Abstract
Translocations for conservation often involve species limited to relict distributions. However, uncertainty can exist regarding the ability of source individuals to acclimatise following a shift to a distant location. We investigated the ability of captive-reared juvenile tuatara (Sphenodon punctatus) of Cook Strait stock (41°S) to adjust to outdoor, predator-protected pens within Orokonui Ecosanctuary (45 °S). We examined potential basking and within burrow temperatures, the influence of temperature on emergence, and growth rates in comparison with other locations. Tuatara at Orokonui reached their preferred temperature when basking over summer, and burrows provided protection from freezing over winter. Emergence was temperature-dependent and essentially ceased during winter. Growth rates of Orokonui-held juveniles were within the range for four other captive-rearing facilities and faster than for wild juveniles from a Cook Strait population. As all Orokonui-held juveniles have survived and grown we conclude that the climate at this southern location is suitable to consider a free-release.
Introduction
Since humans and their associated mammals arrived in New Zealand, many species of endemic reptiles have declined in distribution on the main islands, with some now confined to offshore islands (Towns & Daugherty Citation1994; Towns & Ferreira Citation2001). Tuatara (Sphenodon punctatus), the last living species of the once diverse order Rhynchocephalia (Jones Citation2008) are no exception, having become extinct on mainland New Zealand within the last few hundred years (Cree & Butler Citation1993; Towns & Daugherty Citation1994; Hay et al. Citation2008). Tuatara were once widespread across both North and South Islands (34°S–46°S), but natural populations are now restricted to approximately 32 offshore islands in Cook Strait and northern New Zealand (35°S–41°S), representing in total area about 0.5% of the pre-human distribution (Cree & Butler Citation1993). Conservation management strategies have involved translocations to areas where mammal eradication or control have taken place (Cree et al. Citation1995; Gaze Citation2001; Hay et al. Citation2003). Since 1995, tuatara have been translocated to eight ‘new’ islands (known or presumed reintroductions), and to three fenced ecosanctuaries in the North Island (including Zealandia™, formerly Karori Wildlife Sanctuary; Miller et al. Citation2009). However, all of these reintroductions have been within about the same latitudinal range as the current natural distribution (Besson & Cree Citation2011).
A proposal exists to reintroduce tuatara to the mammal-free Orokonui Ecosanctuary (45°S) in the southern South Island (Besson et al. Citation2012). However, Orokonui is around 570 km south of the most abundant Cook Strait population of tuatara on Stephens Island (Takapourewa; ). Not surprisingly, mean temperatures of models simulating tuatara in forest shade are cooler (by about 3 °C) at Orokonui than on Stephens Island, and the difference in mean daily maximum is greater (up to about 10 °C) for models in the sun (Besson & Cree Citation2010). Therefore, before translocating wild tuatara from their warmer Cook Strait climate (or translocating Cook Strait stock raised in captivity) to the ecosanctuary, it is important to understand the way in which environmental factors (including temperature) might influence behavioural and physiological performance. This is especially pertinent for tuatara, as it is unknown whether extinct populations from the southern mainland were genetically identical to current northern populations; conceivably, tuatara from southern New Zealand may have had special adaptations to cooler temperatures (Besson et al. Citation2012). To date, laboratory investigations of responses to the cooler conditions typical of those provided by Orokonui suggest that tuatara of Cook Strait origin are likely to fare well with the transfer. In particular, tuatara show plasticity of basking behaviour in response to reduced basking opportunity (Besson & Cree Citation2010), are capable of feeding and digesting at fairly cool temperatures, and tolerate short-term exposure to temperatures as low as 0.7 °C (Besson & Cree Citation2011). Additionally, soil temperatures in some parts of the ecosanctuary appear suitable for egg incubation, a process vital for recruitment and population persistence (Besson et al. Citation2012). However, direct observations of the behaviour and performance of juvenile tuatara maintained outdoors under southern South Island conditions have yet to be reported.
In ectotherms, body temperature is directly affected by environmental temperature, which in turn influences all aspects of behaviour and physiology (Huey & Kingsolver Citation1989; Angilletta et al. Citation2002) including growth rates (Autumn & DeNardo Citation1995) and emergence behaviour (Besson & Cree Citation2010). Although tuatara are primarily nocturnal, they also emerge from their burrows to bask (Barwick Citation1982), and juveniles appear especially diurnal (Whitworth Citation2006; Terezow et al. Citation2008). In the wild, nocturnal emergence is rare when air or body temperatures fall below 7 °C (Walls Citation1983; Thompson & Daugherty Citation1998), but increases as air temperature rises (Walls Citation1983). Body temperature can reach as high as 30°C in basking adults (Barwick Citation1982), although captive-held juveniles prefer body temperatures of 21.3±0.9°C (Besson & Cree Citation2011). Tuatara may require a higher body temperature for digestion than for locomotion as digestion appears not to be completed at temperatures below 12 °C (Besson & Cree Citation2011). Environmental temperatures probably affect growth rates, as tuatara from warmer northern populations reach sexual maturity 2–3 years earlier than their Cook Strait counterparts (Castanet et al. Citation1988). Tuatara can live for at least 88 years in the wild (Nelson et al. Citation2002).
In March 2009, 15 juvenile tuatara were transferred from an indoor holding facility at the University of Otago to a mammal-proof outdoor subenclosure at Orokonui Ecosanctuary. This shift towards a thermally more variable outdoor environment created an opportunity to evaluate the effects on behaviour and growth of the juveniles. In particular, we aimed to identify: 1) the body temperatures likely to be experienced by tuatara when basking or within burrows; 2) the influence of air temperature on emergence behaviour; and 3) growth rates before and after release and in comparison with other captive-raised and wild juvenile tuatara.
Methods
History of tuatara bound for Orokonui Ecosanctuary
Fifteen juvenile tuatara (Sphenodon punctatus) were hatched between 2004 and 2008 at the University of Otago (UOO) in Dunedin, New Zealand, from wild or captive-laid eggs of Stephens Island stock (Whitworth Citation2006; Besson et al. Citation2012). The resulting juveniles (toe-clipped for identification) were housed indoors in individual plastic containers (for the 3 months prior to transfer, containers were 36–86L depending on animal size). Basking sites, retreat sites and a water dish were provided on a substrate of soil, sand and leaf litter. The room provided seasonally varying temperatures and photoperiods. Basking lamps allowed tuatara to reach body temperatures of up to 26±1°C during summer, whereas cooler body temperatures (typically 10 °C, rarely 5 °C) were experienced over winter. Arcadia D3 full-spectrum reptile lights mounted about 30 cm above the substrate provided measurable exposure to UVB during basking. Juveniles were fed two to three times per week during spring–autumn and less during winter with live, vitamin-supplemented insects as well as fish-based cat food. As tuatara have temperature-dependent sex determination, sex was able to be inferred for most juveniles (those arising from eggs incubated under constant temperatures) using a well-established and accurate model (Mitchell et al. Citation2006). Laparoscopies confirmed the sex of the eight largest of these juveniles 3 months prior to transfer to Orokonui Ecosanctuary (Besson et al. Citation2012; N.J. Nelson, Victoria University of Wellington, pers. comm. 2008).
Transfer to Orokonui Ecosanctuary (45°46′S, 176°36′E) occurred in March 2009, when juveniles were aged 1–5 years. This 307 ha ecological restoration site located 20 km north of Dunedin is surrounded by a fence designed to keep out predatory exotic mammals (www.orokonui.org.nz). At the ecosanctuary, the juvenile tuatara were housed outdoors in a mesh-walled, rodent-free subenclosure sited for good sun exposure, allowing for maintenance under benign conditions while a proposal for free-release in the wider ecosanctuary is considered by relevant authorities. Within the subenclosure, juveniles were held in groups of two to three similar-sized animals per pen (either mixed sexes or all females). The pens (c. 2×1×0.8 m, l×w×h) contained a soil substrate with artificial burrows made of drainage pipe, plus additional cover in the form of rocks and logs. Grasses and shrubs provided patches of shade, and a water dish was present. In the absence of a forest canopy, when frosts were anticipated a frost cloth was suspended 0.8 m over the pens (however, subsequent monitoring suggested that this made little difference to temperatures inside burrows; V. Fay, pers. obs. 2012). Animals had access to wild invertebrates year-round and were fed additional insects during their spring–autumn activity period ().
Table 1 Conditions under which groups of captive juvenile tuatara of Stephens Island stock (Sphenodon punctatus) were reared.
Operative basking temperatures, burrow temperatures and spot body temperatures at Orokonui Ecosanctuary
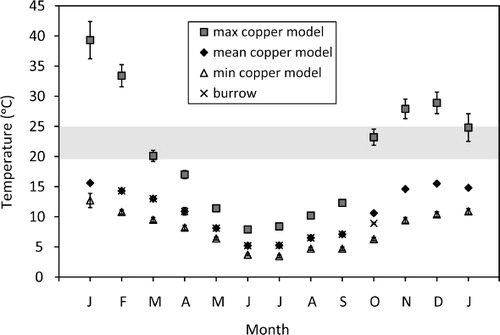
We measured operative temperatures at potential basking sites within the enclosure pens to determine whether temperatures were sufficient for juvenile tuatara to achieve their preferred body temperature (mean±SEM; 21.3±0.9 °C, with the central 50% spanning 19.3–24.9 °C; Besson & Cree Citation2010). Operative temperatures represent the steady-state temperature of an organism (with no metabolic heating or evaporative cooling) at a specific location (Bakken Citation1992; Angilletta Citation2009), and are typically estimated for small reptiles in basking situations using hollow, sealed copper pipes (e.g. Hare et al. Citation2009). To mimic (motionless) basking tuatara, we placed copper models close to the entrance of burrows where they were estimated to experience full sun for at least 5 h per day. Copper models (120×18 mm) were painted a grey-brown colour (Café Royale, Resene Paints) and had previously been calibrated against live juvenile tuatara (Besson & Cree Citation2010). Copper models (two in each of six pens) were installed on January 2010 and retrieved in January 2011 (snout-vent lengths [SVL] of the live tuatara at Orokonui ranged from 71–113 mm at the start of recording). Temperatures inside the models were recorded once per hour using iButton data loggers with a resolution of 0.06 °C (DS 1922, Maxim Integrated Products, Sunnyvale, CA, USA). In addition, burrow temperatures were recorded at c. 20 cm depth, in one burrow per pen, between late summer and spring (February–October 2010). The resulting data were used to calculate mean daily maximum and mean daily minimum temperatures per month by averaging the maximum or minimum temperatures for each 24-h day across loggers in each position (exposed copper model or burrow), and then averaging these across all days per month. Mean daily temperatures in each position were calculated using all temperatures measured hourly throughout the 24-h day.
Body temperatures of tuatara were recorded on two occasions (in NZDST, summer: 28 January 2010, 1200–1330 h; autumn: 26 March 2010, 1210–1348 h; the latter in conjunction with monitoring as part of the national captive management programme for tuatara; Blanchard and the Tuatara Recovery Group Citation2002). Both days were warm and dry, with sunny periods offering opportunity for basking. However, because juveniles generally retreated down burrows to elude capture and required digging out, some measurements probably underestimate what would be achievable during basking. Internal body temperature was recorded within 30 s of capture by inserting a thermocouple into the cloaca (Digi-sense® model 8528-40, Cole-Parmer, Niles, IL, USA).
Seasonal emergence behaviour at Orokonui Ecosanctuary
To determine whether juvenile emergence was related to available basking temperature and/or season, we observed daytime emergence behaviour of five tuatara in autumn (over 10 days between 29 March and 16 April 2010), and in winter (on five days between 3 and 17 June 2010). Emergence was recorded in two pens containing five individuals using a wide-angle camera mounted to the roof of the enclosure (GoPRO® Hero HD 5Mp). Each recording was started manually at 1200 h (NZST) just prior to departure of the caregiver from the enclosure, and continued for c. 2.5 to 3 h (dependent on battery life), with frames taken automatically once a minute.
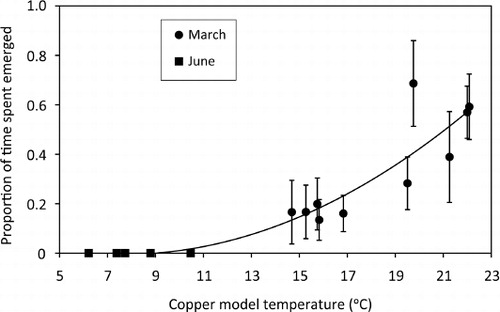
Before the first recording session in March, the five animals were marked with two white paint spots (non toxic, Chromacryl® White, Chroma Ltd) on different parts of their body to aid individual identification. However, as the first recordings showed high site fidelity, and individuals within pens were distinguishable by small differences in size, paint marking was not used for the June recording. For the analyses, the first minute (image) of each recording was discarded. Tuatara were thereafter considered emerged if they had at least the tip of the snout outside the burrow. The proportion of emerged time was considered as the sum of appearances of each individual divided by the number of frames taken. To calculate the (non-linear) relationship between mean temperature of the exposed copper models in basking positions and the mean proportion of time emerged, we fitted second-order polynomial trendlines for the March data, and for the combined March and June data, using a quadratic curve-fitting procedure.
Growth rates at Orokonui Ecosanctuary and elsewhere
To determine whether growth was occurring at an acceptable rate, we compared growth rate of the Orokonui-held juveniles with that for juveniles at four other captive facilities and in the wild on Stephens Island. From the time of hatching at UOO, and including the time after transfer to Orokonui Ecosanctuary (i.e. UOO/OE), SVL and total length (±1 mm with a plastic ruler) were measured twice a year, in late March/April and in October, as required by the captive management programme for tuatara (Blanchard and the Tuatara Recovery Group Citation2002). We also obtained an additional measurement (in January 2010) following transfer of the juveniles to Orokonui.
For each individual, we first calculated growth in SVL from an initial age of 2–6 months until a final age of 2–6 years. By calculating growth over this extended period, the influence of measurement error in any given measurement was minimised. We used linear regression to calculate growth in SVL (in mm/y), and then averaged the rates across individuals. A paired t-test was used to compare growth rates at UOO with those after transfer to Orokonui. Growth rates were compared between four males and seven females (sexes known, or inferred with high confidence from egg incubation temperatures) using an independent sample t-test.
We also compared the mean growth rate with those for juvenile tuatara over a similar range of ages that were held in captivity at four other locations of different latitudes, as well as with wild juvenile tuatara on Stephens Island (). The other captive juveniles (all hatched since 2003) were held at: Nga Manu Nature Reserve (NMNR) in Waikanae, North Island (n=3); at Orana Wildlife Park (OWP) in Christchurch, South Island (n=6); at Peacock Springs Wildlife Park (PSWP) in Christchurch, South Island (n=9); and at Southland Museum and Art Gallery (SMAG) in Invercargill, South Island (n=5). Body measurements were collected by the respective curators and additional information was sought by us about factors that might influence growth rates, including enclosure location (indoor/outdoor), temperature regimes, provision of additional heating, area per tuatara and feeding regime ().
For the wild juveniles (n=8), measurements at first capture in the 1970s or 1980s and at recapture from 2–4 years later were obtained from a database for toe-clipped tuatara on Stephens Island (Christmas et al. Citation1996).The age of the animals was unknown, but animals were considered juvenile if they were less than 180 mm at the final measurement, as this is about the size at which tuatara on Stephens Island reach sexual maturity (Cree et al. Citation1992). Growth rates among all locations (captive and wild) were compared using the non-parametric Kruskal-Wallis test (a parametric test was not used as the assumption of homogeneous variances was seriously violated and could not be rectified with any attempted transformation).
Finally, we examined whether there was any evidence for a reduction in growth rate as animals approached sexual maturity. For the captive juveniles, the mean growth rate (in mm/year between six-monthly measuring periods) was examined in relation to mean initial SVL using linear regression. This analysis was performed for juveniles from NMNR and SMAG only, because: 1) SMAG housed the tuatara in the same indoor enclosure from hatching (i.e. the physical environment was stable); and 2) at NMNR, some tuatara had reached the size of sexual maturity, providing a full range of juvenile growth. For wild animals, which experienced natural conditions throughout, growth rate for each individual was plotted as a function of initial SVL, and examined using linear regression.
Statistical analysis
Analyses were performed using SPSS 16 or IBM SPSS Statistics 20, and significance was assumed at P<0.05.
Results
Operative basking, burrow and body temperatures
The operative temperatures of copper models in potential ‘basking’ positions indicate the temperatures available to tuatara that emerged at Orokonui Ecosanctuary throughout the year of 2010 (). Mean daily temperatures of the models ranged between 15.6 °C in January and December (summer) and 5.1 °C in July (winter). However, given that (unlike the models) tuatara retreat to burrows during cool weather, maximum temperatures are more informative in terms of basking opportunity. Mean daily maximum temperatures of the models ranged between 39.3 °C in January and 7.9 °C in June. Mean daily minimum temperatures ranged between 12.7 °C in January and 3.4 °C in July (the lowest temperature recorded for any model was 0.4 °C). Burrow temperatures showed less variation than copper models, with the mean temperature ranging from 14.3±0.1 °C in February (late summer) to 5.2±0.3 °C in July (winter). The lowest recorded temperature in any burrow was 3.2 °C in July and the highest was 22.2 °C in February. Cloacal temperatures of live tuatara averaged 20.1±0.5 °C in January (range 16.3 °C to 22.7 °C, n=15) and 18.2±0.9 °C in March (range 13.9 °C to 25.1 °C, n=15).
Emergence behaviour
Recordings over 15 d yielded 2805 pictures, spanning approximately 47 h of observation. Weather during the recording days varied from clear to overcast, and sun to rain. During the autumn recording session (29 March–16 April 2010, 1929 pictures over 10 d), initial recording temperatures measured by the copper models ranged from 16.0 °C to 29.4 °C, and final temperatures from 10.9 °C to 22.8 °C. During the winter session (3–17 June 2010, 876 pictures over 5 d), initial recording temperatures ranged from 7.0 °C to 11.7 °C, and final temperatures from 5.3 °C to 9.2 °C.
Behaviour of the tuatara was significantly influenced by mean temperatures as recorded by the copper models (averaged between initial and final values for each recording session; ). The mean proportion of time that tuatara spent emerged increased (in a non-linear fashion) as the mean temperature of the copper models increased during the autumn recordings (F2,9=8.639, r2=0.840, P<0.013) and during the autumn and winter recordings combined (F2,12=34.107, r2=0.850, P<0.001). At the highest mean maximum temperatures measured by the copper models (19.7 °C to 22.0 °C), tuatara spent on average between 57%–68% of their time emerged, with the greatest emergence occurring on days when temperatures showed the least variation during the recording session. During the winter recording in June, mean temperatures of the model remained at or below 10.5 °C and no emergence was detected.
From the recordings, it was apparent that two of the five observed tuatara used more than one burrow, including self-dug burrows as well as artificial burrows. These burrows were within a metre of each other. However, the other three juvenile tuatara remained within a small area close to one burrow (generally no more than two body-lengths in distance). Tuatara sometimes basked with only their head or the front half of the body outside the burrow entrance, and sometimes with the whole body in front of it. Movements were infrequent and tended to follow patches of sunlight.
Growth rate
For the UOO/OE animals, mean growth rate (calculated over periods of 2.5–6 years depending on age at the final measurement) was 12.8 mm year−1 (SEM±0.3, n=15; ). Mean growth rate while indoors at UOO (12.4 mm year−1) differed significantly from that at Orokonui Ecosanctuary (14.2 mm year−1), with growth being faster after animals were transferred to the outdoor enclosure (t14=2.191, P<0.05). At Orokonui, there was no difference in growth rate between animals of different sexes (t=0.494, P>0.6).
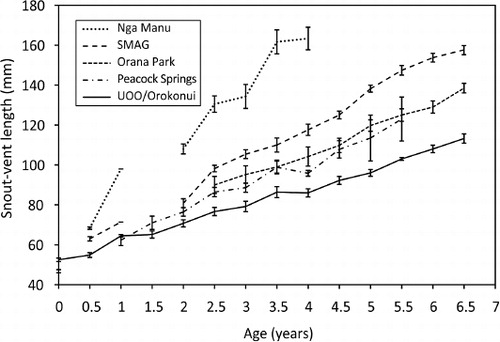
Juvenile tuatara from different locations grew at different rates (H5=27.733, P<0.001; and ). Among the locations for captivity, post-hoc tests revealed no significant difference in growth rate between UOO/OE and OWP (P>0.05), whereas growth rates at UOO/OE were significantly lower than at SMAG, PSWP or NMNR (P<0.05 in all cases). On the other hand, tuatara grew faster at all captive locations (including UOO/OE) than in the wild (P<0.05). Mean growth rates among captive juveniles were highest in tuatara raised at NMNR (18.5 mm year−1) and lowest in tuatara raised at OWP (12.5 mm year−1). No substantial tail loss was reported for any location.
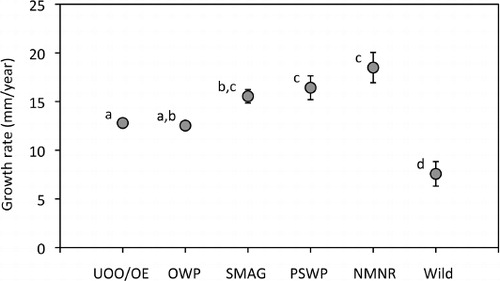
During the first years of life, the growth rate for juvenile tuatara at NMNR and SMAG was variable over time, but showed no consistent relationship with mean SVL (P>0.1 for both locations). In other words, there was no evidence of a decline in growth rate with age or animal size. Among a sample of eight wild tuatara, there was a significant negative relationship between growth rate and SVL (t=2.896, P=0.027); however, this result was strongly influenced by the smallest tuatara. When this animal was removed from the analysis, the linear regression was no longer significant (t=−1.629, P=0.164).
Discussion
Juvenile tuatara appear to have adjusted well to the cool climate experienced at Orokonui Ecosanctuary. For example, they were able to reach preferred body temperature when emerged during the warmer months, they spent proportionally more time emerged on warm days than on cool days, and they grew at a rate similar to some other juvenile tuatara held in outdoor, captive situations.
Environmental temperatures
We investigated the temperatures available to juvenile tuatara at Orokonui Ecosanctuary. Mean daily maximum temperatures recorded in exposed copper models on sunny summer days were similar to those recorded in other open areas at Orokonui in a previous year (Besson & Cree Citation2010). Although these temperatures are typically about 10 °C cooler than in equivalent open habitat on Stephens Island (Besson & Cree Citation2010), they are nonetheless warm enough that tuatara in basking situations should be able to reach their preferred body temperature, which we confirmed by recording body temperatures of juveniles on two occasions.
We also observed that burrows provide protection from near-freezing temperatures. By remaining in burrows (which remain at or above about 3 °C) when outdoor temperatures of copper models fall as low as 0.4 °C, tuatara are likely to maintain body temperatures above their critical minimum of 0.7 °C (the temperature at which the ability to right themselves is lost; Besson & Cree Citation2011). In a previous report, a captive adult tuatara held outdoors in Christchurch was reported to survive air temperatures that fell to −5 °C, although burrow temperature was not measured (Desser Citation1979).
Emergence behaviour
We found that daytime emergence of juvenile tuatara at Orokonui Ecosanctuary is closely related to environmental temperature. Juveniles emerge when operative temperatures in exposed positions are as low as 14.7 °C, but not when such temperatures are below 10.5 °C. Furthermore, juvenile tuatara are more likely to emerge when they can attain body temperatures close to their preferred temperature of 21.3 °C (present study; Besson & Cree Citation2010). These results are consistent with subsequent counts at Orokonui showing that total number of tuatara emerged per day over the spring–summer period (October–March) is positive and increasing over the shade air temperature range of 12–25 °C (V. and M. Fay, Orokonui Ecosanctuary, pers. obs. 2012). Consistent with these findings, observations of wild tuatara on Stephens Island show that numbers emerged at night are more than twice as high at an air temperature of 20 °C than at 12 °C (Walls Citation1983). We also observed tuatara following patches of sunlight suggesting active thermoregulation, as has been seen in wild tuatara (Saint Girons et al. Citation1980; Barwick Citation1982). This is unsurprising as many ectotherms utilise complex microclimatic mosaics to behaviourally regulate their body temperatures (e.g. Kearney et al. Citation2009). The value of thermoregulation to the speed of digestion was shown in a laboratory study of feeding behaviour of juveniles: gut passage time was substantially shorter at air/body temperatures of 20 °C than at 12 °C (Besson & Cree Citation2011).
As in our study, where tuatara were inactive for about 10 weeks (mid-June to mid-August), a lack of emergence of tuatara for several weeks during winter has been reported by other captive facilities, including those from which we obtained growth data (L. Hazley [SMAG], A. Richardson [PSWP], T. Atkinson [OWP] and R. Mills [NMNR], pers. comm. 2010). Similarly, although quiescence in wild tuatara has not been well documented, Walls (Citation1983) reported that tuatara become inert during periods of very low temperature. Our study did not identify whether there are seasonal or diurnal shifts in the effect of potential basking temperatures on daytime emergence, nor how nocturnal temperatures affect emergence in different seasons. These questions would be worth examining in future studies.
Growth rate
A comparison of systematic body measurements from different places (outdoor, indoor and wild tuatara) is a helpful tool to determine growth rate parameters for a possible reintroduction. We found significant differences in growth rate among tuatara held within indoor and outdoor captive enclosures and wild specimens from Stephens Island. Many factors influence growth rate in reptiles, with variation in food supply and temperature during development having a strong influence. For example, juvenile McCann's skinks (Oligosoma maccanni) fed an ad libitum diet grew faster and attained size-based maturity quicker than those fed a restricted diet, with the influence of thermal regime being less important than diet (Hare et al. Citation2012). Differences in temperature and diet probably both contribute to the differences in growth rate among tuatara. In wild populations, those in warmer northern areas (Lady Alice Island) reach size-based sexual maturity 2–3 years earlier than those in Cook Strait (Stephens Island; Castanet et al. Citation1988). Differences in diet composition and quantity could lead to differences in growth, reproduction and survival rates (Cartland et al. Citation1994; Cartland & Grimmond Citation1994). When tuatara from North Brother Island were translocated to another island at similar latitude (and presumably climate), individuals showed an increase in mass and length, suggesting that reduced competition for food at the new site might have provided additional nutrition to be invested in tissue growth (Nelson et al. Citation2002). Differences in thermal regimes, radiation wavelengths, diet, space and social interactions could all have contributed (not necessarily in the same directions) to differences in growth of captive juveniles between indoor housing at UOO and outdoor housing at Orokonui Ecosanctuary.
Similar to other studies using captive tuatara (Tyrrell & Cree Citation1994; Nelson et al. Citation2004), we found no difference in growth rate between sexes for juveniles at Orokonui. In wild tuatara on North Brother Island, growth rates of males and females are also similar during juvenile life; however, growth rates diverge following maturity, with growth subsequently diminishing more rapidly in females than in males (Mitchell et al. Citation2010). Slower growth in females than males was also indicated for adult tuatara on Stephens Island (Dawbin Citation1982; Castanet et al. Citation1988). Sex differences in adult growth rates could be affected by differences in sex steroid concentrations; for example, in summer, adult males typically have much higher concentrations of testosterone (Cree et al. Citation1992).
Sexual maturity of tuatara on Stephens Island is reached at about 170–180 mm SVL (Cree et al. Citation1992; A. Cree, pers. obs.). If juvenile tuatara at Orokonui maintain the mean growth rate of 14.2 mm year−1 observed here, we estimate that they will reach size-based sexual maturity at 13–15 years of age (however, subsequent measurements to March 2012 suggest that this period could be even shorter—perhaps as low as 8 years of age for those spending most of their juvenile lives at the ecosanctuary, provided that food intake remains similar; V. and M. Fay, pers. obs. 2012). These estimates suggest younger ages for maturity at Orokonui than inferred using mark-recapture on Stephens Island, where tuatara reached sexual maturity at about 20 years of age (Dawbin Citation1982). In contrast, Castanet et al. (1998) suggested that tuatara reach size-based sexual maturity between 11 and 13 years on Stephens Island; however, this study used an indirect method (skeletochronology) that involves more assumptions.
In summary, juvenile tuatara at Orokonui Ecosanctuary show a satisfactory adjustment to the local climate, which is cooler than experienced by tuatara at the source location of Stephens Island. Tuatara at Orokonui are showing similar natural behaviours (e.g. thermoregulation, emergence and burrow digging) as tuatara elsewhere, including wild tuatara. Low temperature seems not to be a threat to tuatara at Orokonui, as they have already survived two winters, though with occasional use of frost cloth. They are growing at an intermediate rate between other captive tuatara and wild tuatara. If tuatara are free-released at Orokonui, research should be carried out to examine whether growth rates change once tuatara have to fend for themselves, and to document the amount of free-ranging invertebrates and lizards available as food.
Acknowledgements
Research was undertaken with permission from the New Zealand Department of Conservation and University of Otago Animal Ethics Committee, following consultation with Ngāti Koata (kaitiaki of tuatara from Stephens Island/Takapourewa), Ngāi Tahu, Kāti Huirapa Rūnaka ki Puketeraki, The Tuatara Recovery Group Leader and the Tuatara Captive Management Plan Coordinator. We thank staff, sponsors and volunteers of the Orokonui Ecosanctuary for their support and assistance, especially Kelly Gough for husbandry and Michael Fay for sharing additional analyses. Thanks also to Nicola Nelson for assistance with laparoscopies prior to transfer, Mya Gaby for help with field work, Carey Knox and Sophie Penniket for comments on drafts, and Ken Miller for graphical assistance. We are especially grateful to people and organisations who provided data and personal communications regarding tuatara elsewhere, including Barbara Blanchard (Tuatara Captive Management Plan Coordinator), Tara Atkinson (Orana Wildlife Park), Bruce Benseman and Rhys Mills (Nga Manu Nature Reserve), Anne Richardson (Peacock Springs/Isaac Wildlife Trust), Lindsay Hazley (Southland Museum and Art Gallery) and Mary McIntyre (Stephens Island).
References
- Angilletta MJ Jr, 2009. In: Thermal adaptation: a theoretical and empirical synthesis. Oxford, UK, Oxford University Press.
- Angilletta MJ Jr,, Hill T, Robson M, A. 2002. Is physiological performance optimized by thermoregulatory behavior? A case study of the eastern fence lizard, Sceloporus undulatus. Journal of Thermal Biology 27: 199–204. 10.1016/S0306-4565(01)00084-5
- Autumn K, DeNardo DF 1995. Behavioural thermoregulation increases growth rate in a nocturnal lizard. Journal of Herpetology 29: 157–162. 10.2307/1564552
- Bakken GS 1992. Measurement and application of operative and standard operative temperatures in ecology. American Zoologist 32: 194–216.
- Barwick RE 1982. In: Newman DG (Ed.), New Zealand herpetology. Wellington, New Zealand Wildlife Service. Pp. 225–236. Observations on active thermoregulation in the tuatara, (Reptilia: Rhynchocephalia)
- Besson AA, Cree A 2010. A cold-adapted reptile becomes a more effective thermoregulator in a thermally challenging environment. Oecologia 163: 571–581. 10.1007/s00442-010-1571-y
- Besson AA, Cree A 2011. Integrating physiology into conservation: an approach to help guide translocations of a rare reptile in a warming environment. Animal Conservation14: 28–37.10.1111/j.1469-1795.2010.00386.x
- Besson AA, Nelson NJ, Nottingham CM, Cree A 2012. Is cool egg incubation temperature a limiting factor for the translocation of tuatara to southern New Zealand? New Zealand Journal of Ecology 36: 90–99.
- Blanchard B and the Tuatara Recovery Group 2002. In: Tuatara captive management plan and husbandry manual. Threatened Species Occasional Publication 21. Wellington, New Zealand, Department of Conservation. 75 p.
- Cartland LK, Grimmond NM 1994. The effect of temperature on the metabolism of juvenile tuatara, Sphenodon punctatus. New Zealand Journal of Zoology 21: 373–378. 10.1080/03014223.1994.9518006
- Cartland LK, Cree A, Sutherland WHF, Grimmond NM, Skeaff CM 1994. Plasma concentrations of total cholesterol and triacylglycerol in wild and captive juvenile tuatara (Sphenodon punctatus). New Zealand Journal of Zoology 21: 399–406. 10.1080/03014223.1994.9518009
- Castanet J, Newman DG, Saint Girons H 1988. Skeletochronological data on the growth, age, and population structure of the tuatara, Sphenodon punctatus, on Stephens and Lady Alice Islands, New Zealand. Herpetologica 44: 25–37.
- Christmas E, Coddington E, Cree A 1996. In: A database for toe-clipped tuatara (Sphenodon punctatus) on Stephens Island (Takapourewa). Wildlife Management Report No. 77. Dunedin, New Zealand, University of Otago. 126 p.
- Cree A, Butler D 1993. In: Tuatara recovery plan (Sphenodon spp.). Threatened Species Recovery Plan No. 9. Wellington, New Zealand, Department of Conservation. 71 p.
- Cree A, Cockrem JF, Guillette LJ Jr. 1992. Reproductive cycles of male and female tuatara (Sphenodon punctatus) on Stephens Island, New Zealand. Journal of Zoology (London) 226: 199–217. 10.1111/j.1469-7998.1992.tb03834.x
- Cree A, Daugherty CH, Hay JM 1995. Reproduction of a rare New Zealand reptile, the tuatara Sphenodon punctatus, on rat-free and rat-inhabited islands. Conservation Biology 9: 373–383. 10.1046/j.1523-1739.1995.9020373.x
- Dawbin WH 1982. In: Newman DG (Ed.), New Zealand herpetology. Wellington, New Zealand Wildlife Service. Pp. 237–250. The tuatara : aspects of life history, growth and longevity
- Desser SS 1979. Haematological observations on a hibernating tuatara, Sphenodon punctatus. New Zealand Journal of Zoology 6: 77–78. 10.1080/03014223.1979.10428350
- Gaze P 2001. In: Tuatara recovery plan 2001–2011. Wellington, New Zealand, Department of Conservation.
- Hare JR, Holmes KM, Wilson JL, Cree A 2009. Modelling exposure to selected temperature during pregnancy: the limitations of reptilian viviparity in a cool-climate environment. Biological Journal of the Linnean Society 96: 541–552. 10.1111/j.1095-8312.2008.01151.x
- Hare KM, Caldwell AJ, Cree A 2012. More warmth or more food: effects of early post-natal environment on survival of a lizard. Oecologia 138: 639–649. 10.1007/s00442-011-2145-3
- Hay JM, Daugherty CH, Cree A, Maxson LR 2003. Low genetic divergence obscures phylogeny among populations of Sphenodon, remnant of an ancient reptile lineage. Molecular Phylogenetics and Evolution 29: 1–19. 10.1016/S1055-7903(03)00091-5
- Hay JM, Subramanian S, Millar CD, Mohandesan E, Lambert DM 2008. Rapid molecular evolution in a living fossil. Trends in Genetics 24: 106–109. 10.1016/j.tig.2007.12.002
- Huey RB, Kingsolver JG 1989. Evolution of thermal sensitivity of ectotherm performance. Trends in Ecology and Evolution 4: 131–135. 10.1016/0169-5347(89)90211-5
- Jones MEH 2008. Skull shape and feeding strategy in Sphenodon and other Rhynchocephalia (Diapsida: Lepidosauria). Journal of Morphology 269: 945–966. 10.1002/jmor.10634
- Kearney MR, Shine R, Porter WP 2009. The potential for behavioral thermoregulation to buffer ‘cold-blooded’ animals against climate warming. Proceedings of the National Academy of Sciences, USA 106: 3835–3840. 10.1073/pnas.0808913106
- Miller KA, Nelson NJ, Smith HG, Moore JA 2009. How do reproductive skew and founder group size affect genetic diversity in reintroduced populations? Molecular Ecology 18: 3792–3802. 10.1111/j.1365-294X.2009.04315.x
- Mitchell NJ, Allendorf FW, Keall SN, Daugherty CH, Nelson NJ 2010. Demographic effects of temperature-dependent sex determination: will tuatara survive global warming? Global Change Biology 16: 60–72. 10.1111/j.1365-2486.2009.01964.x
- Mitchell NJ, Nelson NJ, Cree A, Pledger S, Keall SN, Daugherty CH 2006. Support for a rare pattern of temperature-dependent sex determination in archaic reptiles: evidence from two species of tuatara (Sphenodon). Frontiers in Zoology3: doi: 12.1186/1742-9994-3-9.
- Nelson NJ, Keall SN, Brown DD, Daugherty CH 2002. Establishing a new wild population of tuatara (Sphenodon guntheri). Conservation Biology 16: 887–894. 10.1046/j.1523-1739.2002.00381.x
- Nelson NJ, Thompson MB, Pledger S, Keall SN, Daugherty CH 2004. Egg mass determines hatchling size, and incubation temperature influences post-hatching growth, of tuatara Sphenodon punctatus. Journal of Zoology (London) 263: 77–87.
- Saint Girons H, Bell BD, Newman DG 1980. Observations on the activity and thermoregulation of the tuatara, Sphenodon punctatus (Reptilia: Rhynchocephalia), on Stephens Island. New Zealand Journal of Zoology 7: 551–556.
- Terezow MG, Nelson NJ, Markwell TJ 2008. Circadian emergence and movement of captive juvenile tuatara (Sphenodon spp.). New Zealand Journal of Zoology 35: 205–216. 10.1080/03014220809510116
- Thompson MB, Daugherty CH 1998. Metabolism of tuatara, Sphenodon punctatus. Comparative Biochemistry and Physiology 119A: 519–522.
- Towns DR, Daugherty CH 1994. Patterns of range contractions and extinctions in the New Zealand herpetofauna following human colonisation. New Zealand Journal of Zoology 21: 325–339. 10.1080/03014223.1994.9518003
- Towns DR, Ferreira SM 2001. Conservation of New Zealand lizards (Lacertilia: Scincidae) by translocation of small populations. Biological Conservation 98: 211–222. 10.1016/S0006-3207(00)00156-7
- Tyrrell C, Cree A 1994. Plasma corticosterone concentrations in wild and captive juvenile tuatara (Sphenodon punctatus). New Zealand Journal of Zoology 21: 407–416. 10.1080/03014223.1994.9518010
- Walls GY 1983. Activity of the tuatara and its relationship to weather conditions on Stephens Island, Cook Strait, with observations on geckos and invertebrates. New Zealand Journal of Zoology 10: 309–318.
- Whitworth E 2006. Photothermal orientation and factors associated with egg incubation success in tuatara (Sphenodon punctatus). Unpublished MSc thesis, University of Otago, Dunedin, New Zealand.