Abstract
Racumin® paste is an anticoagulant toxin increasingly being used for rodent control throughout New Zealand. Sachets containing Racumin® paste were distributed within the Eglinton Valley, Fiordland between July and November 2006 as part of a control operation targeting ship rats (Rattus rattus). Although the paste sachets were contained within bait stations, rats and possums were able to pull the sachets out of the stations exposing birds to the toxin. During a study of the breeding success of South Island robins (Petroica australis) within the Eglinton Valley, 50% of the robin pairs were known to be exposed to Racumin®. Direct consumption of baits or traces of Racumin® in several dead nestlings were noted. If bait sachets are unable to be secured, the use of this form of the toxin in areas where robins are present needs to be carefully considered or alternative toxins and matrices used.
Introduction
New Zealand robins (Petroica australis) are commonly monitored as an indicator species for the success of predator management. Robins are relatively easy to monitor and they are particularly sensitive to predators such as ship rats (Rattus rattus) (Powlesland et al. Citation2000; Innes Citation2005; Armstrong et al. Citation2006). In South Island beech forest (Nothofagus spp.), rat numbers can irrupt to high levels in response to mast beech seedfall and can have a significant negative impact on native birds (King & Moller Citation1997; Dilks et al. Citation2003). The Department of Conservation (DOC) is currently investigating the most efficient way to control rat irruptions in beech forest (Elliott & Suggate Citation2007). Following a mast beech seeding event in 2006 in the Eglinton Valley, rat control was initiated at several sites using a variety of toxins (Smith et al. Citation2009).
One of the products used to control rats was Racumin® paste, which contains the toxin coumatetralyl. It is a first-generation anticoagulant that achieves maximum potency through multiple feeds rather than a single dose. The use of Racumin® has increased in recent years, as it is less likely to cause secondary poisoning of non-target species and has a lower environmental persistence than second-generation anticoagulants such as brodifacoum (Pospischil & Schnorbach Citation1994; Eason et al. Citation2010). Bird species are thought to be far less susceptible to Racumin® than mammals (Madden Citation2002).
New Zealand robins have been monitored in the Eglinton Valley since 2005 as part of a larger project examining their population dynamics within managed and unmanaged sites. This short note highlights observations of robin foraging behaviour at bait stations in one of the managed areas within the Eglinton Valley.
Methods
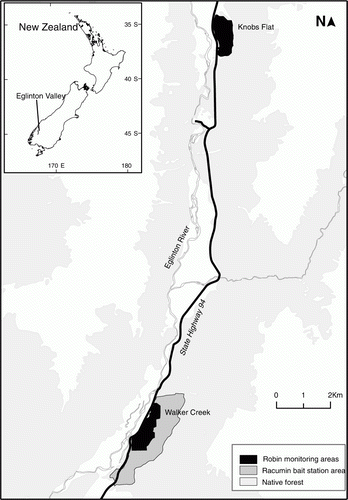
The Eglinton Valley is in Fiordland National Park (44°58′S, 168°01′E) (). The valley is about 50 km long and 1–1.5 km wide and mountains on either side up to 2000 m. Temperate rainforest dominated by red, silver and mountain beech (Nothofagus fusca, Nothofagus menziesii and Nothofagus solandri var. cliffortoides) covers glacial terraces, outwash fans on lower hill slopes and steeply rising mountain slopes up to the tree line at 1000–1200 m.
Walker Creek is located at the lower end of the Eglinton Valley and consists of mixed stands of red and silver beech on a series of river terraces. Robins are intensively monitored in a 100-ha grid within a larger 450-ha area managed for rodents. A similar sized non-treatment area at Knobs Flat is located 16 km north of Walker Creek ().
In June 2006, rat control was initiated in the Eglinton Valley in response to a mast beech seedfall and rising rodent levels. 1080 pellets were initially deployed for 1 month in mini-Philproof bait stations laid out in a 100×100-m grid throughout the Walker Creek area. Racumin® was then distributed in 10-g paste sachets between July and November 2006. Approximately 250–300 g of Racumin® (0.375 g/kg coumatetralyl) was used per station during each fill (Smith et al. Citation2009). 1080 was used again in November and January 2007, followed by a repeated application of Racumin® in February 2007.
The majority of robins within Walker Creek were individually colour banded. Robin nests and individual birds were located and checked twice a week for survival. Foraging observations of any birds around bait stations were noted. Any dead birds (adults, nestlings and juveniles) found were frozen and sent to the Toxicology Laboratory at Landcare Research (Gerald St, Lincoln, New Zealand) for analysis. Tissue analysis was carried out using the TLM076 test for coumarin rodenticides in animal tissue with a method limit of detection (MDL) of 0.01 µg/g for coumatetralyl (Jones Citation1996).
Results
Twelve pairs of robins and three single males were monitored in the Walker Creek study area from August 2006 until April 2007. During this period, sachets of Racumin® bait were removed from bait stations (presumably by rats), torn open and scattered on the forest floor. Observations of eight robins (two adult females, four adult males and two juveniles) eating or feeding Racumin® to their mates or fledglings were subsequently noted. On 31 August, a male was observed courtship feeding Racumin® to a female. Between 16 October and 3 November, three male robins were observed feeding Racumin® to fledglings, and two adult females and two juveniles were observed feeding directly on Racumin® paste. In addition, four robin nestlings found freshly dead in nests were analysed for coumatetralyl and all were found to have residues (). Of the eight robins directly observed foraging on toxic baits, four (one adult male and female, one juvenile male and female) had disappeared within 1 month of the observations. In total, six of the 12 pairs of robins monitored were directly exposed to Racumin® baits.
Table 1 Levels of coumatetralyl found in freshly dead robins
Within the Walker Creek treatment area, six breeding adults disappeared, (25% of the breeding population) while in the non-treatment area at Knobs Flat, 11 breeding adults disappeared (31% of the breeding population) over the 2006–07 breeding season.
Discussion
Predation by introduced mammals is the primary reason for the current decline in native forest birds in New Zealand and it is predicted few native species will persist on the mainland without predator control (Innes et al. Citation2010). Ship rats are a significant predator, and anticoagulant toxins are commonly used as a control tool. Second-generation anticoagulants, such as brodifacoum, were commonly used throughout the New Zealand but because of increasing concern about environmental persistence their use by the DOC has been reduced on the mainland (Eason et al. Citation2010). First-generation anticoagulants, such as coumatetralyl, have a lower environmental persistence and are being used more frequently, despite the disadvantage of multiple feeds of the toxin being needed and the increased expense (Eason et al. Citation2010). The lower toxicity and persistence means the risks to non-target species (e.g. native birds) is lower but not mitigated entirely (Erickson & Urban Citation2004).
Little information is available on the toxicity of coumatetralyl on native birds (Eason & Wickstrom Citation2001). All the dead robin nestlings tested in this study had coumatetralyl in their livers but it is not known whether that was the cause of death for all or some of the birds. Two robin nestlings showed sign of being eaten but it is unclear whether they had been killed by rats or scavenged after exposure to lethal doses of coumatetralyl. The secondary poisoning effects of coumatetralyl have been tested on wild caught ferrets (Mustela furo) and weka (Gallirallus australis). Two of the 10 ferrets died after eating poisoned rats. These animals had liver residues of 0.27 and 0.28 mg/kg—levels below that found in one of the robin nestlings. There were no obvious effects on weka (O'Connor et al. Citation2003).
Early studies investigating the effects of Racumin® calculated a single acute oral LD50 for Racumin® of >3000 mg/kg body weight for chickens (Gallus gallus domesticus) (Hermann 1963, as cited in Madden Citation2002). However, a chronic oral LD50 for chickens is only 50 mg/kg/day for 8 days and for blackbirds (likely Turdus merula) 6.73 mg/bird/day for 23 days (Hermann 1963, as cited in Madden Citation2002). If robins had a similar susceptibility to chickens, they would need to eat 5 g of bait/day for 8 days to obtain a LD50, or 2–3 g/day for 23 days if similar to blackbirds. Given the observed quantity of Racumin® being eaten, it is likely that robins could eat a lethal dose.
While the loss of robins at Walker Creek is greater than expected given the programme of pest control, the loss of robins at Knobs Flat (the non-treatment site) was marginally higher. Therefore, despite the possibility that Racumin® killed some robins at Walker Creek, robin survival without pest control would probably have been lower. Indeed, the survival of other bird species present at Walker Creek that do not consume baits appears to have benefitted from the use of Racumin® (M Pryde, DOC, unpubl. data).
If robins are able to access Racumin® baits directly in the paste formulation used in the Eglinton Valley, then robin deaths are likely to occur. For this reason, the use of this particular form of the toxin in areas where robins are present needs to be carefully considered. If bait sachets are unable to be secured within bait stations, alternative toxins and bait matrices (e.g. harder, drier and less crumbly cereal-based pellets) should be used. In 2009, cereal-based pellets containing the toxin pindone (another first-generation anticoagulant) was used in the same study area at Walker Creek and no observable impact on robins was detected (M Pryde, DOC, unpubl. data). Research should also continue to investigate effective colour and chemical repellents (Hartley et al. Citation1999; Day et al. Citation2003) that can be added to poison baits to reduce the risk to native birds.
Acknowledgements
We thank R Hay, L Hartley and R Earl for help with bird monitoring. Bait station layout and poison delivery was undertaken by Contract Wild Animal Control (A and D Gutsell, Te Anau). Samples were analysed by the Pest Control Technologies team at Landcare Research, Lincoln. The manuscript was improved with the help of Colin O'Donnell and an anonymous referee.
References
- Armstrong , DP , Raeburn , EH , Lewis , RM and Ravine , D . 2006 . Modelling vital rates of a reintroduced New Zealand robin population as a function of predator control . Journal of Wildlife Management , 70 : 1028 – 1036 .
- Day , TD , Mathews , LR and Waas , JR . 2003 . Repellants to deter New Zealand's North Island robin Petroica australis longipes from pest control baits . Biological Conservation , 114 : 309 – 316 .
- Dilks , P , Willans , M , Pryde , M and Fraser , I . 2003 . Large scale stoat control to protect mohua (Mohoua ochrocephala) and kaka (Nestor meridionalis) in the Eglinton Valley, Fiordland, New Zealand . New Zealand Journal of Ecology , 27 : 1 – 9 .
- Eason , C , Henderson , R , Hix , S , MacMorran , D , Miller , A , Murphy , E , Ross , J and Ogilvie , S . 2010 . Alternatives to brodifacoum and 1080 for possum and rodent control—how and why? . New Zealand Journal of Zoology , 37 : 175 – 183 .
- Eason CT , Wickstrom M 2001 . Vertebrate and pesticide toxicology manual (poisons) . Department of Conservation Technical Series 23 .
- Elliott G , Suggate R 2007 . Operation Ark Three year progress report . Christchurch , Department of Conservation .
- Erickson W , Urban D 2004 . Potential risks of nine rodenticides to birds and nontarget mammals: A comparative approach . Washington , DC , US Environmental Protection Agency .
- Hartley , L , O'Connor , C , Waas , J and Matthews , I . 1999 . Colour preferences in North Island robins (Petroica australis): implications for deterring birds from poisonous baits . New Zealand Journal of Ecology , 23 : 255 – 259 .
- Innes , J . 2005 . “ Ship rat ” . In The handbook of New Zealand mammals , 2nd edition , Edited by: King , CM . 98 – 109 . Melbourne : Oxford University Press .
- Innes , J , Kelly , D , Overton , JM and Gillies , C . 2010 . Predation and other factors currently limiting New Zealand forest birds . New Zealand Journal of Ecology , 34 : 86 – 114 .
- Jones , A . 1996 . HPLC determination of anticoagulant rodenticide residues in animal livers . Bulletin of Environmental Contamination and Toxicology , 56 : 8 – 15 .
- King , CM and Moller , H . 1997 . Distribution and response of rats Rattus rattus, R. exulans to seedfall in New Zealand beech forests . Pacific Conservation Biology , 3 : 143 – 155 .
- Madden , W . 2002 . “ Racumin rodenticide—potential environmental impact on birds ” . In Ecology and Conservation of owls , Edited by: Newton , I , Kavanagh , R , Olsen , J and Taylor , I . 296 – 301 . Melbourne : CSIRO .
- O'Connor , CE , Eason , ET and Endepols , S . 2003 . Evaluation of secondary poisoning hazards to ferrets and weka from the rodenticide coumatetralyl . Wildlife Research , 30 : 143 – 146 .
- Pospischil R , Schnorbach HJ 1994 . Racumin Plus, a new promising rodenticide against rats and mice . In : Halverson WS , Crabb AC . Proceedings of the Sixteenth Vertebrate Pest Conference , California , 28 February–3 March .
- Powlesland , RG , Knegtmans , JW and Marshall , ISJ . 2000 . Breeding biology of North Island robins (Petroica australis longpipes) in Pureora Forest Park . Notornis , 47 : 97 – 105 .
- Smith , DHV , Murphy , EC , Christie , JC and Hill , GS . 2009 . The effectiveness of poison bait stations at reducing ship rat abundance during an irruption in a Nothofagus forest . New Zealand Journal of Zoology , 36 : 13 – 21 .