhakapeka was on the margins of the wetlands within a dense zone (approximately 20 m wide) of mature grey willow (6–8 m in height and approximately 20 years old) that formed a woody canopy cover of over 80% (required threshold for inclusion of a plot). Other abundant plant species were Rubus fruticosus, Phormium tenax, Baumea rubiginosa, Carex secta and Coprosma propinqua. Leaf litter and coarse woody debris (CWD) were present on the ground.
Figure 1 Map of the study area in the Waikato, North Island, New Zealand, showing the locations of the three wetlands (Whangamarino, Toreparu and two Horsham Downs peat lakes—Lake Kaituna and Lake Tunawhakapeka) in which beetle communities were sampled using Malaise traps.
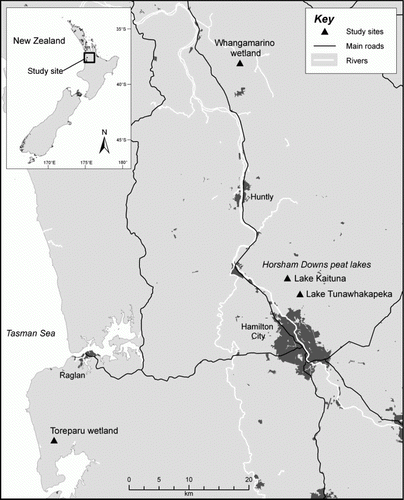
Table 1 Number of plots in each vegetation type within the Waikato wetlands.
The native wetland vegetation undergoing grey willow invasion at Toreparu was approximately 30 m from the wetland edge where young willows (<2.5 m in height and approximately 10 years old) were scattered among the native wetland species, which included P. tenax, Baumea arthrophylla, B. rubiginosa, C. secta, Leptospermum scoparium and Coprosma tenuicaulis. Native wetland vegetation undergoing grey willow invasion was sampled only at Toreparu, as the transition zone between grey willow and native wetland vegetation was abrupt at Whangamarino and Lake Tunawhakapeka, and was too narrow (<5 m) to meet the sampling plot criterion of 10 m in diameter.
The native wetland vegetation at Whangamarino and Toreparu was dominated by B. rubiginosa. Other common species were C. secta, P. tenax, L. scoparium, C. tenuicaulis and at Whangamarino, Schoenus brevifolius and Baumea teretifolia. The ground cover was sparse, apart from scattered Sphagnum mounds and Drosera binata at Whangamarino. Native wetland vegetation was less than 2 m in height and the plots were located approximately 90 m from the wetland edge.
In 1999, grey willows were removed from the margins of Lake Kaituna, located 1.2 km from Lake Tunawhakapeka within the Horsham Downs peat lakes. The restored wetland was dominated by Carex virgata, and P. tenax, B. arthrophylla and Cordyline australis were also common. The native wetland vegetation was less than 2 m high and occurred in a 20-m zone around the edge of the lake.
Efficiency of entomological sampling techniques in wetlands
A variety of monitoring techniques, such as pitfall traps, light traps, standard Malaise traps, modified Malaise traps, hand searching, sweep netting and emergence traps, has been used to sample the invertebrate fauna within the Waikato wetlands (Watts Citation2009; Watts & Hoare Citation2009; Watts unpubl. data). Pitfall traps were susceptible to flooding from fluctuating water levels, and emergence traps were prone to falling over in heavy rain and winds. Light traps, hand searching and sweep netting were time-consuming and, compared with other techniques, produced few specimens. The standard Malaise traps (1.8 m long×1.1 m wide×1.9 m high) were difficult to erect in the low native wetland vegetation and frequently blew over or became distorted. In addition, large temporal and spatial variation in beetle catches have been observed from traps placed in shrub and forest habitats in New Zealand (Hutcheson Citation1990; Hutcheson & Kimberley Citation1999; Hutcheson & Jones Citation1999). However, analyses carried out by Toft et al. (Citation2001) differentiated between plots in forest fragments with and without a dense weed cover by their fungus gnat communities, and to a lesser extent, beetle species. Smaller, commercially available Malaise traps (1.1 m long×0.7 m wide×1.7 m high) have been found to provide catches too small for robust classification of communities (Dugdale & Hutcheson Citation1997). However, a modified Malaise trap was designed by Richard Toft (Entecol Ltd, Nelson) specifically for the very windy environments and boggy ground found in low vegetation at higher altitudes on the West Coast, New Zealand (Richard Toft, Entecol Ltd, pers. comm.). While the design of the fine gauze Malaise tent remained the same, the dimensions of the trap were 1.0 m long×0.7 m wide×1.3 m high with modifications to increase catch efficiency. The Malaise tent was secured at each end to two T-shaped aluminium poles, which provided a rigid frame to hold the Malaise traps in a reliable shape, and the end poles were secured to a flat wooden plate (30×30 cm) on the ground for increased stability. Additional floor panels extend 15 cm out from the central wall of the trap to provide a more consistent catch of species that crawl over the ground. The collecting jar was then secured onto one of the end poles. This modified Malaise trap appeared to be the most efficient trapping technique for wetlands, and beetle samples were of an adequate size to allow discrimination between wetland vegetation types (Watts unpubl. data). These traps successfully revealed differences in the invertebrate communities in a study comparing habitat restoration techniques at the Stockton coal mine (Ross et al. Citation2000).
Beetle sampling, sorting and identification
Beetles were sampled using one modified Malaise trap in the approximate centre of each vegetation plot within the four vegetation types (grey willow-dominated vegetation, native wetland vegetation undergoing grey willow invasion, native wetland vegetation and restored native wetland vegetation). Each trap requires a relatively flat, log-free, open area of approximately 1.0 m2. Each Malaise trap was orientated with collecting jar pointing northwards and contained 100 ml monopropylene glycol to preserve specimens until collection. A total of 14 Malaise traps were set from 21 November 2007 to 17 January 2008 and were emptied twice at 30-day intervals. Beetles were removed and preserved in 70% alcohol until identified and counted. They were first sorted on the basis of external morphology to morphospecies or recognised taxonomic units (hereafter referred to as species) and then, where possible, given generic and species-level identifications by a taxonomic expert (Stephen Thorpe). Beetle species were assigned to three trophic groups according to Klimaszewski & Watt (Citation1997) (see ): predators, herbivores (includes xylophages) and detritivores (includes fungivores, saprophages and scavengers). Where only one feeding biology was known for a family, all species were assigned to that trophic group. In other cases, where multiple feeding biologies were known to occur, species were assigned on an individual basis using published details of the feeding biology of the species or genera. Each beetle species was classified as native, introduced or unknown status using Klimaszewski & Watt (Citation1997) and Kuschel (Citation1990).
Measurement of environmental variables
At each vegetation type, 10 environmental and habitat variables [% total vegetation cover (%TC),% total grey willow cover (%SC), number of vascular plant species (SPP), canopy height (CH), canopy density (CD), litter depth (LD), litter biomass (LB), amount of CWD, pH and moisture content] were measured within a plot (10 m in diameter) around each Malaise trap. These measurements were conducted within one week after the final beetle sampling. At each plot, the maximum height (m) of each canopy plant species was recorded, with percentage cover estimated using a modified Braun–Blanquet cover class number (Mueller-Dombois & Ellenburg Citation1974): 1:<1%; 2: between 1% and 5%; 3: between 5% and 25%; 4: between 25% and 50%; 5: between 50% and 75%; 6: between 75% and 100%. The variables analysed were %TC, %SC and SPP. In addition, the native or introduced status of each plant species sampled from within the plots was determined. CH was estimated against the height of a 2.5-m sighting pole. CD was measured at each trap at 1.5 m above ground level using a convex spherical canopy densitometer (Model 8, Ben Meadows Company, USA).
Two leaf-litter variables, LD and LB, were measured at each sampling period. LD (mm) was measured to the bottom of the F-horizon at four random points around each Malaise trap and then averaged. One LB sample was randomly collected within a circular metal ring (area = 0.025 m2) within 2 m of each trap. Another sample was also randomly collected using the same circular metal ring adjacent to each trap to determine the biomass of CWD in each plot. The litter and CWD samples were oven dried for at least 72 h at 60 °C until samples reached a constant weight. One soil core was obtained from the surface layer of each plot by cutting a steel cylinder (100 mm diameter×75 mm deep) into the substrate; the core was then sealed in a plastic bag. In the laboratory, substrate pH (PH) was determined by mixing 10 g fresh weight of substrate with 25 ml of distilled water and left for 1 h before reading with a standard pH meter. The rest of the soil core was weighed, and then oven-dried for at least 72 h at 105 °C until the samples reached a constant weight to determine moisture content (MOIST%), calculated as ((wet mass–dry mass)/dry mass)×100.
Data analysis
Beetle abundance
Differences in beetle abundance between the four vegetation types and three wetlands were examined using the total sample per Malaise trap over the 2-month period and analysed using a general linear model (GLM) under the Poisson model with a log link in the statistical software R version 2.9. We used Akaike information criterion (AIC) to choose the best model (minimum adequate model) from three possible models (). The investigation of interaction effects for these two covariates was difficult because not all the possible combinations of interactions were sampled and only seven possible interactions could be investigated (). To address the interaction effects, vegetation type and wetland were therefore combined into a single covariate with seven levels. We analysed the relationship between the beetle abundance and the new covariate using a Poisson model with a log link. The result from the model was used to determine the difference among these seven levels.
Table 2 Akaike information criterion (AIC) values for three possible models fitted to beetle abundance data.
Beetle species richness
Differences in beetle-species richness between the four vegetation types and three wetlands were examined using the total sample per Malaise trap over the 2-month period and analysed using a Poisson model with a log link. Again, AIC was used to find the best model of three possible models to explain the variation of beetle species richness.
Native versus introduced beetles and trophic structure of the beetle community
The proportions of native versus introduced beetles and the proportion of each trophic group within the beetle community were compared in terms of the number of beetle species and individuals across each vegetation type.
Multivariate analyses
Variation in beetle species composition and relative abundance between vegetation types were analysed using cluster analysis and ordination techniques within the PATN multivariate analysis package (Belbin Citation1995). The total beetle sample from each Malaise trap was used for analysis (=plot). Nine rare species, defined as having only one specimen in the total dataset, were omitted from the dataset. Plant species were classified into four vegetation types using an agglomerative hierarchical fusion technique (FUSE). To compare the similarity of beetle composition and abundance between vegetation type and to define the principal environmental gradient(s) structuring beetle assemblages, we conducted a multivariate analysis using semi-strong hybrid multidimensional scaling (SSH). The SSH hybrid scaling ordination technique implements an improved version of hybrid scaling that combines metric and non-metric criteria, as defined by Faith et al. (Citation1987). Because it is more flexible and fits output distances to input distances rather than squared input and output distances, the SSH technique is considered to have some advantages over other ordination techniques, such as principal components analysis, correspondence analysis/reciprocal averaging and other multidimensional scaling programmes for measuring ecological distance (Minchin Citation1987; Belbin Citation1995). SSH does not rotate the final axes to principal components, so maximum variation does not necessarily occur along the first axis (Belbin Citation1995). In all analyses we used the flexible Unweighted Pair-Group Method and the Bray–Curtis association measure using Arithmetic averages (UPGMA) clustering method (with β=−0.1), where equal weight is given to objects, not groups. Plot similarity in SSH ordination scores were correlated with the distributions of beetle species using the Principal Component Correlation (PCC) routine, in which taxa with the highest correlations have the most influence on the ordination patterns. Two-dimensional ordinations provided an adequate summary of the data sets; the stress value for the ordination (a measure of the fit between the similarity measure and the resulting configuration of plots in the ordination; Belbin Citation1995) was 0.1702. As increasing the number of dimensions (up to four) resulted in minor reductions in the stress value but did not change ecological interpretability (Belbin Citation1995), the addition of more than two axes was not warranted.
The environmental and habitat variables (%TC,%SC, SPP, CH, CD, LD, LB, amount of CWD, pH, and moisture content) and plot ordination scores were then analysed using a vector-fitting approach to examine species–environment responses within PATN. Additional variables included in the ordination analyses were vegetation type (VEG), plot distance from wetland edge (DIST) and wetland of each replicated Malaise trap within each vegetation type (LOC). Treatment and environmental variables that explained significant (P<0.01) variation in beetle community composition and abundance among plots were illustrated by plotting vectors in ordination space to show graphically the gradients represented by environmental variation.
Results
A total of 1505 beetles, comprising 90 species, were collected (see ). The most species-rich families in the samples were Curculionidae and Anthribidae (11 and nine species, respectively). The most abundant beetle species were Scirtidae sp. 1 (34.4% of all samples) and Notagonum lawsoni (Carabidae; 7.5% of all samples).
Beetle abundance
Overall, beetle abundance was lowest in the native wetland vegetation and highest in the willow-dominated vegetation (; ). There was no significant difference in the abundance of beetles collected in native wetland vegetation at Whangamarino and Toreparu (Z=0.71, P=0.4750; ; ), and the abundance of beetles at these sites was significantly lower than for all other combinations of vegetation types and wetland sites (; ).
Figure 2 Total abundance of beetles collected from a Malaise trap within two plots at each vegetation type in the Waikato wetlands. N, native wetland vegetation; R, restored native wetland vegetation; I, native wetland vegetation undergoing grey willow invasion; W, dense grey willow-dominated vegetation; WH, Whangamarino; TO, Toreparu; HD, Horsham Downs peat lakes.
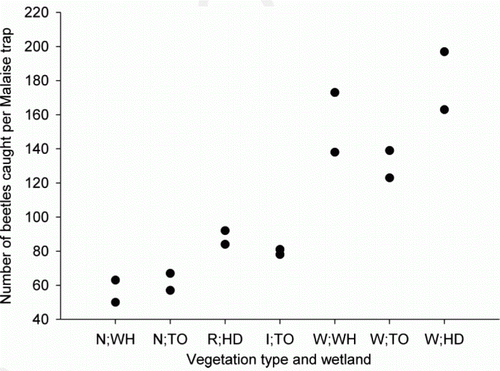
Table 3 General linear model (GLM) of beetle abundance at the four vegetation types and wetland sites.
Beetle species richness
The model with beetle species richness against vegetation type gave the smallest AIC = 79.38 (). Hence this was considered the best model to explain the variation in beetle species richness, and was used to determine the differences among the four vegetation types.
Table 4 Akaike information criterion (AIC) values for three possible models fitted to beetle species richness data.
Significantly higher beetle species richness was sampled in the restored native wetland vegetation (Z=2.02, P=0.0426; ; ) and the willow-dominated vegetation (Z=3.89, P<0.0001; ; ), when compared with the native wetland vegetation. There was marginal evidence of differences in the beetle species richness sampled between native wetland vegetation and native wetland vegetation undergoing grey willow invasion (Z=1.64, P=0.0992; ).
Figure 3 Beetle species richness sampled from a Malaise trap and plant species richness recorded from the sampling plot within each vegetation type. Closed circles, beetle species richness; open circles, plant species richness; Native, native wetland vegetation; Restored, restored native wetland vegetation; Invaded, native wetland vegetation undergoing grey willow invasion; Willow, dense grey willow-dominated vegetation.
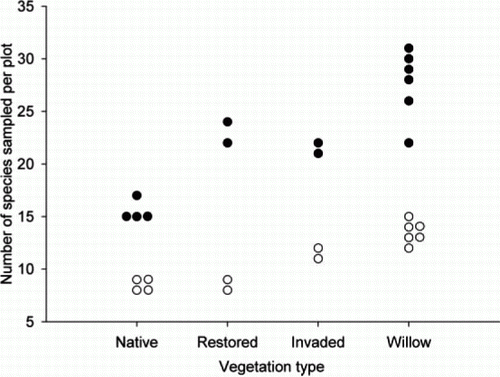
Table 5 General linear model (GLM) of beetle species richness at the four vegetation types.
Plant species richness
Plant species richness showed similar trends to beetle species richness with the highest number of species being sampled in willow-dominated vegetation and the lowest in native wetland vegetation ().
Native and introduced beetles and plants
Of the total 90 beetle species caught, 57 were native species, 25 were introduced species and the status of eight species was unknown (see ). Native beetle species accounted for 63% of the total sample.
Native beetles dominated the samples from native wetland vegetation (species 79% and abundance 92%) and restored native wetland vegetation (species 67% and abundance 80%; ). The proportion of native beetles caught in traps placed in grey willow-dominated vegetation and native wetland vegetation undergoing grey willow invasion was smaller (species 42% and abundance 45% and species 37% and abundance 63%, respectively; ). Introduced beetles were more common in the willow-dominated wetlands (species 46% and abundance 51%) and, in terms of species caught, in the native wetland vegetation undergoing grey willow invasion (species 51%) than the other vegetation types sampled ().
Figure 4 Proportion of native and introduced taxa sampled from each vegetation type. A, Proportion of beetle species caught per vegetation type. B, Proportion of beetles caught per vegetation type. C, Proportion of plant species sampled per vegetation type. Black bars, native species; light grey bars, introduced species; dark grey bars, status unknown. Note: n, the number of plots/Malaise traps per vegetation type ().
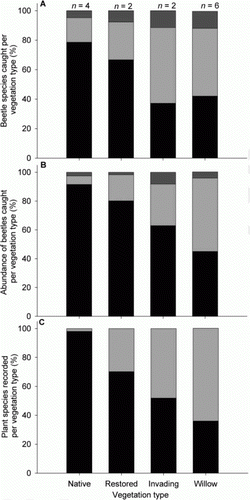
Native plant species dominated the plots within the native wetland vegetation (98%; ). The proportion of native plant species found in the plots within the restored native wetland vegetation and native wetland vegetation undergoing grey willow invasion was smaller than in the native wetland vegetation (70% and 51%, respectively; ). Within the willow-dominated vegetation, only 35% of plant species recorded were native ().
Trophic structure of the beetle assemblages
Of the beetle species caught, 42% (37 species) were detritivores, 36% (33 species) were herbivores and 22% (20 species) were predators (see ).
Trophic structure at the level of species was consistent between the native wetland and restored native wetland vegetation, with herbivorous beetles accounting for approximately half the species caught (47% and 45%, respectively; A). Beetle species sampled from the grey willow-dominated vegetation and native wetland vegetation undergoing grey willow invasion had a similar trophic structure, with detritivorous beetles being most common (45% and 40%, respectively; A).
Figure 5 Trophic structure of beetles caught from Malaise traps in each vegetation type. A, Proportion of beetle species caught per vegetation type. B, Proportion of beetles caught per vegetation type. Black bars, detritivores; light grey bars, predators; dark grey bars, herbivores. Note: n, the number of plots/Malaise traps per vegetation type ().
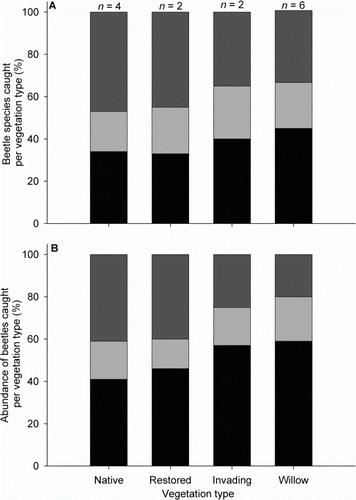
An alternative way to examine these data is to look at the total number of individuals in each trophic group, irrespective of species. The proportions of beetles caught from the native wetland and restored native wetland vegetation were comparable, with similar proportions of detritivores and herbivores sampled (41% herbivores and 41% detritivores in native wetland vegetation and 40% herbivores and 46% detritivores in restored native wetland vegetation; B). Beetle abundances sampled from the grey willow-dominated vegetation and native wetland vegetation undergoing grey willow invasion, had a similar trophic structure, with detritivorous beetles being dominant (59% and 57%, respectively; B).
Beetle community composition
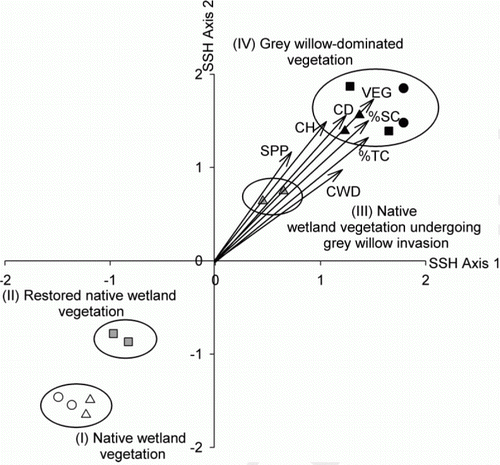
The ordination based on species composition and abundance showed that beetle communities differed in different vegetation types (). Four groups with differing beetle species composition were identified by the FUSE clustering analysis, and these groupings were overlaid onto the SSH ordination to identify trends in beetle community composition within the study (). The groups form a gradation from wetlands dominated by native vegetation (Group I), through to restored wetlands (Group II), to native wetlands characterised by invading grey willow (Group III) and grey willow-dominated wetlands (Group IV).
Figure 6 Two-dimensional semi-strong hybrid multidimensional scaling (SSH) ordination of beetles caught in 14 Malaise traps (symbols) and significant (P<0.01) environmental variables (arrows). Four vegetation type groupings identified by the FUSE clustering analysis have been superimposed as ellipses around plots with similar species composition: (Group I) plots dominated by native wetland species, (Group II) restored native wetland plots, (Group III) native wetland plots undergoing grey willow invasion and (Group IV) grey willow-dominated plots. The vegetation type and wetland sampled are: open circles, native wetland vegetation at Whangamarino; open triangles, native wetland vegetation at Toreparu; grey filled squares, restored native wetlands at a Horsham Downs peat lake; grey filled triangles, invading grey willow plots at Toreparu; closed squares, grey willow-dominated vegetation at a Horsham Downs peat lake; closed circles, grey willow-dominated vegetation at Whangamarino; closed triangles, grey willow-dominated vegetation at Toreparu. See and text for environmental codes. The angle between environmental arrows indicates the degree of intercorrelation in their effects on beetle community composition.
Relationship of species composition to environmental gradients
Variation in beetle species composition was explained by vegetation type (R=0.823, P<0.01; ) and by changes in the structural complexity of the vegetation, as measured by the variables listed in . These variables included %SC, CD, CH, %TC, amount of CWD and SPP (). As might be expected, many of the environmental variables are significantly correlated with each other. In particular, the grey willow-dominated vegetation types are associated with a higher and denser canopy, increased S. cinerea cover and %TC, and increased amount of CWD ().
Table 6 Environmental variables and their correlations with vectors in ordination space.
The abundances of only 25 (out of 81) beetle species were significantly correlated with plots along SSH axes in relation to increasing complexity of vegetation structure. This was most clearly seen for Scirtidae sp. 1, N. lawsoni, Halmus chalybeus (Coccinellidae), Rhyzobius fagus (Coccinellidae), Byrrhopsis gravidus (Scritidae), which were more abundant in the grey willow-dominated plots with a more complex vegetation structure (increased canopy cover, density and height) and a more heterogeneous ground habitat (increased amount of CWD). Conversely, Selenopalpus cyaneus (Oedemeridae), Asilis sp. 1 (Cantharidae), and Conoderus sp. 1 (Elateridae) were associated with native wetland vegetation with sparse vegetation cover and habitat complexity.
Discussion
Impacts of grey willows on the beetle community
The presence of mature grey willow trees was clearly associated with an increase in terrestrial beetle species richness and abundance. Although we believe this trend to be meaningful, the lack of replication in our dataset was unavoidable. This is particularly true for the restored native wetland vegetation and the native wetland vegetation undergoing grey willow invasion, given that these were the only locations within Waikato wetlands that could be sampled. This reservation notwithstanding, our results agree with other studies that have shown similar effects of riparian willows (Salix spp.) on other invertebrate taxa, e.g. Ephemeroptera, Trichoptera and Diptera, by Glova & Sagar (Citation1994), who noted that the diversity, abundance and biomass of the benthic invertebrates were greater in stream sections with willows than without them. In contrast, other studies (Lester et al. Citation1994; Read & Barmuta Citation1999) reported that the presence of riparian willow trees (S. fragilis) clearly reduced macroinvertebrate abundance and biomass in streams. In addition, Greenwood et al. (Citation2004) observed that willow-invaded river sections in Australia had significantly lower abundance and diversity of canopy arthropods than native river sections.
We have evidence that grey willow trees alter the composition of the beetle communities present in the Waikato wetlands we studied. For example, the beetle community composition differed significantly between grey willow-dominated and native wetlands, and this was related to changes in the structural complexity of the vegetation. Those study plots with a higher proportion of native plant species, i.e. the native wetlands, had a high proportion of native beetles present. However, when grey willows dominated, the proportions of native versus introduced beetles changed, with introduced beetles being more common. Similarly, Watts & Gibbs (Citation2000) and Kuschel (Citation1990) found a positive relationship between the proportion of native beetles collected and the proportion of native plant species present within different aged forests. In addition, the functional structure of the beetle community differed between the grey willow-dominated plots and native wetlands. Herbivorous beetles were more commonly caught within native wetland vegetation, while detritivorous beetles dominated the samples from grey willow-dominated wetlands. The dominance of detritivores could result from the increased amounts of CWD within the willow-dominated wetlands.
Environmental factors influencing the occurrence of beetle species at grey willow-dominated wetlands
Beetle community composition differed significantly between grey willow-dominated and native wetlands. Total S. cinerea cover, CD, CH, %TC, amount of CWD and SPP have been shown to influence beetle species’ occurrance, illustrating that changes in the beetle community were associated with the increasing complexity of the vegetation structure related to the invasion of grey willow. The abundances of 40% of beetle species were significantly related to the increasing complexity of vegetation structure. For example, while no specimens of N. lawsoni were caught in the native wetland vegetation and only a few individuals were collected within the native vegetation undergoing grey willow invasion, this species was abundant within the grey willow-dominated plots. Nocturnal and thought to be gregarious, this predacious ground beetle lives in dense leaf litter, fallen branches and logs in wet habitats, including lowland swamp forests (Larochelle & Larivière Citation2001). Conversely, the metallic lax beetle, S. cyaneus, known to be abundant in open, exposed habitats (Kuschel Citation1990), dominated samples collected from the native wetland vegetation. As the vegetation structure and complexity increased, however, the abundance of S. cyaneus sampled was lower, with only a few specimens captured from plots within the native vegetation undergoing grey willow invasion. No specimens were caught within the grey willow-dominated plots.
Our results agree with those of other similar studies, which suggest that the composition of beetles present at a site is related to environmental factors such as vegetative physiognomy (vegetation age, diversity, height and density) (Watts & Gibbs Citation2002; Grimbacher & Catterall Citation2007), presence of dead wood on the ground (Reay & Norton Citation1999) and LD (Sanderson et al. Citation1995). With increasing age, restored plant communities in a mined peat bog at Torehape (35 km east of Whangamarino) become more diverse and structurally complex, resulting in the beetle community composition converging rapidly on the target community structure of an undisturbed peat bog (Watts et al. Citation2008). Of the 90 species collected in the present study, 20 species were also sampled from restored peat islands at Torehape (Watts et al. Citation2008). The species collected in both these studies were found in wet swampy habitats (Scopodes fossulatus), were fully winged mobile species (S. cyaneus), and/or were common widely distributed species (Sitona lepidus).
Beetle species richness and abundance within grey willow-dominated vegetation were approximately twice those found in native wetlands between November 2007 and January 2008. A more diverse, abundant beetle community could result from an increased variety of habitats, including increased amounts of dead wood and a palatable food source (willow leaves) during summer, which were observed in grey willow-dominant vegetation. Greenwood et al. (Citation2004) found that the effect of willow invasion of the riparian zone on terrestrial arthropod communities was seasonal, and similar trends have been observed in benthic invertebrate communities (Lester et al. Citation1994; Read & Barmuta Citation1999). Since both resources and habitat structure can change seasonally with plant phenology (e.g. leaf flushing, flowering), differences in invertebrate abundance and diversity between seasons, particularly between summer and winter, are not unexpected. We would expect evergreen native wetlands, which lack a clear dormant phase, to exhibit fewer seasonal effects on abundance and diversity of herbivorous invertebrates compared with deciduous willow-dominated wetlands. A more complete assessment should be undertaken to test this hypothesis.
Implications for restoration
There was no difference in species richness and abundance of beetles in the plots where native wetlands had been restored by removing grey willow in 1999 compared with plots where grey willow had invaded native wetlands within the last 12 years. However, the proportion of native versus introduced species, trophic structure and composition of the beetle community sampled from the restored native wetland vegetation were most similar to the beetle communities found within the native wetlands. Willow-induced change in the native beetle community may affect higher trophic taxa, such as native fish, birds and invertebrates, which may depend on the native beetle communities as a food source. Willow invasion within the riparian zone alters inputs of terrestrial arthropods used as food by fish (Bridcut Citation2000). Native galaxids in southern Australia that depend on terrestrial arthropods from riparian vegetation in spring and summer, could be forced to alter their diet in winter when willows have dropped their leaves, with cascading implications for in-stream community structure (Greenwood et al. Citation2004). This may also apply to wetland ecosystems and further research is clearly required.
Our study supports a small but growing body of evidence suggesting that restoration of native vegetation can promote the reestablishment of the associated native beetle community. Watts et al. (Citation2008) predicted that beetle communities on restored peat islands at Torehape peat mine would converge on the average community composition of an undisturbed peat bog within just 7–8.5 years. From a conservation perspective, our results are encouraging and suggest that, although grey willows dramatically alter the composition of the beetle community present, communities can be restored to ones similar to those found in native wetlands by the removal of willows.
Acknowledgements
Research funds were provided by the Foundation for Research, Science and Technology New Zealand (under contract C09X0508). Thanks to Scott Bartlam, Bev Clarkson, Jordan Edgar and Toni Johnston who assisted with fieldwork. Particular thanks to Kerry Bodmin who helped with the wetland vegetation surveys. We are grateful to Richard Toft for design plans and advice on constructing the modified Malaise traps and discussions about the variability in Malaise trap catches. Thanks to Stephen Thorpe who identified the beetles. We thank the Hayes, Hendersen, Givens and Munns families for access to their land to allow us entry into the wetlands. Ian Stringer, Bev Clarkson, Anne Austin, John Marris and two anonymous referees provided helpful criticism.
References
- Belbin , L . 1995 . PATN: Pattern analysis package. Technical reference , Canberra : CSIRO Division of Wildlife and Ecology .
- Bridcut , EE . 2000 . A study of terrestrial and aerial macroinvertbrate on river banks and their contribution to drifting fauna and salmon diets in a Scottish catchment . Hydrobiologia , 427 : 83 – 100 .
- Clarkson , BR . 2002 . “ Swamps, fens and bogs ” . In Botany of the Waikato , Edited by: Clarkson , B , Merrett , M and Downs , T . 49 – 57 . Hamilton : Waikato Botanical Society Inc .
- Cox , GW . 1999 . Alien species in North America and Hawaii: Impacts on natural Ecosystems , Washington , D.C : Island Press .
- Dugdale J , Hutcheson J. 1997 . Invertebrate values of kanuka (Kunzea ericoides) stands, Gisborne Region . Science for Conservation 55 . Wellington : Department of Conservation .
- Ewel , KC , Cressa , C , Kneib , RT , Lake , PS , Levin , LA , Palmer , MA , Snelgrove , P and Wall , DH . 2001 . Managing critical transition zones . Ecosystems , 4 : 452 – 460 .
- Faith , DP , Minchin , PR and Belbin , L . 1987 . Compositional dissimilarity as a robust measure of ecological distance . Vegetatio , 69 : 57 – 68 .
- Glova , GJ and Sagar , PM . 1994 . Comparison of fish and macroinvertebrate standing socks in relation to riparian willows (Salix spp.) in three New Zealand streams . New Zealand Journal of Marine and Freshwater Research , 28 : 255 – 266 .
- Greenwood , H , O'Dowd , DJ and Lake , PS . 2004 . Willow (Salix×rubens) invasion of the riparian zone in south-eastern Australia: reduced abundance and altered composition of terrestrial arthropods . Diversity & Distributions , 10 : 485 – 492 .
- Grimbacher , PS and Catterall , CP . 2007 . How much do site age, habitat structure and spatial isolation influence the restoration of rainforest beetle species assemblages? . Biological Conservation , 135 : 107 – 118 .
- Houlahan , JE and Findlay , CS . 2004 . Effect of invasive plant species on temperate wetland plant diversity . Conservation Biology , 18 : 1132 – 1138 .
- Hutcheson , J . 1990 . Characterization of terrestrial insect communities using quantified, Malaise-trapped Coleoptera . Ecological Entomology , 15 : 143 – 151 .
- Hutcheson , J and Jones , D . 1999 . Spatial variability of insect communities in a homogenous system: Measuring biodiversity using Malaise trapped beetle in a Pinus radiata plantation in New Zealand . Forest Ecology and Management , 118 : 93 – 105 .
- Hutcheson , J and Kimberley , MO . 1999 . A pragmatic approach to characterising insect communities in New Zealand: Malaise trapped beetles . New Zealand Journal of Ecology , 23 : 69 – 79 .
- Klimaszewski J , Watt JC 1997 . Coleoptera. Family-group review and keys to identification . Fauna of New Zealand 37 . Lincoln : Manaaki Whenua Press .
- Kuschel G 1990 . Beetles in a suburban environment: a New Zealand case study . Wellington : Department of Scientific and Industrial Research .
- Larochelle A , Larivière M-C 2001 . Carabidae (Insecta: Coleoptera) catalogue . Fauna of New Zealand 43 . Lincoln : Manaaki Whenua Press .
- Lester , PJ , Mitchell , SF and Scott , D . 1994 . Effects of riparian willow trees (Salix fragilis) on macroinvertebrate densities in two small Central Otago, New Zealand, streams . New Zealand Journal of Marine and Freshwater Research , 28 : 267 – 276 .
- Minchin , PR . 1987 . An evaluation of the robustness of techniques for ecological ordination . Vegetatio , 69 : 89 – 107 .
- Mueller-Dombois D , Ellenburg H 1974 . Aims and methods of vegetation ecology . New York : John Wiley and Sons .
- Read , MG and Barmuta , LA . 1999 . Comparisons of benthic communities adjacent to riparian native eucalypt and introduced willow vegetation . Freshwater Biology , 42 : 359 – 374 .
- Reay , SD and Norton , DA . 1999 . Assessing the success of restoration plantings in a temperate New Zealand forest . Restoration Ecology , 7 : 298 – 308 .
- Ross C , Simcock R , Williams P , Toft R , Flynn S , Birchfield R , Comeskey P 2000 . Salvage and direct transfer for accelerating restoration of Native ecosystem on mine sites in New Zealand . 2000 New Zealand Minerals & Mining Conference Proceedings , 29–31 October 2000 .
- Sanderson , RA , Ruston , S , Cherrill , AJ and Byrne , JP . 1995 . Soil, vegetation and space: an analysis of their effects on the invertebrate communities of a moorland in north-east England . Journal of Applied Ecology , 32 : 506 – 518 .
- Schulze , DJ and Walker , KF . 1997 . Riparian eucalypts and willows and their significant for aquatic invertebrates in the River Murray, South Australia . Regulated Rivers: Research & Management , 13 : 557 – 577 .
- Serbesoff-King , K . 2003 . Melaleuca in Florida: a literature review on the taxonomy, distribution, biology, ecology, economic importance and control measures . Journal of Aquatic Plant Management , 41 : 98 – 112 .
- Toft , RJ , Harris , RJ and Williams , PA . 2001 . Impacts of the weed Tradescentia fluminensis on insect communities in fragmented forests in New Zealand . Biological Conservation , 102 : 31 – 46 .
- Vitousek , PM . 1990 . Biological invasions and ecosystem processes: towards an integration of population biology and ecosystem studies . Oikos , 57 : 7 – 13 .
- Watts , CH and Gibbs , GW . 2000 . Species richness of indigenous beetles in restored plant communities on Matiu-Somes Island, Wellington Harbour, New Zealand . New Zealand Journal of Ecology , 24 : 195 – 201 .
- Watts , CH and Patrick , B. 2001 . Creatures of the swamp . Forest & Bird February , 299 : 32 – 35 .
- Watts , CH and Gibbs , GW . 2002 . Revegetation and its effect on the ground-dwelling beetle fauna of Matiu-Somes Island, New Zealand . Restoration Ecology , 10 : 96 – 106 .
- Watts , CH , Clarkson , BR and Didham , RK . 2008 . Rapid beetle community convergence following experimental habitat restoration in a mined peat bog . Biological Conservation , 141 : 568 – 579 .
- Watts CH 2009 . The invertebrate fauna of Whangamarino wetland, Waikato . Hamilton : Landcare Research , LC0809/121 .
- Watts CH , Hoare R 2009 . The invertebrate fauna of Kopuatai wetland, Waikato . Hamilton : Landcare Research , LC0809/124 .
- Zedler , JB and Kercher , S . 2004 . Causes and consequences of invasive plants in wetlands: Opportunities, opportunists and outcomes . Critical Reviews in Plant Science , 23 : 431 – 452 .
- Zedler , JB and Rea , N . 1998 . Introduction, ecology and management of wetland plant invasions. Wetlands . Ecology and Management , 5 : 161 – 163 .