Abstract
Almost all individuals from the only remaining population of a newly discovered species of large land snail were taken into captivity when their habitat was mined in 2007. Growth rates and population dynamics of a sample of 318 of the snails remaining in captivity in 2007–2009 were calculated and compared with those of the wild population at the time it was removed using both deterministic and stochastic modelling methods. Snails in the captive population appeared to be exhibiting slower growth, particularly amongst hatchlings, than the wild population and, although adult mortality is low in captivity, the hatchling mortality rates were higher than expected. Furthermore, we found the predicted long-term size distribution for the captive population differed markedly from that of the population at capture, suggesting the dynamics of captive population are significantly different from those of the wild.
Introduction
Powelliphanta (O'Connor, 1945) are large, carnivorous land snails endemic to New Zealand. Almost all Powelliphanta species have small, discrete distributions, a pattern thought to reflect New Zealand's complex bio-geographical history (Walker Citation2003). The restricted spatial and ecological range of most Powelliphanta makes them especially vulnerable to habitat loss and predation by introduced predators (Meads et al. Citation1984), with most Powelliphanta taxa being classified as endangered or declining (Hitchmough et al. Citation2007).
Powelliphanta augusta is a recently described alpine species, endemic to Mount Augustus on the Stockton Plateau (600–1100 m asl), just north of Westport in New Zealand's South Island (Trewick et al. Citation2008; Walker et al. Citation2008). Powelliphanta augusta was not discovered until 2004, by which time a substantial proportion of its habitat and population had been lost to mining. From July 2006 to May 2007, substantial efforts were made to recover individual snails, resulting in 6139 snails being taken into captivity. Subsequently, 3913 of the snails were translocated to three sites nearby that had not previously supported P. augusta, while the remainder were retained in captivity as a safeguard against failure of the translocated populations.
There are few threatened snail taxa for which captive breeding programmes have been established and these are predominantly from Oceanic Islands in the SW Pacific, where predation and habitat loss have been the major factors contributing to large-scale decline and extinction of many land snails species. One example is the Polynesian tree snails (Partula spp.), which suffered severe decline on many islands following the introduction of the carnivorous snails Euglandina rosea. Conservation efforts for Partula commenced in 1986 with the establishment of an international breeding programme (Tonge & Bloxam Citation1991). Captive populations of Partula spp. have since been housed successfully at zoos and universities throughout the world, but many initial attempts to establish populations were unsuccessful and three species have become extinct in captivity (Cunningham & Daszak Citation1998). More recently, some Partula spp. populations have been reintroduced to the wild with mixed results (Pearce-Kelly et al. Citation1995; Mace et al. Citation1998; Coote et al. Citation2004).
Early efforts to breed Placostylus snails successfully in New Zealand and New Caledonia were also wrought with difficulty (Salas et al. Citation1997; Stringer & Parrish Citation2003, Citation2008). However, following many years of monitoring and refining captive management methods, Placostylus fibratus have now been successfully captive-bred for large-scale reintroductions (Brescia et al. Citation2008).
Powelliphanta had not been successfully reared to a second generation in captivity before the establishment of the P. augusta captive programme in July 2006, so there was concern a captive population was an unreliable safety net for the survival of the species. Previous captive rearing attempts by KJ Walker had been in permeable outdoor containers in which maintaining live earthworm food had been problematic, with low long-term survival of snails, particularly hatchlings (unpublished data). To try and overcome these problems, the P. augusta captive population was established in indoor containers where food and micro-climate could be artificially maintained. An initial constant housing temperature of 7 °C was selected based on average annual temperatures from the snails' alpine habitat at Stockton and in an effort to minimise the risk of bacterial growth and disease. A series of trials was subsequently established to determine optimum rearing conditions (Allan Citation2011; KA Weston unpublished data).
As is often the case when threatened invertebrates are taken into captivity, little background biological information was available for P. augusta prior to the establishment of the captive programme in July 2006. Important life history parameters such as growth rates and age at reproductive maturity are poorly documented within the genus due to the apparent long life spans of Powelliphanta species. Powell (Citation1946) stated (without giving evidence) that there was ‘15 years to a generation’ and hypothesised that Powelliphanta may have ‘a life span of up to 40 years’. Walker (Citation2003) suggested an average life span of 12–14 years, with some individuals living up to 20 years following the study of marked wild Powelliphanta hochstetteri bicolor and Powelliphanta lignaria johnstoni, and many lowland Powelliphanta species kept in captivity. Sexual maturity in captivity appeared to be around 5–6 years (Walker Citation2003). Powelliphanta augusta, like other Powelliphanta species, are normally cross-fertilising hermaphrodites, although individuals housed apart appear capable of either sperm storage or self-fertilisation (Walker et al. Citation2008). Their eggs are relatively large (~8 mm), hard-shelled and laid in clutches of two or three, which, in captivity, have an incubation period of 12–15 months (Walker et al. Citation2008).
Estimates of P. augusta growth rates and age at reproductive maturity are vital parameters for informing captive management effort. The only substantial work on P. augusta is that of Allan (Citation2011), who examined many aspects of the captive breeding programme including an initial model of individual adult growth based on a subset of the data used here. This paper substantially extends that work by comparing a range of growth curves comprising all sizes of P. augusta, over a longer period. Survival and reproductive age in the first few years of captivity are also examined. Together, this provides a first step in provision of key population parameters for P. augusta. Finally, all these aspects are combined in a population level model that compares the wild population demographics with the captive population.
Methods
By 2008, approximately 2000 snails remained in captivity in a containment facility at the Department of Conservation, Hokitika. These individuals were housed individually in 2-l plastic containers partly filled with dampened Sphagnum cristatum moss. Snails were kept in dark cool stores at a constant temperature of 7 °C and fed one exotic compost worm (Eisenia andrei) per month. The size of this worm was selected to suit the size of the snail. Snails were rehoused each month and any eggs found were recorded. The majority of snails were measured on collection, and there was then a gap in measurements of between 15 and 18 months. Subsequent measurements were taken in captivity at intervals of approximately 1–2 months. Maximum shell height, width and diameter, as defined by Walker et al. (Citation2008), were measured to the nearest 0.01 mm using digital calipers.
In 2009, the size and weight details of a sample of 238 snails, all measured on collection (July 2006–May 2007) and again within 2 months of July 2008 and regularly thereafter, were entered onto an electronic database for detailed study. These individuals were selected through stratified random sampling for equal representation of all sizes greater than 11 mm and geographic areas of origin. In addition to this selection, a further 80 individuals that had hatched in captivity (hatchlings) were included. Of these, 40 were P. augusta eggs collected from the Stockton mine and 40 were eggs laid in captivity. This sample dataset consisted of over 4700 individual records collected between July 2006 and June 2009. Although the original data contained measurements of width, height, weight and maximum shell diameter, only the maximum shell diameter measurements were used, as this was the largest data set, and was subject to the least random ‘noise’ (primarily measurement error). Allan (Citation2011) compared results using shell diameter and weight and concluded that, although both measurements showed a good correlation, the shell diameter measurement was more accurate in predicting the asymptotic size of an individual and hence a better predictor for adulthood. Each individual had between two and six usable maximum diameter records in total, with an average individual having three records (many records were empty or did not contain the required measurement); 15 individuals had only two records available. The parent size for a selection of eggs laid in captivity was also recorded. Unfortunately, data were not collected that allowed an estimation of the probability of an egg producing a viable offspring.
In summary, three datasets were used in the analysis:
| • | Dataset 1: Records concerning 318 individuals with each record consisting of a measurement of the maximum shell diameter for each individual at capture or hatching as well as subsequent measurements (1–5) at given dates and whether the snail was alive at those dates. | ||||
| • | Dataset 2: The maximum diameter of all 5973 individuals when they were first collected from the wild on Stockton plateau. This dataset is used to estimate the population size distribution upon capture. | ||||
| • | Dataset 3: The size of the parent for 523 eggs laid during the study period. This dataset is used to give an estimate of the minimum size of egg-laying individuals. | ||||
Analysis
Individuals collected from the mine site were analysed separately from those that had hatched in captivity. All individuals collected from the mine site had a maximum diameter greater than 11 mm; conversely, all individuals raised in captivity had a maximum diameter at birth of less than 11 mm. To allow for an analysis of the different regimes, the models distinguished between hatchling and non-hatchling snails, where hatchlings were classed as any individual with maximum diameter less than 11 mm.
Growth curves were computed using size increments computed from pairs of size measurements. For a pair of measurements we define the size increment as the difference in size divided by the length of the time interval.
Hatchling growth curves
Within dataset 1, data from 80 individuals that hatched during the first 2 years of the study period were analysed separately. Each of these ‘hatchlings’ measured <11 mm in maximum shell diameter during the entire study period. They were analysed separately for two reasons; first, for these snails, age and initial size were known, making the available growth data more complete, and second, these individuals were raised entirely in captivity, which may lead to different dynamics. The mean maximum shell diameter at hatching of the 80 individuals in the sample was measured and the growth rate in mm/year (of the surviving individuals) was calculated by computing size increments as for the rest of the data set. Measuring hatchlings accurately was difficult due to their very soft and easily damaged shells and the size of the error in this measurement was clear from the small number of individuals whose shells were recorded as decreasing in their maximum diameter.
Non-hatchling growth curves
Using dataset 1, the increase in shell diameter measured for the 238 non-hatchling snails over the first 2 years in captivity was used to estimate growth curves for an average individual, thus providing a prediction of an individual's age. The expected size of an individual, x(t) can be described by a number of common growth models of which three were tested
These models have been used widely for individual growth (e.g. Gamito Citation1998; Lopez et al. Citation2004; Karkach Citation2006) and in particular for snails (Plorin & Gilbertson Citation1984; Fernandez & Esch Citation1991; Hadfield et al. Citation1993; Dybdahl & Kane Citation2005; Gimbert et al. Citation2008). Different methods of fitting have been proposed and analysed (Rafail Citation1973). For each function, K is the maximum size an individual would be expected to reach and r is an intrinsic growth parameter. By plotting the size increment against the average size of the individual during the corresponding period, the parameters K and r can be determined using non-linear regression. The initial size x 0 at time t=0 does not affect the model fit and was chosen to be the mean (±SD) maximum shell diameter at hatching (8.5±0.6 mm, see below). Size increments were measured using the first and last available measurements for each individual to help minimise measurement errors. To compare the best fit between models, r-squared values were used.
Changes in growth during captive period
The growth model described above used the first and last data point for every individual to calculate the size increment for that period. Whilst this method has the advantage of minimising measurement errors, it can mask temporal changes. To look at differences in growth rates within the study period in captivity, we compared changes in growth using the first two available measurements (i.e. at capture and the first subsequent measurement) against change in growth using the last two available measurements from the end of the 3-year study period. Note that for a small number of individuals with only two recorded measurements (2% of the sample) these two quantities are the same. The effect of discounting these individuals was tested.
Population model
A stochastic population model following the work of Law et al. (Citation2008) was used to study the dynamics of both the wild P. augusta population upon capture and the captive population. The advantage of this style of modelling is that the results, in conjunction with the estimated parameter values from the previous sections, can be used to make predictions about the viability of the captive population without requiring comparison to data from the same or similar species.
The initial population model parameters were derived from the data as described below:
| • | Each individual starts with an initial size taken from a normal distribution with parameters calculated using the hatchling data. | ||||
| • | An individual's growth is deterministic and (for all sizes of individual) follows the growth model with the highest r 2 value found previously, with an intrinsic growth rate, r, and expected maximum individual size, K. | ||||
| • | To allow for some variability in final size, it was assumed that each individual's maximum size is taken from a normal distribution with mean K and coefficient of variation 10%. | ||||
| • | Mortality occurs as a Poisson process with constant rate in each of two growth stages, hatchling and non-hatchling, where hatchlings are defined to be all snails with maximum shell diameter less than 11 mm. These rates were estimated from the proportion of individuals in each group (hatchlings and non-hatchlings) that died during their first year in captivity. | ||||
| • | Reproduction is also a Poisson process and the average number of viable offspring produced per mature adult per year was estimated at b=1. Note that this figure is lower than the number of eggs laid per mature adult per year. The model does not explicitly include the egg incubation period. | ||||
| • | Reproductively mature snails were defined as having maximum diameter over 32 mm. | ||||
By applying these parameters to an individual based model as described by Law et al. (Citation2008), with a time step of 0.1 years and allowing the model to run until a stable size distribution is reached, we can get an estimate for the distribution of the size of individuals in the population (the population size distribution). The model also predicts whether the total number of individuals in the population will grow exponentially or diminish to extinction and gives an estimate for the time taken for the population either to double or to halve in size as appropriate.
Upon finding incongruence between the estimated size distribution of the captive population and the observed size distribution of the population upon capture, the model was then used to estimate a set of parameter ranges that could reproduce the observed population size distribution at capture. The model solution was compared with the data using the Kolmogorov–Smirnov statistic and the sum of residuals of the cumulative distribution function. To avoid inaccuracy due to the low capture probability of very small individuals, the comparison was limited to individuals with maximum diameter greater than 20 mm. It should be noted that as the model is a stochastic one finding a ‘best’ fit solution is not feasible. By exhaustively searching for parameter combinations where the maximum residual between the cumulative distributions was less than 0.03 (i.e. Kolmogorov–Smirnov statistic less than 0.03), a range of parameter values that gave a ‘good’ fit to the observed size distribution of the wild population at capture were obtained.
All statistical analysis was carried out using tools available in MATLAB R2010a. The population model and associated analysis was also performed using MATLAB R2010a.
Results
Survival and growth (excluding hatchlings)
The first dataset included records for 238 captive individuals born in the wild. Of these individuals, only two died during the study period, giving an annual survival rate of over 99% for non-hatchling P. augusta snails kept in captivity. shows the best fit parameter values for each growth model. The logistic model is the best fit to the data (as measured by r 2 value) with the Gompertz model, as used by Allan (Citation2011), a close second. A shows the increment data with the three best fit growth models and B the corresponding growth curves. Using either the logistic or the Gompertz model, the growth curves predict that an average snail born in captivity will take approximately 20 years to grow to reach full size (~39 mm). Over 98% of eggs were laid by parents with a maximum shell diameter greater than 32 mm. All three models predict it takes an average captive snail approximately 12 years to grow from 8.5 mm to 32 mm under the environmental conditions experienced in the captive facility.
Figure 1 A, Increment data with the three best fit functions for the growth rates of non-hatchling individuals. B, The subsequent growth curves predicted by these models.
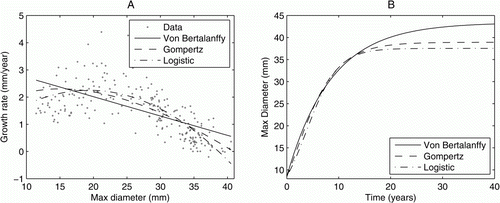
Table 1 Best fit parameter values and R 2 values for each growth model for non-hatchling individuals
Hatchling snails: survival and growth
The maximum diameter of a newly hatched snail was 8.5±0.6 mm, with a subsequent growth rate of 0.6±0.8 mm/year. Of the 80 hatchling individuals in the dataset, 13 died before they reached 1 year old. This gives an initial estimate of annual hatchling survival of 84%.
Changes in growth during captive period
shows the change in size for each individual (cf. ), with non-hatchling snails of all sizes exhibiting faster growth rates in panels A and B where early measurements were used compared with those in panels C and D where measurements from the end of the 3-year study period were used. gives the best fit parameter values for the growth curves from these two intervals. If growth were consistent throughout the study period, one would expect these results to show simply different amounts of measurement error. In this case, they show a marked decrease in the growth rates during their time in captivity. Discounting individuals with only two available measurements did not affect this result.
Figure 2 A and B, The growth model predictions for non-hatchling individuals as predicted by early data. C and D, The same predictions made with data from the end of the study period. A and C give the increment data and best fit functions, B and D give the subsequent growth curves. Data from later in the study period predicts much slower growth for non-hatching individuals.
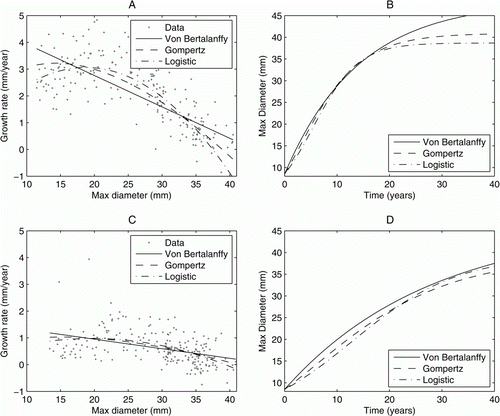
Table 2 Temporal changes in growth curve parameters for non-hatchling individuals during the captive period
Population model
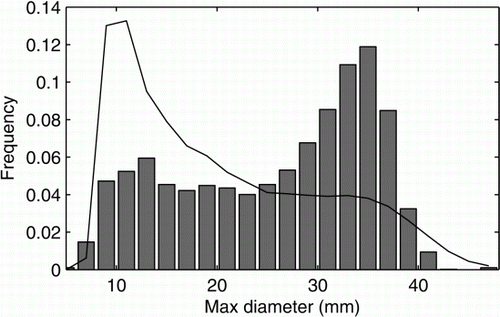
shows the size (maximum shell diameter) distribution of all individuals at capture when taken from the Stockton plateau (bars) and the long-term captive population size distribution predicted by the model parameters derived from the captive data described above (red line). At these parameter values, the model predicts that the captive population is stable and will increase exponentially. However, the population size distribution predicted by the captive model parameters is very different from the distribution of the wild population upon capture (Kolmogorov–Smirnov statistic=0.32), indicating that the model is not a good fit to the data. shows that the wild population at capture was skewed towards larger snails, whereas for the captive population, the model predicts the long-term size distribution to be skewed towards smaller snails.
Figure 3 The demographic structure of the population at capture (bars). The demographic structure predicted by the model using data obtained from the population whilst in captivity (line). It is clear that the structure will be significantly altered whilst in captivity.
shows an example of a solution from the population model, which provides a good fit (Kolmogorov–Smirnov<0.03) to the population size distribution at capture. The range of parameter values that gives a good fit to the observed population is shown in . Some parameter values outside but still close to the ones given here can also give a good fit to the data; however if one moves too far from this range the goodness of fit quickly deteriorates. Within this predicted parameter range, the majority of populations are unstable, i.e. the number of individuals in the population will decrease in time, and have a population halving time between 10 and 100 years. Note that this distribution was derived by comparing the model results to data for large individuals only to avoid any problems with the low capture probability of small individuals. Despite this limited comparison, the best fit model parameters provide a solution which is a reasonable estimate for the entire population, i.e. including small individuals.
Figure 4 The demographic structure of the population at capture (bars). An example good fit provided by the model (r=0.005 year−1, b=0.12, µA=µj=0.1, K=35.5).
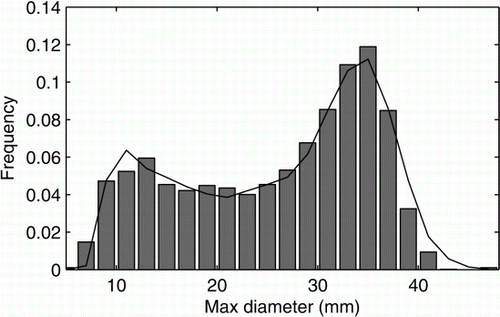
Table 3 Parameter values as predicted by the captive data (column 2)
Discussion
Growth rates
The results of the growth curves are relatively consistent with earlier growth rate estimates for Powelliphanta by Powell (Citation1946) of ‘15 years to a generation’. More recently, however, Walker (Citation2003) found sexual maturity in lowland Powelliphanta species in captivity appeared to be around 5–6 years. This contrasts with our estimate of 12 years to reproductive maturity for P. augusta in captivity. Given that snail growth rate is influenced by climatic conditions, a species that lives in high altitude such as P. augusta is likely to have a slower development than lowland species, as has been shown for other land snails (Sulikowska-Drozd Citation2011).
The hatchling growth rates are more contradictory, with the captive hatchling growth rate being far lower than that predicted by extrapolation from the growth model. By considering the non-hatchling growth curves in , one would expect the hatchlings to have an average annual growth of between 1 and 2 mm. However, the mean growth rate of the individuals hatched in captivity was around 0.6 mm/year. This implies that an average individual will take approximately 5 years to grow from 8.5 mm at hatching to 11 mm and a large proportion of individuals will spend over 10 years in this growth stage. Walker (Citation2003) reported shell growth in captive Powelliphanta to be most rapid in the first 3–4 years and then continuing more slowly. Again, P. augusta hatchling growth rates in captivity contradict this finding, suggesting that during the study period, the hatchling snails were not thriving in the captive environment. Studies of early rearing efforts for the land snail Placostylus fibratus also reported a very slow growth rate for captive juveniles (Salas et al. Citation1997). However, through many years of adaptive management, with changes to substrate, light and diet (including calcium supplementation), growth rates in captivity have now been accelerated (Brescia et al. Citation2008). Informed by the finding that hatchling P. augusta did not appear to be thriving in captivity, changes to the diet and substrate of the captive P. augusta have been made in an attempt to improve hatchling survival and growth. Early indications are that hatchling survival rates have improved. Hatchling temperature trials will also start soon at the captive facility using custom-built environmental chambers (Rodney Phillips, DOC Hokitika pers. comm.).
It also appears that the individual growth rate of all non-hatchling snails of different sizes within the population has decreased during the captive period. The growth rates predicted by Allan (Citation2011) used data centred on July 2008, which is very close to the start of the study period considered here (May 2008–June 2009). As would be expected, the results of Allan (Citation2011) correspond with the results of the earlier data shown here. It is possible that growth in captive P. augusta has slowed due to low temperatures or photoperiod, as has been shown for other land snails in captivity (Gomot de Vaufleury Citation2001). A subset of the captive non-hatchling P. augusta population are now housed in custom-built environmental chambers and different photoperiod/temperature and diet regimes are being trialled to establish optimal conditions for growth, reproduction and survival.
Population model
The survival rates of snails (excluding hatchlings) observed in captivity were far higher than would be expected in the wild; the captive mortality rate was of the order 1%, implying some individuals may reach over 100 years old, compared with our estimated 8–15% mortality rate for the wild population. The primary cause of this difference is the absence of predation leading individuals to die from old age rather than external mortality. If the study were extended over a longer period, one would expect to see the death rate rise significantly as individuals reached old age. Once again, the captive hatchlings were markedly different from the larger individuals and had a higher mortality rate than that estimated for hatchlings in the wild population (16% versus 8–15%). Despite the low reproductive and hatchling growth rate, the parameters predicted by the captive data do give a stable population that will increase exponentially. This population growth is largely caused by the very low adult mortality rate that is unlikely to be representative of the long-term mortality rate. Without further investigation into the reproductive rate and the long-term survival rate, this result should not be relied upon.
By comparing the overall demographic structure of the population under captive conditions and wild conditions, we found that the population size distribution predicted by the captive data was very different from the size distribution of the wild population upon capture. Within the best fit parameter range, we found that some populations were unstable (i.e. in declining numbers) whilst others were stable, implying the wild population existed on the borderline of stability. It may be that the population parameters in captivity are significantly different from those in the wild. This is supported by our finding that the captive hatchlings are very slow growing, and these retarded growth rates may be producing the accumulation of smaller-sized captive snails observed in .
In October 2011, 826 snails died at the Hokitika captive facility as a result of a cool room malfunction. This loss represented almost half of the captive population and snails of all sizes were lost (though the exact size distribution of affected individuals is not available). This loss does not affect the validity of the model presented here and the model shows that if the lost individuals were predominantly mature adults then the time to recover from the malfunction will be longer than if those lost were predominantly juveniles and hatchlings. Overall, with a smaller starting population, the captive snails are now at an even greater risk of extinction in the event of further catastrophes such as disease outbreaks or freak incidents. Changes have been made to captive management as a result of the key findings presented in this study, i.e. that snails in the captive population appeared to be exhibiting slower growth than those in the wild, particularly amongst hatchlings, and that hatchling mortality rates were higher than expected. It is clear, however, in light of the recent accident that continued improvement to captive management, informed by research, is required to provide the best prognosis for the snails into the future.
Acknowledgements
Thanks to Matt Botur, Clare Bycroft and Harry Moyse for studying the snails as part of their university programmes. In particular, thanks to Clare for first drawing our attention to the changing dynamics. Also thanks to Ingrid Gruner, Tom Allan, Rodney Phillips and many others at DOC Hokitika for their time and patience and seemingly infinite snail knowledge.
References
- Allan TE 2011 . Husbandry of the carnivorous land snail , Powelliphanta augusta ( Gastropoda : Pulmonata: Rhytdidae ). Unpublished Master's thesis. Wellington, Victoria University of Wellington .
- Brescia , FM , Pollabauer , CM , Potter , MA and Robertson , AW . 2008 . A review of the ecology and conservation of Placostylus (Mollusca: Gastropoda: Bulimulidae) in New Caledonia . Molluscan Research , 28 : 111 – 122 .
- Coote , T , Clarke , D , Hickman , CJS , Murray , J and Pearce-Kelly , P . 2004 . Experimental release of endemic Partula species, extinct in the wild, into a protected area of natural habitat on Moorea . Pacific Science , 58 : 429 – 434 .
- Cunningham , AA and Daszak , P . 1998 . Extinction of a species of land snail due to infection with a microsporidian parasite . Conservation Biology , 12 : 1139 – 1141 .
- Dybdahl , MF and Kane , SL . 2005 . Adaptation vs. phenotypic plasticity in the success of a clonal invader . Ecology , 86 : 1592 – 1601 .
- Fernandez , J and Esch , GW . 1991 . Effect of parasitism on the growth rate of the pulmonate snail Helisoma anceps . The Journal of Parasitology , 77 : 937 – 944 .
- Gamito , S . 1998 . Growth models and their use in ecological modelling: an application to a fish population . Ecological Modelling , 113 : 83 – 94 .
- Gimbert , F , de Vaufleury , A , Douay , F , Coeurdassier , M , Scheifler , R and Badot , PM . 2008 . Long-term responses of snails exposed to cadmium-contaminated soils in a partial life-cycle experiment . Ecotoxicology and Environmental Safety , 70 : 138 – 146 .
- Gomot de Vaufleiry , A . 2001 . “ Regulation of growth and reproduction ” . In The biology of terrestrial molluscs , Edited by: Barker , GM . 331 – 355 . New York : CAB International .
- Hadfield , MG , Miller , SE and Carwile , AH . 1993 . The decimation of endemic Hawaiian tree snails by alien predators . American Zoologist , 33 : 610 – 622 .
- Hitchmough , R , Bull , L and Cromarty , P . 2007 . New Zealand threat classification system lists 2005 , Wellington : New Zealand Department of Conservation .
- Karkach , AS . 2006 . Trajectories and models of individual growth . Demographic Research , 15 : 348 – 400 .
- Law , R , Plank , MJ and James , A . 2008 . Size-spectra dynamics from stochastic predation and growth of individuals: derivation and asymptotic properties . Ecology , 90 : 802 – 811 .
- Lopez , S , Prieto , M , Dijkstr , J , Dhanoa , MS and France , J . 2004 . Statistical evaluation of mathematical models for microbial growth . International Journal of Food Microbiology , 96 : 289 – 300 .
- Mace , GM , Pearce-Kelly , P and Clarke , D . 1998 . An integrated conservation programme for the tree snails (Partulidae) of polynesia: a review of captive and wild elements . Journal of Conchology Special Publication , 2 : 89 – 96 .
- Meads , MJ , Walker , KJ and Elliott , GP . 1984 . Status, conservation, and management of the land snails of the genus Powelliphanta (Mollusca: Pulmonata) . New Zealand Journal of Zoology , 11 : 277
- Pearce-Kelly , P , Mace , GM and Clarke , D . 1995 . The release of captive bred snails (Partula taeniata) into a seminatural environment . Biodiversity and Conservation , 4 : 645 – 663 .
- Plorin , GG and Gilbertson , DE . 1984 . Equations for describing growth of the schistosome host snail Biomphalaria glabrata . Journal of Parasitology , 70 : 43
- Powell , AWB . 1946 . The Paryphantidae of New Zealand. No. V. further new species of Paryphanta, Wainuia and Rhytida . Records of the Auckland Institute and Museum , 3 : 99 – 136 .
- Rafail , SZ . 1973 . A simple and precise method for fitting a von Bertalanffy growth curve . Marine Biology (Berlin) , 19 : 354 – 358 .
- Salas , M , Bonnaut , C , LeBel , S and Chardonnet , L . 1997 . Activity and food intake of captive Placostylus fibratus (Gastropoda: Bulimulidae) in New Caledonia . New Zealand Journal of Zoology , 24 : 257 – 264 .
- Sulikowska-Drozd , A . 2011 . Population dynamics of the carpathian clausiliid Vestia gulo (e. a. bielz 1859) (pulmonata: Clausiliidae) under various climatic conditions . Journal of Conchology , 40 : 462 – 470 .
- Stringer IAN , Parrish GR 2003 . Unsuccessful transfer of captive-bred land snails to a cage at Te Paki Farm Park, North Auckland . DOC Science Internal Series 97 .
- Stringer IAN , Parrish GR 2008 . Transfer of captive-bred Placostylus hongii snails to Limestone Island . DOC Research and Development series 302 .
- Trewick , SA , Walker , KJ and Jordan , CJ . 2008 . Taxonomic and conservation status of a newly discovered giant landsnail from Mount Augustus, New Zealand . Conservation Genetics , 9 : 1563
- Tonge , S and Bloxam , Q . 1991 . A review of the captive-breeding programme for Polynesian tree snails Partula spp . International Zoo Yearbook , 30 : 51 – 59 .
- Walker KJ 2003 . Recovery plans for Powelliphanta land snails . Threatened Species Recovery Plan 49. Department of Conservation , Wellington .
- Walker , KJ , Trewick , SA and Barker , GM . 2008 . Powelliphanta augusta, a new species of land snail, with a description of its former habitat, Stockton coal Plateau, New Zealand . Journal of the Royal Society of New Zealand , 38 : 16