Abstract
The aim of the study was to obtain preliminary data on the occurrence of helminths in New Zealand endemic passerines. We also examined a small number of non-endemic native and introduced birds. Altogether, of 388 samples of faeces or dead birds, 5.4% contained evidence of trematode infections, 7.7% of cestode infections and 1.5% of Capillaria. Trematodes found in the gall bladders of saddlebacks and one song thrush were tentatively identified as belonging to the family Dicrocoeliidae but definitive identifications have yet to be made. The taxonomic position of the trematodes found in tui is unknown. However, these are the first records of trematodes in New Zealand endemic passerines. The presence of cestode infections in saddlebacks, tui and the North Island robin, and of Capillaria in tui, appears not to have been recorded previously.
Introduction
Of the thousands of bird species known today, only a few hundred and their parasites have been studied in detail, and only a small number of these studies have focused specifically on passerines. However, publications from all over the world have documented an extraordinary spectrum of helminths inhabiting the passerine hosts so far examined (Binder Citation1971; Cooper & Crites Citation1975, Citation1976a, Citation1976b; Misof Citation2005).
In the wild, most infections with helminths are mild, with only a few parasites, and birds suffer few ill effects. Exceptions are found in birds kept in captivity, or under crowded conditions in quarantine aviaries before translocation. In these circumstances helminths may have a negative effect on the birds’ condition and serious infections with a great number of parasites may sometimes occur.
Most of the work on the identification of passerine helminths has been done in the Northern hemisphere. In New Zealand, checklists compiled by Weekes (Citation1982) and McKenna (Citation2010) list the published records of helminths in both native and introduced passerines. The lists include helminths in both wild and captive passerines, as well as endemic and native species including the native silvereye (Zosterops lateralis lateralis) and the endemic North Island saddleback (Philesturnus carunculatus rufusater), kokako (Callaeas cinerea) and hihi (Notiomystis cincta). The prevalence and epidemiology of diseases caused by internal parasites in New Zealand birds are unknown.
Amongst the introduced passerine species in New Zealand, blackbirds (Turdus merula) are hosts to three nematode species (Capillaria sp., Porrocaecum ensicaudatum, Syngamus trachea) and four species of cestode (Anomotaenia verulamii, Aploparaksis dujardinii, Dilepis undula, Hymenolepis serpentulus); song thrushes (Turdus philomenos) are known to host two nematode species (Capillaria sp., Porrocaecum ensicaudatum) and three species of cestodes (Anomotaenia verulamii, Aploparaksis dujardinii, Dilepsis undula); and starlings (Sturnus vulgaris) host Syngamus trachea (Weekes Citation1982). Some helminths commonly found overseas, such as the acanthocephalan Prosthorhynchus formosus, have not been recorded in passerines from New Zealand.
Although helminths rarely cause health problems, there are examples of severe effects on native New Zealand hosts, such as nematodes of the ascarid family, genus Porrocaecum, causing death in saddlebacks (Alley et al. Citation2007), visceral and neural larva migrans in North Island brown kiwi (Apteryx mantelli) (Alley et al. Citation2004) and Acanthocephala spp. infection in a pied stilt (Himantopus himantopus) (Hunter et al. Citation2011).
A point that should not be overlooked is that parasites in New Zealand native birds are, in general, a poorly documented and understood part of the native faunal diversity. Since many of the hosts are endangered, these parasites may also be at risk of disappearing before they have been recognised or studied.
In this study, 388 faecal and tissue samples were examined from a range of species including: four endemic birds, tui (Prosthemadera novaeseelandiae), North Island saddleback (Philesturnus carunculatus rufusater), hihi (Notiomystis cincta) and North Island robin (Petroica australis longipes), two self-introduced native species, silvereye (Zosterops lateralis) and fantail (Rhipidura fuliginosa), as well as three European introductions, house sparrow (Passer domesticus), blackbird (Turdus merula) and song thrush (Turdus philomenos). The aim was to develop some preliminary data on the diversity of helminths that these birds host in New Zealand.
Material and methods
Samples
The majority of samples from native birds came from Mokoia Island (38°05′S, 176°16′E), located in Lake Rotorua in the North Island of New Zealand. In addition, samples were obtained from other sites in the North Island. A dead fantail and 2 silvereyes were collected in Palmerston North, Manawatu (40°21′S, 175°37′E). One dead tui was received from near Masterton in the Wairarapa (40°57′S, 175°39′E). One faecal sample and one dead saddleback were received from a captive population from Orana Park in Christchurch (43°28′S, 172°27′E), South Island. Seven dead tui were received from Raoul Island (29°27′S, 177°92′W) in the Kermadec group approximately 750 to 1000 km north-north-east of New Zealand. Hihi faecal samples from the Mt. Bruce National Wildlife Centre (NWC), in Masterton, Wairarapa, North Island (42°43′S, 171°19′E) are routinely examined by the parasitology laboratory in the Institute of Veterinary, Animal and Biomedical Sciences (IVABS) at Massey University and the results were made available to the authors. Formalin fixed organs and tissues archived by IVABS from 29 saddleback (27 North Island and two South Island saddleback) were also examined grossly and histologically for trematodes. Sources of faecal samples are summarised in .
Table 1 Results of the examination of 358 faecal samples of New Zealand passerines for helminths.
Capture, handling and faecal sampling
Seven trips were made for capturing and sampling of birds on Mokoia Island, in January 2007, May 2007, October 2007, March 2008, November 2008, December 2008 and February 2009. Birds were caught in mist nets and held temporarily in cotton holding bags until examined. North Island saddlebacks and robins were attracted to the net with the help of recorded songs of their own species. Other species were caught by placing the mist nets at sites frequented by the birds.
Faecal samples from saddlebacks and robins were obtained from the holding bags. Tui were placed inside a cardboard box (measuring 31×25×22 cm, L×W×H) containing a perch for 10 minutes until defecation. Nectarivorous species such as saddlebacks and tui were given freshly prepared sugar water prior to release.
Faecal samples from hihi were received by IVABS/ Massey University over the period between 23 November 2007 and 28 October 2009. Samples were collected by staff at Mt. Bruce NWC, mostly during the morning feed rounds between 8 and 10 am, and either taken from the roost boxes or from black polythene sheeting placed under the feeding stations. Whenever possible, individual samples were collected, particularly from the adult birds that were often kept separated. In the case of nestlings, pooled samples consisting of four to six single droppings were collected. A total of 136 faecal samples from a population of six adults and pooled samples of 11 nesting attempts at NWC were submitted. The initial faecal flotation and examination of the samples was undertaken by the senior technician in the laboratory.
Where possible, faecal samples were weighed before processing so that parasite stages per gram faeces could be estimated. Faecal samples from all species were placed in 1.5 ml micro centrifuge tubes and either examined within 24 hours or stored at 4 °C until examination. The samples were subjected to a standard flotation technique using saturated zinc sulphate solution at a specific density of 1.32 and examined at 100x magnification.
Pathology
The dead birds were received chilled. After arrival they were weighed and the body condition assessed before examination using a standard necropsy technique. Samples of pectoral muscle and all internal organs were collected for histology and both pectoral muscle and liver were frozen for later PCR analysis. Intestinal contents were collected for parasitological examination. Samples of all visceral organs were fixed in 10% buffered formalin before being processed routinely and embedded in paraffin wax. Sections were cut at three µm and stained with haematoxylin and eosin. Archived saddleback livers with gallbladders were examined under low magnification grossly, as well as under the dissection microscope for adult worms before processing for histopathology.
Statistics
An independent-samples t-test was used to examine the relationship between length and length/width ratios of cestode eggs found in saddleback from Mokoia Island and Orana Park. The data referring to the cestode egg sizes are given as mean±SD (standard deviation). Analyses were carried out using WinSTAT® for Microsoft Excel version 2007.1. P-values of <0.05 were considered significant.
Results
We examined 359 samples (fresh faeces and dead specimens) from nine different species; four endemic, two native and three introduced. Trematode eggs were found in 16 individual birds (4.5%), cestode eggs in 29 (8.1%) and Capillaria spp. eggs in six (1.7%) (see ). Internal organs including livers, gallbladders and alimentary tracts from 31 birds (15 tui, eight blackbirds, three saddlebacks, two silvereyes, two house sparrows and one song thrush) were examined grossly and histologically. Of these, 22 (71.1%) birds were infected with one or more helminth species. In addition, archived tissues from 29 saddlebacks were examined; three (10.3%) had trematodes in their gallbladders.
Capillaria spp. in sensu lato
There were Capillaria spp. eggs in six of 56 (9.33%) tui samples. In addition, Capillaria were found in 37 of 66 (56.1%) individual faecal samples from hihi and in four of 70 (5.7%) pooled samples from hihi nestlings (11 nestling attempts). The Capillaria spp. eggs were morphologically typical of the group, with those eggs from hihi being more rounded than those eggs from tui (). Eggs from hihi had a mean length of 68.6 µm (SD: 1.97, n=7) and a width of 29.3 µm (SD: 1.22, n=7) (). Capillaria eggs from tui were not measured.
Figure 1 Capillaria spp. eggs from two different New Zealand passerines. A, Capillaria sp. egg of a tui. B, Capillaria sp. egg from a hihi from Mt. Bruce NWC.
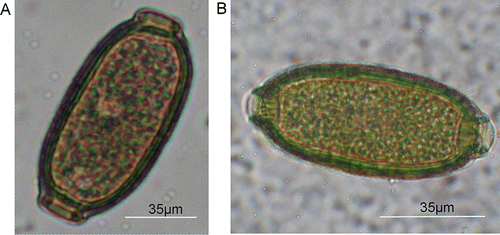
Table 2 Descriptive statistics of the Capillaria eggs recovered from hihi from Mt. Bruce NWC.
The estimated faecal egg counts varied widely: in tui counts ranged up to 10,000 eggs/g (mean 1711 eggs/g, SD 4069) and in hihi up to 77,145 eggs/g (mean 4815 eggs/g, SD: 12930). The records of the IVABS parasitology laboratory show that the highest faecal counts for Capillaria in hihi were in the period between January and March, with mostly negative results in other months. Gravid Capillaria spp. worms were found in the gut contents of both hihi and tui.
Other nematodes
In the dead saddleback received from Orana Park, an unidentified nematode was found encapsulated in the wall of the ruptured duodenum.
Cestodes
Cestode eggs were found in faecal samples from one of 38 (2.63%) North Island robins, three of 56 (5.4%) tui, 23 of 232 (9.9%) North Island saddlebacks, two of 11 (18%) blackbirds and three of 136 (2.2%) hihi faecal samples representing six adults. In addition, an unknown number of nestlings from 11 nesting attempts also contained cestode eggs.
Cestode eggs from tui, blackbird and the one saddleback from Orana Park were observed to have a delicate transparent membrane (‘oncospheral envelope’) around them. Cestode eggs from saddlebacks on Mokoia did not show this membrane (). All cestode eggs contained clearly visible hexacanth larvae. Eggs from tui were subspherical, transparent and, on average, 29.4 µm (SD = 4.8, n=14)×25.8 µm (SD = 2.6, n=14). Cestode eggs from Mokoia saddleback were subspherical, yellowish-brown in colour and, on average, 43.91 µm (SD = 5.9, n=16)×36.1 µm (SD = 3.41, n=16). Eggs from the Orana Park saddleback were ellipsoid, transparent and, on average, 65.5 µm (SD = 13.9, n=20)×37.1 µm (SD = 2.3, n=20) (). The dimensions of the eggs from Mokoia and Orana Park birds ( and ) differed significantly in length (independent-sample t-test t = − 5.921845354, n=16, 20 respectively, P<0.01) and length/width ratios (independent samples t-test t = − 6.265862682, n=16, 20, P<0.01) indicating that they belong to two different species. Cestode eggs from North Island robin were not measured.
Figure 2 Cestode eggs from different passerine species. Note the hexacanth larvae inside the eggs (arrow). A, Blackbird. B, Tui. C, North Island saddleback (Mokoia). D, North Island saddleback (Orana Park).
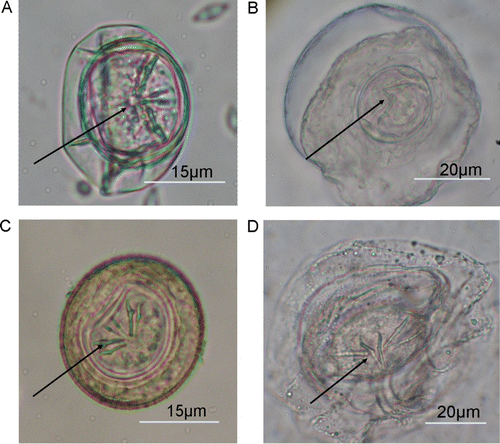
Figure 3 Comparison of the length of cestode eggs of North Island saddleback from Mokoia and Orana Park. The x-axis shows the egg size in µm, the y axis presents the frequency of the findings (number of eggs). Mokoia (white), Orana Park (black).
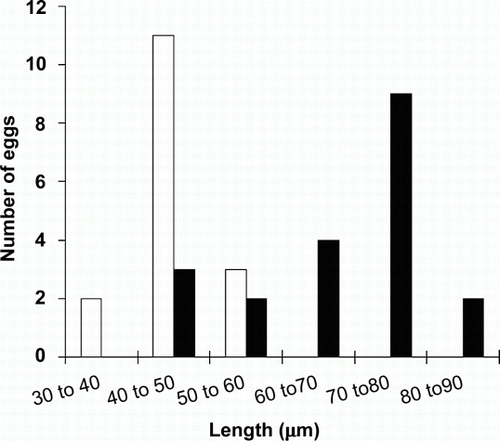
Figure 4 Comparison of the length/width ratio of cestode eggs of North Island saddleback from Mokoia and Orana Park. The x-axis shows the egg size in µm, the y axis presents the frequency of the findings (number of eggs). Mokoia (white), Orana Park (black).
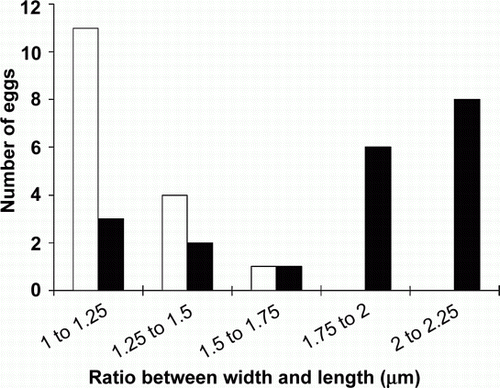
Table 3 Descriptive statistics of cestode eggs from North Island saddleback and tui.
Trematodes
Faecal samples from three of 56 (5.36%) tui and 13 of 232 (5.6%) North Island saddleback contained trematode eggs. No trematode eggs were recovered from any of the other species examined. The eggs from both tui and saddleback species were ellipsoidal in shape with an operculum at one end (A, B). The eggs of the saddleback trematode were more rounded than those from the tui and each contained a clearly visible miracidium. Miracidia were not visible in the eggs from tui. The mean size of the eggs from tui was 21.8 µm (SD = 2.8, n=17)×13 µm (SD = 2.11, n=17) (see ). The eggs from saddlebacks had an average size of 42.2 µm (SD = 6.05, n=19) in length and 19.74 µm (SD = 3.19, n=19) in width (see ).
Figure 5 Trematode eggs. A, Tui. B, North Island Saddleback/Mokoia Island. Abbreviations: M, Miracidium larvae; O, operculum.

Table 4 Descriptive statistics of the trematode eggs from tui and North Island saddleback.
Post mortem findings
Three saddlebacks and one song thrush that had died from various causes were collected during the study period. All birds were in good body condition. The gallbladders in all four birds contained several adult trematodes and their eggs (), with the trematodes found in the thrush similar to the ones found in the saddlebacks. In addition, three more saddlebacks with infected gallbladders were found in archived material (). Trematode worms were recovered from the large intestine of three of seven (42.9%) tui from Raoul Island examined post mortem.
Figure 6 Adult trematodes containing numerous eggs in the gallbladder of a North Island saddleback from Mokoia. H&E for haematoxilin and eosin, staining method.
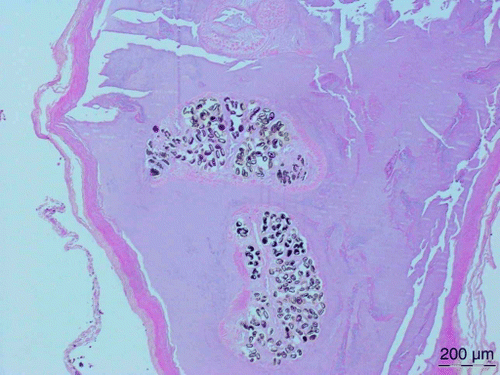
Table 5 Post mortem findings for birds with gallbladders infected with trematodes.
The source locations of the birds, year, gross post mortem findings and gallbladder measurements are compiled in .
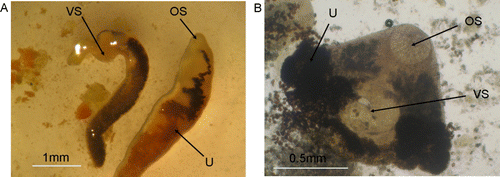
The trematodes recovered from saddleback and song thrush gallbladders were adults that were up to 4 mm in length and had a prominent ventral sucker (A). The numerous adult trematodes recovered from the tui large intestine were roughly triangular in shape and up to 2 mm long (B).
Figure 7 Adult trematodes in New Zealand passerines. A, Adult trematodes from the gallbladder of a North Island saddleback. B, Adult trematode found in the large intestine of a tui. OS, oral sucker; BS, ventral sucker; U, uterus.
Histopathology
The liver of birds infected with trematodes showed a mild chronic cholangiohepatitis with disseminated foci of inflammation composed mainly of lymphocytes and plasma cells associated with the bile ducts. The bile ducts in affected areas showed variable epithelial hyperplasia and contained necrotic debris. There was mild haemosiderin accumulation in hepatocytes as well as focal areas of bile pigment accumulation in sinusoids. The gallbladders of these birds contained pale basophilic shales of sloughed epithelium and the remaining epithelium was folded and hyperplastic. In the part of the gallbladder closest to the liver, there were numerous hyperplastic glands secreting excess basophilic mucus. The liver lesions were most severe in the saddleback that died during blood sampling, although the other birds showed similar histological changes.
Discussion
This is a preliminary investigation into the spectrum of helminth parasites in some New Zealand passerines. The recent parasite checklist of McKenna (Citation2010) lists the few species recorded previously. Although only a small number of species were examined in this study, the results indicate that there is likely to be a considerable variety of helminths yet to be identified. Although species identification of the helminths and helminth eggs observed still has to be performed, it appears that this is the first record of trematodes in native passerines and the first record of cestode infections in tui and saddleback.
Nematodes and cestodes
The nematodes found in tui and hihi have been provisionally identified as belonging to the genus Capillaria in sensu lato. According to (Anderson Citation1992), the classification of the subfamily Capillariinae is one of the most difficult and unsatisfactory in the class Nematoda. So far, around 300 species have been described in the subfamily and between five and 16 genera are recognised. The definitive identification of the helminths described in this study might, therefore, prove to be difficult.
Cestodes have been reported from passerine birds in New Zealand previously and Weekes (Citation1982) listed several in introduced birds including blackbird and song thrush carrying cestodes. A later report by Johnstone and Cork (Citation1993) mentioned tapeworms in a saddleback, but without any description. In 1994, Clemance reported the cestodes found in North Island saddleback as Choanotaenia infundibulum, a tapeworm of poultry (Clemance Citation1995), but the identification is open to question. The dimensions of the cestode eggs collected from saddlebacks on Mokoia differed significantly from those from saddlebacks from Orana Park. Both are unlike the eggs of Choanotaenia. Both cestodes in saddlebacks, and the one found in the tui from which the eggs originate, remain unidentified. Complete adult specimens are necessary for identification, which usually needs to be carried out by a specialist.
The cestode eggs from the North Island robin found in the initial part of this study were not measured or photographed, as focus of the study at the time was on coccidia. Similarly, the cestode eggs in hihi faeces were found during routine examination in the IVABS parasitology laboratory and neither photographs nor measurements were taken. Further samples are needed to recover more specimens from both these species.
Trematodes
One of the most interesting findings in the current investigation was the discovery of trematodes in the gallbladders of both North and South Island saddlebacks and the large intestine of tui. From the appearance of the trematodes and their eggs found in the gallbladders of the North Island saddleback it seems likely that they belong to the family Dicrocoeliidae. The family to which the trematodes found in tui belong was not determined.
Members of the dicrocoeliid genera Lutztrema, Brachylecithum and Oswaldoia have been recorded in the biliary systems of a variety of passerines overseas (Carney Citation1970; Binder Citation1971) and it is possible that the trematodes recorded from the North Island saddleback in this study belong to one of these genera. These trematodes are very common in other parts of the world. For example in the Czech Republic and Poland, Turdus merula, T. pilaris, T. philomelos, Sturnus vulgaris and Sylvia atricapilla were found to be infected with Lutztrema attenuatum (formerly Brachylecithum attenuatum) (Sitko et al. Citation2000). The song thrush examined in the present study presented similar pathological findings as the saddlebacks and the trematodes found were similar in morphology and size.
The occurrence of these apparently similar trematodes in two very different hosts, one endemic and the other introduced, raises a series of questions that need investigating. The first issue is to determine their generic and species identities and whether the parasites from the two hosts are the same. If this is the case, and particularly if they are a parasite species found in song thrushes overseas, it can be reasonably concluded that the trematode was introduced. As saddlebacks and thrushes are both present on Mokoia and at Orana Park, transfer of parasites between them is theoretically possible. If, on the other hand, the parasites from the two hosts prove to be different and/or previously undescribed, the question then is whether or not they are endemic to New Zealand.
Assuming that the trematodes belong to the family Dicrocoeliidae, as suspected, intriguing questions as to their life cycle(s) arise. Characteristically, the life cycles of dicrocoeliid trematodes involve two intermediate hosts, a mollusc and an arthropod. For example, Brachylecithum mosquensis, which infects birds, utilises ants as the second intermediate host (Carney Citation1970) as does the type species of the family, Dicrocoelium dendriticum, primarily a parasite of ruminants. Identifying the intermediate hosts involved in New Zealand presents an interesting challenge.
The trematodes in the tui were found in the colon, but it is possible that this is not the site they inhabit naturally. The birds had been dead for several days before post mortem examination and they could have been carried down the gut at the time of death or subsequently. Trematodes inhabiting the intestines of their definitive hosts are normally found in the small intestine.
Conclusion
The primary objective of the study was to develop preliminary data on the occurrence of helminths in some New Zealand endemic passerines. The opportunity was also taken to examine a small number of native and introduced birds; the significance of observations on these is limited by the small numbers examined. However, the results indicate that, as might be expected, a wide variety of helminths are present in New Zealand's endemic birds. Given New Zealand's unique bird life and the superimposed introduced avifauna, a host of fascinating questions arise as to the identity, origin and biology of these parasites. Clearly, a considerable research effort will be needed to investigate these, research that is made difficult by the scarcity and vulnerability of many of the host species involved.
Acknowledgements
The authors would like to thank Te Arawa iwi for access to Mokoia Island, Darren Page, aviculturalist at Mt. Bruce NWC for providing details of the history and management of hihi at Mt. Bruce, as well as the residents of the New Zealand Wildlife Heath Centre at Massey University/Palmerston North for providing clinical histories. We acknowledge the outstanding technical help from staff at the Institute of Veterinary, Animal, and Biomedical Sciences, including Barbara Adlington (senior parasitology technician), Anne Tunnicliffe, Elaine Booker, Evelyn Lupton and Nicola Wallace. Funding for this study was received from the IVABS Research Fund for Postgraduate Students to Ellen Schoener; Massey University Research Fund and Department of Conservation Grants to Laryssa Howe and Isabel Castro; and indirectly by Biosecurity NZ through contract 10424/2 to Isabel Castro.
References
- Alley , MR , Gartrell , BD and Morgan , KJ . 2004 . Wildlife cases from Massey University October 2003–April 2004. Visceral and neural larval migrans in kiwi, Apteryx australis . Kokako , 11 : 13
- Alley , MR , Gartrell , BD and Scott , I . 2007 . Deaths in saddlebacks, Philesturnus carunculatus, due to the migrating nematode Porrocecum sp . Kokako , 29 : 15
- Anderson , RC . 1992 . Nematode parasites of vertebrates: their development and transmission , 2nd edition , Wallingford , , UK : CABI Publishing .
- Binder , N . 1971 . Beitraege zur morphologie, invasionsdynamik und entwicklung der helminthen in der amsel (Turdus merula L) . Zoologische Beitraege , 17 : 83 – 150 .
- Carney , WP . 1970 . Brachylecithum mosquensis: infections in vertebrate, molluscan and arthropod hosts . Transactions of the American Microscopial Society , 89 : 233 – 250 .
- Clemance , M . 1995 . Veterinary cases from the kiwi house . Kokako , 2 : 15 – 18 .
- Cooper , CL and Crites , JL . 1975 . Helminth parasites of starling (Sturnus vulgaris) from South Bass Island, Ohio . Journal of Parasitology , 61 : 161 – 161 .
- Cooper , CL and Crites , JL . 1976a . Additional check list of helminths of starling (Sturnus vulgaris L) . American Midland Naturalist , 95 : 191 – 194 .
- Cooper , CL and Crites , JL . 1976b . Check list of helminth parasites of robin, Turdus migratorius Ridgway . American Midland Naturalist , 95 : 194 – 198 .
- Hunter , SA , Jensen , MA and Alley , MR . 2011 . Severe internal parasitism in a pied stilt, Himantopus himantopus . Kokako , 18 : 17
- Johnstone , AC and Cork , SC . 1993 . Diseases of aviary and native birds in New Zealand . Surveillance , 20 : 35 – 36 .
- McKenna PB 2010 . An updated checklist of helminth and protozoan parasites of birds in New Zealand . WebmedCentral Parasitology 1 9 : WMC00705 .
- Misof K 2005 . Eurasian blackbirds (Turdus merula) and their gastrointestinal parasites: a role for parasites in life history decisions? Published PhD thesis , Mathematisch-Naturwissenschaftliche Fakultaet, Rheinische Friedrich-Wilhelms-Universitaet , Bonn , Germany .
- Sitko , J , Okulewicz , J and Noga , L . 2000 . Variability and systematic status of Lutztrema attenuatum (Dujardin, 1845) comb. n. (Trematoda: Dicrocoeliidae) parasitizing passeriform birds . Helminthologia , 37 : 97 – 111 .
- Weekes , PJ . 1982 . Checklist of helminth parasites of birds in New Zealand . New Zealand Journal of Zoology , 9 : 451 – 460 .