Abstract
The secretion of defensive chemicals onto the skin is a widely used mechanism for predator defence in anurans. Secretions often consist of a mixture of bioactive peptides with cytotoxic or neurotoxic effects. In New Zealand, introduced rodents have been suggested as main drivers for declines and extinctions of endemic frogs. We demonstrate the efficacy of Leiopelma pakeka secretions in deterring rats (Rattus norvegicus Long-Evans) from ingesting secretion-covered food and showed that they can successfully lyse rat erythrocytes. When offered a choice, rats displayed a significant preference for food pellets coated with water over those covered in frog secretions. Direct oral exposure to the secretions has no significant effects on water or food intake of rats. Video analysis showed no significant difference in the proportion of time rats spent grooming, rising on hind legs, motionless or investigating associated with exposure to the secretions. This study provides new insight into the defensive function of leiopelmatid skin secretions.
Introduction
The release of skin secretions in response to predator attack is an integral mechanism of the wide suite of defences in anurans. Often, bulk discharge of toxic secretions act synergistically with aposematic colouration, defensive postures and distress calls (Williams et al. Citation2000). The skin secretions of anurans often contain a potent mixture of peptides, biogenic amines or alkaloids with an extensive range of pharmacological activities (e.g. Clarke Citation1997; Daly et al. Citation2005). While such peptides are often studied in the context of novel drug development, the activities of these compounds indicate they act in defensive roles against predation in their natural context (Toledo & Jared Citation1995; Conlon et al. Citation2007). Many peptides isolated from anuran skin are characterised by their effects on muscle control, but they also act as neuromodulators or neurotransmitters and often have analgesic properties (Pukala et al. Citation2006; You et al. Citation2009). In addition, many peptides display high levels of cytotoxicity, which is a result of rupturing plasma and lysosomal membranes and can be estimated by measuring the rate of haemolysis (eg. Conlon et al. Citation2005; Simmaco et al. Citation2009). The ability of peptides to lyse vertebrate cells may therefore play a vital part in their defensive role.
Peptides can disrupt normal cell functioning by either causing the formation of pores in the cell membrane or by binding to intracellular targets, such as nucleic acids (Oren & Shai Citation1998; Park et al. Citation1998). Neurotoxic and cytotoxic anuran peptides, such as caeruleins or bombesins, are important in the host defence system, in particular their effects on muscle control and the central nervous system (e.g. Pukala et al. Citation2006). For example, bradykinins can stimulate immune effects, activate nociceptive pathways in mammals and affect muscle control of respiratory pathways (Basir et al. Citation2000). Neuropeptides such as tryptophyllins and tachykinins, are produced in amphibian skin, gut or brain, and some are known to induce sleep in mice (Das et al. Citation2000; Severini et al. Citation2002; Li et al. Citation2006).
Amphibians commonly produce defensive chemicals in their skin, but the function of these secretions in predator defence is rarely studied empirically. Even though the activity of peptides may be studied in vitro with relative ease, it is difficult to study their effects on predators in the natural environment. In fact, specific predators of anuran species often remain unidentified because anuran predators taking prey items are rarely observed. The limited empirical data on the impact of specific predators on frog populations is mostly restricted to anecdotal reports attributing the release or rejection of prey to the frogs’ toxic skin secretions (Green Citation1988; Karraker Citation2001). One of the major problems for many populations of endemic amphibians is the lack of evolutionary history with newly introduced predators. As a result, previously dependable mechanisms of defence can be ineffective against introduced species, even if they are similar to native predators (Kats & Ferrer Citation2003).
The introduction of non-native predators into naive ecosystems, mostly mediated by human activities, has played a major role in the declines of numerous amphibian populations (Kats & Ferrer Citation2003). The extinction of many endemic birds, reptiles and anurans in New Zealand has been linked to the arrival of human settlers and the associated introduction of predators (e.g. Gibbs Citation2009; Towns Citation2009). In New Zealand, rats and mice have been suggested as one of the main drivers for the declines and extinctions of Leiopelma species (Worthy Citation1987; Bell Citation1994; Towns & Daugherty Citation1994). The Pacific rat (Rattus exulans) was introduced to New Zealand at about AD 1280 with the arrival of humans, while the Norway rat (Rattus norvegicus) and ship rat (Rattus rattus) became widespread in the eighteenth and nineteenth centuries after European settlement had begun (Wilmshurst et al. Citation2008). Although evidence for rat predation is largely circumstantial, rats and mice are still perceived as a major threat to endemic frogs. However, the potential protective properties of Leiopelma skin secretions have not been critically evaluated.
In order to clarify the role of skin secretions of native frogs in predator deterrence, it is essential to examine the secretions in isolation from other factors contributing to the defensive repertoire of L. pakeka. We accomplished this by: (a) observing the effects of direct oral exposure to the secretion on rat behaviour; (b) using choice experiments to determine the potential of frog skin secretions to act as feeding deterrents to rats; and (c) using an in vitro haemolytic assay to test the toxicity of L. pakeka defensive skin secretions against rat red blood cells.
Methods
Animal husbandry
In May 2009, male Long Evans rats (Rattus norvegicus, n=23; Hercus-Taieri Resource Unit, Dunedin, New Zealand) were housed in individual cages (35×20×28 cm; L×W×H) with wood shavings and shredded paper on a L:D cycle of 12 hours at 20°C. Individual rats were weighed to the nearest gram twice a week throughout. Water was freely accessible to the animals at any time, while standard rat food pellets (Speciality Feed, Glen Forest, Australia) were offered ad libitum during a two-week acclimation period. After this period, daily access to food was restricted to two hours (1700 h–1900 h) to no less than 85% of the free-feeding weight of the rats. A pilot study conducted in preparation for the present experiments showed that 22 hours of food deprivation were needed to increase interest in food during the choice trials. We did not observe any visible signs of stress or significant weight loss during the four weeks on this feeding schedule. Animals were allowed to acclimatise to the laboratory conditions for 14 days prior to initiating experiments. Rats were re-housed as pets after the experimental trials.
Maud Island frogs (Leiopelma pakeka, n=15) from a captive colony at Otago University were housed in individual plastic containers (30×21×9 cm; L×W×H) containing paper towels moistened with purified water at 100% humidity and fed small locusts and crickets ad libitum once a week. Light and temperature were set to reflect natural fluctuations on Maud Island. At the time of the experiment, temperatures were 10°C during the day and 4.5°C at night on a L:D cycle of 10:14 hours. Frogs were returned to the captive colony after experimental procedures. All handling and testing of animals were approved by the University of Otago Animal Ethics Committee and the New Zealand Department of Conservation (AEC 62/08, AEC 80/05, NM-19892-RES).
Skin secretion collection of L. pakeka
To collect skin secretions, individual frogs were held by the back legs, their skin was moistened with ultra-purified, deionised water (hereafter MQW; Millipore, Molsheim, France) and a battery-powered bipolar platinum electrode (21G) connected to a multimeter was gently applied to the glands on the back of the frogs. The skin of each frog was electrically stimulated at 1.5 V (AC) for 30 seconds and the secretions either gently scraped off with a metal spatula or washed off with MQW. This non-invasive method has no adverse effects on frogs (Tyler et al. Citation1992; Smith et al. Citation2004).
Behavioural effects of L. pakeka skin secretions on rats
To test if the frog secretions induce aversion behaviour in rats, freshly collected secretions were gently scraped off individual frog skin (n=4) with a small metal spatula after mild electric stimulation. Rats were assigned to either treatment (n=4; oral treatment with frog skin secretions) or control groups (n=4; oral treatment with water). The water and food intake of each rat was monitored for three days before and four days after treatment with either water or frog skin secretions.
The secretions of each frog were collected separately and directly applied to the individual rats’ tongue and cheeks (n=4). To apply treatments orally, rats were gently grasped and restrained with a small towel. Water on a metal spatula was applied to the tongue of individual rats (n=4) to control for the physical presence of liquid. Each rat was introduced into an observation cage with clear plastic walls (35×20×30 cm; L×W×H) and wood shavings. After 10 minutes of acclimation their behaviour was filmed for 15 minutes before and after treatment with either water or frog skin secretions. An ethogram was constructed and behaviour was categorised as: stillness (motionless with no behaviour evident); investigating the surroundings (flaring of nostrils with movement of vibrissae and walking around); rising on hind legs (of any form where both front paws are lifted off the ground); and grooming (of any form). Linear mixed-effects models (restricted maximum likelihood fit [REML]) were used to test variations in water and food consumption, with treatment (water control, exposed to skin secretions) and before–after (B, before treatment; A, after treatment) as fixed factors and day∣rat as the random factor to account for the repeated measures taken from individuals. The water consumption data was natural log transformed and centred prior to analysis to fit normality. The proportions of time that secretion-exposed and control rats spent motionless, sniffing with locomotion, rising on hind legs, or grooming before and after treatment were analysed as generalised linear mixed-effects models with a quasipoisson distribution in the lme4 library in R (Bates et al. Citation2008). In the model, treatment (water control, secretion-exposed) and BA (before treatment, after treatment) were considered fixed factors and BA|rat as the random factor.
Effect of defensive frog skin secretions on palatability of rat food
During an acclimation period, each rat was introduced into an experimental cage (35×20×30 cm; L×W×H, plastic tank with air holes in the lid) for 20 minutes per day for 10 days. The cage contained two identical porcelain bowls (10 cm diameter) marked as A and B, which held two standard food pellets each. The amount of food consumed from bowl A and B was recorded to detect preferences for a specific bowl or position.
Before each trial, frog secretions were freshly collected from individual L. pakeka (n=15) as described above. After electrically stimulating the skin of each frog for 30 seconds, the animal was rinsed in a re-sealable plastic bag with 2 ml of MQW. After removing the frog, four standard rat pellets were added to the plastic bag and allowed to absorb the rinsed-off skin secretions, while control pellets were exposed to an equal volume of MQW. All pellets were weighed to the nearest gram before the trial.
At the start of each trial, rats previously unexposed to frog secretions (n=19) were introduced individually to the experimental cage and given a choice between food pellets with frog skin secretions (bowl A) and pellets without skin secretions (bowl B). Each trial commenced when the rat started feeding (within 10 seconds to two minutes) and the animal was allowed to feed for four-minute intervals after which the bowls were weighed to the nearest gram and their position switched around in the following pattern (ABBA/BAAB). The rat was removed from the experimental cage after each four-minute interval and offered access to water while the bowls were weighed. This procedure was repeated until half of the available food had been consumed. A one-way independent ANOVA test was used to determine if there was a difference in the amount of food pellets eaten, with and without skin secretions. All statistical tests were two-tailed. Omega (ω) values are reported as a measure of effect size, where 0.01 is considered a small, 0.06 a medium and anything equal to or greater than 0.14 a large effect size (Kirk Citation1996).
Haemolytic activity of skin secretions
Skin secretions were collected from L. pakeka (n=4) by electric stimulation as described above. The combined secretions were washed into a polypropylene beaker with MQW and acidified with hydrochloric acid (HCl) to a final concentration of 1% to inactivate endogenous peptidases (Steinborner et al. Citation1997). The sample was passed over C-18 Sep-Pak cartridges (Waters Corporation, Milford, Massachussetts, USA) that had been activated with methanol and rinsed with 0.1% HCl (v/v/v) and eluted with 70% acetonitrile, 29.9% water, and 0.1% trifluoroacetic acid (v/v/v). The volume of the elute was recorded, and 1 ml removed for subsequent calculation of peptide concentration and the sample lyophilised. The total amount of peptides present in each sample was determined by Micro BCA Assay (Pierce, Rockford, Illinois, USA). Manufacturer's instructions were followed, except that bradykinin (RPPGFSPFR; Sigma) was used to establish a standard curve (Rollins-Smith et al. Citation2002). The sample was reconstituted with Dulbecco's phosphate-buffered saline (D-PBS), pH 7.4 at a concentration of 1 mg/ml and filter sterilised (0.2 µm syringe filter, Corning, New York, USA).
Adult male Long Evans rats (Hercus-Taieri Resource Unit, Dunedin, New Zealand) were anaesthetised by intraperitoneal injection of pentobarbital and, subsequently, blood was drawn from the vena cava. Serial dilutions of the frog skin peptides were incubated with rat erythrocytes (2×107 cells) in Dulbecco's phosphate-buffered saline, pH 7.4 for one hour at 22°C. After centrifugation (13,000×g for two minutes), 100 µl of supernatant was plated in 96 well microtiter plates (Cellstar, Greiner Bio-One, Frickenhausen, Germany) in replicates of eight and the absorbance at 450 nm was measured with a Fluostar Omega spectrophotometer (Alphatech Systems, Auckland, New Zealand). A parallel incubation in the presence of MQW (no peptides present) was carried out as a control to determine the absorbance associated with 100% haemolysis in three independent experiments (Castro et al. Citation2005). The absorbance of the D-PBS blank was subtracted in order to calculate haemolysis induced by the skin secretions. The LD50 value was taken as the mean concentration of peptides producing 50% haemolysis.
Results
Behavioural effects of L. pakeka skin secretions on rats
The volume of water that rats drank before treatment did not differ significantly from that drank after treatment with either force-fed water control or the rodent secretions (P=0.6545, , ). The direct oral application of water had no effect on drinking behaviour before and after exposure (P=0.9360, , ). Drinking levels of rats exposed to frog skin secretions after treatment were also not significantly different from rats exposed to water before exposure (P=0.6565, ).
Figure 1 Water consumption (g) of rats (mean±SEM, n=8) before and after treatment with either defensive frog skin secretions (triangles) or water control (circles).
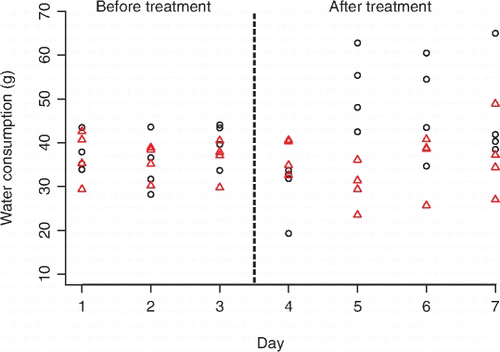
Table 1 Summary of linear mixed-effects models for changes in water and food consumption of rats before and after (BA) treatment with water or Leiopelma pakeka skin secretions.
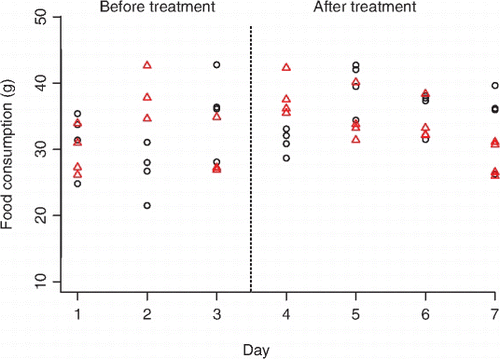
Food consumption of rats exposed to frog skin secretions did not differ significantly from that of rats exposed to water (P=0.8165, , ). Rats exposed to frog skin secretions did not differ in the food consumption from the water-exposed group before treatment (P=0.3113, , ). However, rats consumed significantly more food after being exposed to the water treatment when compared to before treatment (P=0.0138, , ).
Figure 2 Food consumption (g) of rats (n=8) before and after treatment with either defensive frog skin secretions (triangles) or water control (circles).
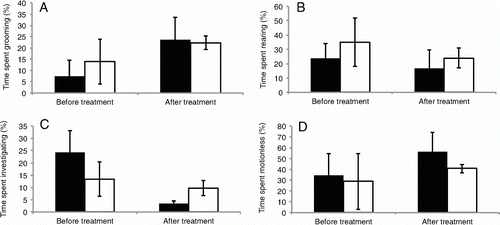
Generalised linear mixed-effect models for each behaviour showed that rats did not significantly change their behaviour before and after treatment with either L. pakeka skin secretions or water (). Rats generally spent more time grooming after treatment, but this effect was non-significant (P=0.6782, t=0.5534, a). Time spent grooming was also not significantly different between treatments (P=0.7444, t=−0.4245). There was no significant difference in the proportion of time rats spent rising on hind legs before and after treatments (P=0.7411, t=−0.3389) or between rats exposed to secretions and water (P=0.9163, t=−0.1076, b). There was no significant difference in the proportion of time rats spent investigating before and after treatments (P=0.7800, t=−0.3600, c) or between rats exposed to secretions and water (P=0.5289, t=0.9130). However, the proportion of time spent investigating the surroundings appeared less after treatment with frog secretions and water. There was no significant difference in the proportion of time rats spent motionless before and after treatments (P=0.7732, t=0.3722) or between rats exposed to secretions and water (P=0.9225, t=0.1224, d).
Figure 3 Behaviour of rats before and after treatment with either skin secretions from Leiopelma pakeka (black bars) or water (white bars). Bars show the mean percentage of time (±95% confidence intervals) spent in these behaviours. A, Grooming. B, Rising on hind legs. C, Investigating. D, Motionless.
Effect of defensive frog skin secretions on palatability of rat food
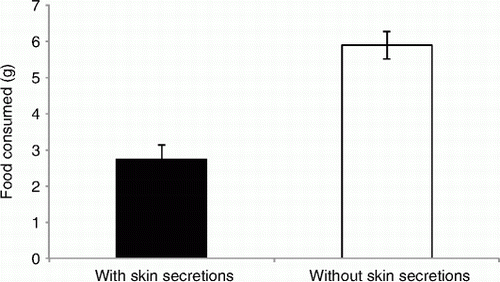
When offered the choice between food pellets coated with either frog skin secretions or water, rats consumed significantly less of the secretion-treated food compared with control food (F1, 36=33.72, P<0.001, ω=0.68, ).
Haemolytic assay of skin secretions
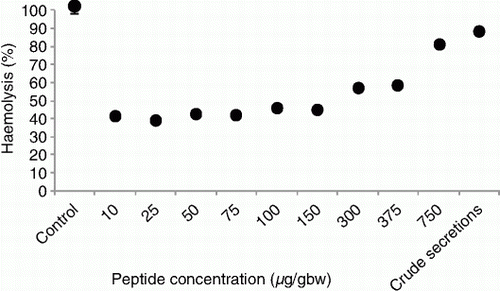
The partially purified skin secretion of L. pakeka showed haemolytic activity against rat erythrocytes with an LD50 of 225 µg/gram body weight (). The secretions effectively lysed erythrocytes at all tested concentrations, even though crude, un-purified secretions showed slightly higher activity than purified secretions at 750 µg/gram body weight.
Figure 5 Haemolytic activity of crude and partially purified skin secretions of Leiopelma pakeka against rat erythrocytes (% haemolysis±SEM). Haemolysis was determined by measurement of the optical density at 450 nm of the supernatant and compared to complete haemolysis achieved with a water control. Error bars are not visible when less than symbol size.
Discussion
Toxicity is a widely used predator defence mechanism (e.g. Cameron & Endean Citation1973; Lass & Spaak Citation2003), but the significance of this defence system in its ecological context remains largely untested in anurans. As the chemistry of anuran skin secretions is more widely investigated, there is an increasing appreciation for the functional diversity of these compounds. We show that the skin secretions of L. pakeka effectively lysed rat erythrocytes and effectively deterred feeding by a potential mammalian predator. Rats displayed a significant preference for food pellets coated with water over those covered in defensive frog skin secretions (see ). Rats frequently and consistently investigated both types of food before making a choice, indicating that they can detect the secretions and are deterred from ingesting them.
However, the ingestion of skin secretions had no apparent ill effects on the rats. The direct oral application of frog granular secretions did not significantly change their water and food intake or behaviour compared to control animals (see , ). The trend to lower investigating behaviours of the surroundings after direct oral exposure to the secretions may possibly be explained by a mild neurotoxic effect, but further analyses are needed to confirm this. Interestingly, the direct oral application of water resulted in increased food consumption in comparison to before the treatment. It is possible that the concentration of active compounds in the secretion was too low to cause any observable detrimental effects to the rats. Additionally, the mass of a laboratory rat is larger than that of rats in the wild, where food is scarcer. Therefore, higher concentrations of secretions may be needed to cause significant behavioural changes.
We chose to test natural mixtures of secretions instead of isolated compounds because this is a more accurate reflection of the natural conditions. The skin secretions of L. pakeka contain numerous novel peptides (Melzer et al. Citation2011) and their structural traits, such as hydrophobicity and C-terminal amidation, facilitate the lysis of red blood cells. The presence of peptides with hydrophobic amino acid residues is positively correlated with haemolytic activity and increased cytotoxicity to mammalian cells (Conlon et al. Citation2007; Strandberg et al. Citation2007). This in turn indicates an important defensive role of the secretions by ultimately impacting survival of predators through increased risk of infection and loss of oxygen binding capacity (Boronow & Langkilde Citation2010; Biardi & Coss Citation2011). However, further studies are needed to link the haemolytic activity of L. pakeka skin secretions to individual peptides.
It has been suggested that the original function of granular glands in frog skin was to maintain skin homeostasis, but they subsequently evolved to produce compounds that would provide protection from predator attacks (Flier et al. Citation1980). The skin secretions of Leiopelma species appear to play a vital role in the anti-predator response of these frogs because predators may reject them in the wild (Bell Citation1985a; Beauchamp Citation1996). This behaviour has often been observed for other anuran species that produce skin peptides as a defensive mechanism. A closely related species, Ascaphus truei, which produces a number of bradykinin peptides that can affect the smooth muscles, heart and vascular system of snakes (Conlon et al. Citation2005), has reportedly been rejected by garter snakes (Thamnophis spp.).
In addition to the discharge of granular secretions, Leiopelma species utilise a combination of defensive postures and calls in response to physical stimuli (Green Citation1988; S. Melzer pers. obs. 2010). Individuals of L. pakeka and L. archeyi inflate and elevate the body by slightly raising both front and hind legs with a humped back, ventriflexing the head and leaning towards the stimulus. As a result, dorsal areas of high gland densities are presented to the predator when these species assume their defensive postures (Green Citation1988; S. Melzer pers. obs. 2010). The glands of the semi-aquatic L. hochstetteri are additionally distributed across the ventral surfaces and this species pulls up its legs close to the body and ceases all movement when agitated, even when turned onto its back. Feigning death may detract attention of predators and is observed in many anuran species (Williams et al. Citation2000; Honma et al. Citation2006). In addition to defensive postures, Leiopelma species may emit high frequency chirps, which are purely defensive since leiopelmatid frogs lack external eardrums and do not vocalise to communicate (Bell Citation1985b). Not only do behavioural adaptations suggest skin secretions are important in predator defence, but morphological aspects of the granular glands themselves are further evidence for their defensive function (Melzer et al. Citation2011).
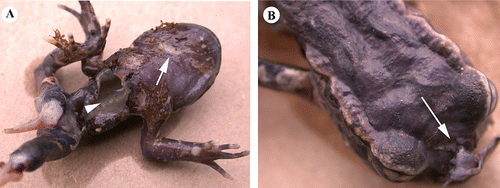
Although the introduction of rats has been linked to the extinction of two anuran species (Towns Citation2009), there is currently no direct evidence that predation is actually a major cause of loss of native frogs at the population level. Before the introduction of invasive mammals to New Zealand, only birds, fish or reptiles could have predated upon native frogs. Stable isotope analysis suggests that fish, such as shortfin eels (Anguilla australis) and banded kokopu (Galaxias fasciatus), ingest L. hochstetteri more often than rats (Najera-Hillman et al. Citation2009). However, L. hochstetteri probably do not constitute a major part in banded kokopu diet, because out of 180 fish examined only one was found to have ingested a frog (West et al. Citation2005). For terrestrial Leiopelma species, there is anecdotal evidence for tuatara, robin and weka predation (Newman Citation1977; Beauchamp Citation1996), even though it remains unclear if these predators have a big impact on native frog populations. Evidence for the negative impact of introduced rats on native New Zealand frog fauna remains largely circumstantial (Thurley & Bell Citation1994). The presence of rat bite marks on frog carcasses suggest that rats may feed on native frogs (), but it remains unclear if the death of these frogs was indeed caused by rats.
Figure 6 Deceased Leiopelma archeyi found in the wild. A, Ventral side shows rat bite marks in the throat (white arrow) and opened body cavity (arrowhead). B, Dorsal side shows no bite marks, except at the snout (white arrow). Crown copyright: Department of Conservation–Te Papa Atawhai.
The peptide cocktail released onto the skin of many anurans as a defensive response may correspond to the different mechanisms required to deter a specific suite of predators encountered in their habitat. While the toxic properties of many peptides act as an effective defence system, a variety of anuran species do not produce peptides in their granular glands. For example, the red-banded rubber frog (Phrynomerus bifasciatus) often burrows into soil during the day and, even though it produces copious skin secretions when agitated, these do not contain any peptides (C. Shaw, Queens University Belfast, pers. comm. 2 April 2008). Species occupying different niches inevitably face a different suite of predators, requiring a discrete array of defences. For example, the South American flying frogs Rhacophorus reinwardtii and R. dennysi spend considerable time in the canopy and were found to not produce any toxic skin secretions (C. Shaw, Queens University Belfast, pers. comm. 2 April 2008).
Chemical skin defences are widespread among amphibian species, but the function of these secretions in predator defence is rarely studied empirically. Given that this anti-predator mechanism not only involves the detection of a broad spectrum of threats but also the production and release of a complex mixture of defensive compounds, it is beneficial to study chemical, morphological and behavioural aspects of the response. While we have previously investigated leiopelmatid skin peptides (Melzer et al. Citation2011), the presence of other toxic components remains unknown. This line of investigations can be continued with a comparative study of the skin secretion content in relation to the behavioural response of a variety of native and introduced predators. Future investigations should determine the deterrent effects of defensive skin secretions to wild-caught rats as well as testing the behavioural response of a variety of native and introduced predators.
In conclusion, we show that direct exposure to L. pakeka skin secretions did not cause any significant observable changes in rat behaviour, but the secretions effectively deterred rats from feeding and were strongly haemolytic to rat erythrocytes. While our results indicate that the secretions of L. pakeka may induce aversion behaviour in rats, it is not certain if they can act as an effective deterrence against rat predation. In order to accurately assess the actual risk to endemic frogs from introduced predators in the wild, further study of anti-predation deterrents of frogs under natural conditions is warranted.
Acknowledgements
This work was supported by the University of Otago. The authors acknowledge Kim Garrett for his help with rat husbandry. We thank Professor Neil McNaughton for advice on experimental design, Sarah Herbert for help with statistical analyses and Tommy Leung for helpful comments on the manuscript.
References
- Basir , YJ , Knoop , FC , Dulka , J and Conlon , JM . 2000 . Multiple antimicrobial peptides and peptides related to bradykinin and neuromedin N isolated from skin secretions of the pickerel frog, Rana palustris . Biochimica et Biophysica Acta , 1543 : 95 – 105 . doi: 10.1016/S0167-4838(00)00191-6
- Bates D , Maechler M , Dai B 2008 . lme4: Linear-mixed effects models using S4 classes . R package version 0.999375-27 .
- Beauchamp , AJ . 1996 . Weka (Gallirallus australis) and Leiopelma frogs: a risk assessment . Notornis , 43 : 59 – 65 .
- Bell , BD . 1985a . “ Conservation status of the endemic New Zealand frogs ” . In Biology of Australasian frogs and reptiles , Edited by: Grigg , G , Shine , R and Ehmann , H . 449 – 458 . New South Wales : Royal Zoological Society of New South Wales .
- Bell , BD . 1985b . “ Development and parental-care in the endemic New Zealand frogs ” . In Biology of Australasian frogs and reptiles , Edited by: Grigg , G , Shine , R and Ehmann , H . 269 – 278 . New South Wales : Royal Zoological Society of New South Wales .
- Bell , BD . 1994 . A review of the status of New Zealand Leiopelma species (Anura: Leiopelmatidae), including a summary of demographic studies in Coromandel and on Maud Island . New Zealand Journal of Zoology, Special Issue: Second World Congress of Herpetology , 21 : 341 – 349 .
- Biardi , JE and Coss , RG . 2011 . Rock squirrel (Spermophilus variegatus) blood sera affects proteolytic and hemolytic activities of rattlesnake venoms . Toxicon , 57 : 323 – 331 . doi: 10.1016/j.toxicon.2010.12.011
- Boronow , KE and Langkilde , T . 2010 . Sublethal effects of invasive fire ant venom on a native lizard . Journal of Experimental Zoology Part A: Ecological Genetics and Physiology , 313A : 17 – 23 . doi: 10.1002/jez.570
- Cameron , AM and Endean , R . 1973 . Epidermal secretions and evolution of venom glands in fishes . Toxicon , 11 : 401 – 410 . doi: 10.1016/0041-0101(73)90115-3
- Castro , MS , Matsushita , RH , Sebben , A , Sousa , MV and Fontes , W . 2005 . Hylins: Bombinins H structurally related peptides from the skin secretion of the Brazilian tree-frog Hyla biobeba . Protein and Peptide Letters , 12 : 89 – 93 .
- Clarke , BT . 1997 . The natural history of amphibian skin secretions, their normal functioning and potential medical applications . Biological Reviews , 72 : 365 – 379 .
- Conlon , JM , Abraham , B , Sonnevend , A , Jouenne , T , Cosette , P , Leprince , J , Vaudry , H and Bevier , CR . 2005 . Purification and characterization of antimicrobial peptides from the skin secretions of the carpenter frog Rana virgatipes (Ranidae, Aquarana) . Regulatory Peptides , 131 : 38 – 45 . doi: 10.1016/j.regpep.2005.06.003
- Conlon , JM , Al-Ghaferi , N , Abraham , B and Leprince , J . 2007 . Strategies for transformation of naturally-occurring amphibian antimicrobial peptides into therapeutically valuable anti-infective agents . Methods , 42 : 349 – 357 . doi: 10.1016/j.ymeth.2007.01.004
- Conlon , MJ , Jouenne , T , Cosette , P , Cosquer , D , Vaudry , H , Taylor , CK and Abel , PW . 2005 . Bradykinin-related peptides and tryptophyllins in the skin secretions of the most primitive extant frog, Ascaphus truei . General and Comparative Endocrinology , 143 : 193 – 199 .
- Daly , JW , Spande , TF and Garraffo , HM . 2005 . Alkaloids from amphibian skin: a tabulation of over eight-hundred compounds . Journal of Natural Products , 68 : 1556 – 1575 . doi: 10.1021/np0580560
- Das , M , Mallick , BN , Dasgupta , SC and Gomes , A . 2000 . A sleep inducing factor from common Indian toad (Bufo melanostictus Schneider) skin extract . Toxicon , 38 : 1267 – 1281 . doi: 10.1016/S0041-0101(99)00229-9
- Flier , J , Edwards , MW , Daly , JW and Myers , CW . 1980 . Widespread occurrence in frogs and toads of skin compounds interacting with the ouabain site of Na+, K+-ATPase . Science , 208 : 503 – 505 . doi: 10.1126/science.6245447
- Gibbs , GW . 2009 . The end of an 80-million year experiment: a review of evidence describing the impact of introduced rodents on New Zealand's ‘mammal-free’ invertebrate fauna . Biological Invasions , 11 : 1587 – 1593 . doi: 10.1007/s10530-008-9408-x
- Green , DM . 1988 . Antipredator behaviour and skin glands in the New Zealand native frogs, genus Leiopelma . New Zealand Journal of Zoology , 15 : 39 – 45 .
- Honma , A , Oku , S and Nishida , T . 2006 . Adaptive significance of death feigning posture as a specialized inducible defence against gape-limited predators . Proceedings of the Royal Society B – Biological Sciences , 273 : 1631 – 1636 . doi: 10.1098/rspb.2006.3501
- Karraker , N . 2001 . Acaphus truei (tailed frog) predation . Herptetological Review , 32 : 100 – 101 .
- Kats , L and Ferrer , R . 2003 . Alien predators and amphibian declines: review of two decades of science and the transition to conservation . Diversity and Distribution , 9 : 99 – 110 . doi: 10.1046/j.1472-4642.2003.00013.x
- Kirk , RE . 1996 . Practical significance: a concept whose time has come . Educational and Psychological Measurement , 56 : 746 – 759 . doi: 10.1177/0013164496056005002
- Lass , S and Spaak , P . 2003 . Chemically induced anti-predator defences in plankton: a review . Hydrobiologia , 491 : 221 – 239 . doi: 10.1023/A:1024487804497
- Li , J , Liu , T , Xu , X , Wang , X , Wu , M , Yang , H and Lai , R . 2006 . Amphibian tachykinin precursor . Biochemical and Biophysical Research Communications , 350 : 983 – 986 . doi: 10.1016/j.bbrc.2006.09.150
- Melzer , S , Clerens , S and Bishop , PJ . 2011 . Differential polymorphism in cutaneous glands of archaic Leiopelma species . Journal of Morphology , 272 : 1116 – 1130 . doi: 10.1002/jmor.10960
- Najera-Hillman , E , Alfaro , AC , Breen , BB and O'Shea , S . 2009 . Characterisation (delta C-13 and delta N-15 isotopes) of the food webs in a New Zealand stream in the Waitakere Ranges, with emphasis on the trophic level of the endemic frog Leiopelma hochstetteri . New Zealand Journal of Zoology , 36 : 165 – 176 .
- Newman , DG . 1977 . Some evidence of the predation of Hamilton's frog (Leiopelma hamiltoni (McCulloch)) by tuatara (Sphenodon punctatus (Grey)) on Stephens Island . Proceedings of the New Zealand Ecological Society , 24 : 43 – 47 .
- Oren , Z and Shai , Y . 1998 . Mode of action of linear amphipathic alpha-helical antimicrobial peptides . Biopolymers , 47 : 451 – 463 . doi: 10.1002/(SICI)1097-0282(1998)47:6<451::AID-BIP4>3.0.CO;2-F
- Park , CB , Kim , HS and Kim , SC . 1998 . Mechanism of action of the antimicrobial peptide buforin II: Buforin II kills microorganisms by penetrating the cell membrane and inhibiting cellular functions . Biochemical and Biophysical Research Communications , 244 : 253 – 257 . doi: 10.1006/bbrc.1998.8159
- Pukala , TL , Bowie , JH , Maselli , VM , Musgrave , IF and Tylerc , MJ . 2006 . Host-defence peptides from the glandular secretions of amphibians: structure and activity . Natural Product Reports , 23 : 368 – 393 . doi: 10.1039/b512118n
- Rollins-Smith , LA , Reinert , LK , Miera , V and Conlon , JM . 2002 . Antimicrobial peptide defenses of the Tarahumara frog, Rana tarahumarae . Biochemical and Biophysical Research Communications , 297 : 361 – 367 . doi: 10.1016/S0006-291X(02)02217-9
- Severini , C , Improta , G , Falconieri-Erspamer , G , Salvadori , S and Erspamer , V . 2002 . The tachykinin peptide family . Pharmacological Reviews , 54 : 285 – 322 . doi: 10.1124/pr.54.2.285
- Simmaco , M , Kreil , G and Barra , D . 2009 . Bombinins, antimicrobial peptides from Bombina species . Biochimica et Biophysica Acta (BBA) – Biomembranes , 1788 : 1551 – 1555 . doi: 10.1016/j.bbamem.2009.01.004
- Smith , B , Hayasaka , Y , Tyler , M and Williams , B . 2004 . Beta-caryophyllene in the skin secretion of the Australian green tree frog, Litoria caerulea: an investigation of dietary sources . Australian Journal of Zoology , 52 : 521 – 530 . doi: 10.1071/ZO04019
- Steinborner , ST , Waugh , RJ , Bowie , JH , Wallace , JC , Tyler , MJ and Ramsay , SL . 1997 . New caerin antibacterial peptides from the skin glands of the Australian tree frog Litoria xanthomera . Journal of Peptide Science , 3 : 181 – 185 . doi: 10.1002/(SICI)1099-1387(199705)3:3<181::AID-PSC97>3.0.CO;2-K
- Strandberg , E , Tiltak , D , Ieronimo , M , Kanithasen , N , Wadhwani , P and Ulrich , AS . 2007 . Influence of C-terminal amidation on the antimicrobial and hemolytic activities of cationic alpha-helical peptides . Pure and Applied Chemistry , 79 : 717 – 728 . doi: 10.1351/pac200779040717
- Thurley , T and Bell , BD . 1994 . Habitat distribution and predation on a western population of terrestrial Leiopelma (Anura: Leiopelmatidae) in the northern King Country, New Zealand . New Zealand Journal of Zoology , 21 : 431 – 436 .
- Toledo , RC and Jared , C . 1995 . Cutaneous granular glands and amphibian venom . Comparative Biochemistry and Physiology Part A: Physiology , 111 : 1 – 29 . doi: 10.1016/0300-9629(95)98515-I
- Towns , DR . 2009 . Eradications as reverse invasions: lessons from Pacific rat (Rattus exulans) removals on New Zealand islands . Biological Invasions , 11 : 1719 – 1733 . doi: 10.1007/s10530-008-9399-7
- Towns , DR and Daugherty , CH . 1994 . Patterns of range contractions and extinctions in the New Zealand herpetofauna following human colonisation . New Zealand Journal of Zoology, Special Issue: Second World Congress of Herpetology , 21 : 325 – 339 .
- Tyler , M , Stone , D and Bowie , J . 1992 . A novel method for the release and collection of dermal, glandular secretions from the skin of frogs . Journal of Pharmacolocial and Toxicological Methods , 28 : 199 – 200 . doi: 10.1016/1056-8719(92)90004-K
- West , DW , Jowett , IG and Richardson , J . 2005 . Growth, diet, movement, and abundance of adult banded kokopu (Galaxias fasciatus) in five Coromandel, New Zealand streams . New Zealand Journal of Marine and Freshwater Research , 39 : 915 – 929 .
- Williams , CR , Brodie , E , Tyler , M and Walker , S . 2000 . Antipredator mechanisms of Australian frogs . Journal of Herpetology , 34 : 431 – 443 . doi: 10.2307/1565367
- Wilmshurst , JM , Anderson , AJ , Higham , TFG and Worthy , TH . 2008 . Dating the late prehistoric dispersal of Polynesians to New Zealand using the commensal Pacific rat . PNAS , 105 : 7676 – 7680 . doi: 10.1073/pnas.0801507105
- Worthy , TH . 1987 . Osteology of Leiopelma (Amphibia: Leiopelmatidae) and description of three new subfossil Leiopelma species . Journal of the Royal Society of New Zealand , 17 : 201 – 251 .
- You , D , Hong , J , Rong , M , Yu , H , Liang , S , Ma , Y , Yang , H , Wu , J , Lin , D and Lai , R . 2009 . The first gene-encoded amphibian neurotoxin . The Journal of Biological Chemistry , 284 : 22079 – 22086 . doi: 10.1074/jbc.M109.013276