Abstract
Tubulanus mawsoni (Wheeler 1940) comb. nov. is redescribed based on the material collected during the Spanish Antarctic expedition Bentart-03 through the Bellingshausen Sea (Antarctica). Previously belonging to the genus Carinina Hubrecht 1885, the species is transferred to the genus Tubulanus based on the situation of the brain and lateral nerves between the basement membrane and the outer circular muscle layer, the presence of muscle crosses in the body wall, the position of the blood and excretory systems, the presence of proboscis nerves and the structure of the cerebral sensory organs, the rhynchocoel wall and the rhynchodaeal and buccal nerves. Microphotographs of the different regions of the body and the proboscis structure are provided for the first time. Based on the ambiguities between Tubulanus diagnosis and the species descriptions, as well as the possible paraphyletic status of the genus, the need for new morphological and molecular studies is suggested.
http://zoobank.org/urn:lsid:zoobank.org:pub:544458B6-F448-4CE4-B0D6-946D78903C86
Introduction
In the most recent revision of Antarctic marine benthic diversity by Clarke and Johnston (Citation2003), the Nemertea are considered to be a relatively well-known group in this geographic area. Mahon et al. (Citation2010), using molecular methods, have shown the underestimation of Antarctic nemertean diversity. Although a large number of species of this phylum have been identified from Antarctic and sub-Antarctic waters (Bürger Citation1904; Joubin Citation1910; Baylis Citation1915; Dawson Citation1957; Gibson Citation1985), the class Palaeonemertea is poorly represented in Antarctic waters, by two species (Dawson Citation1969) of the genus Carinina Hubrecht 1885: C. antarctica Bürger Citation1904 and C. mawsoni Wheeler Citation1940. Müller (Citation1965) reviewed the genus and regarded the attribution of these species to this genus doubtful. Carinina antarctica Bürger Citation1904 was considered a nomen dubium by Sundberg and Hylbom (Citation1994).
During the Spanish Antarctic expedition (Bentart-03) nemerteans were collected from several locations, mainly in the Bellingshausen Sea and in surrounding areas at depths ranging from 10 to 2045 m. The Bellingshausen Sea, in the western sector of Antarctica, is one of the least explored Antarctic seas because of the prevalence of ice (Saíz et al. Citation2008, Citationin press); its benthic communities have been researched relatively poorly. The collection includes hoplonemerteans (Uz et al. Citation2010), heteronemerteans (Fernández-Álvarez & Anadón Citation2012) and two specimens of palaeonemerteans. In the current study, one species of Tubulanidae Bürger Citation1904 (Palaeonemertea Hubrecht 1879) is redescribed on the basis of histological sections of the two specimens. Photomicrographs of the different regions of the body and the proboscis structure are provided for the first time. We conclude that morphological characteristics clearly place C. mawsoni in the genus Tubulanus. Therefore, the following new combination is proposed: Tubulanus mawsoni (Wheeler Citation1940) comb. nov.
Material and methods
The material examined was obtained during the ‘Bentart-03’ Spanish Antarctic expedition in February 2003, from the Bellingshausen Sea (Antarctica)—off Peter I Island (Antarctica)—68°56′47″ S, 90°35′13″ W (station 5), aboard the RV Hespérides. Peter I Island is an isolated oceanic island of volcanic origin (Kimura Citation1982) in the Antarctic sector corresponding to the Bellingshausen Sea ().
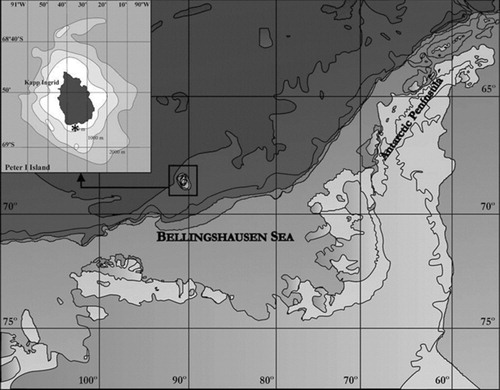
The two specimens available were collected at a depth of 128 m with a Box-corer, from dark muddy-sand and gravel bed (81.1% silt and 0.3% gravel); organic matter (%) 1.431; redox (Eh) value 199.25.
The living animals were anaesthetized in 7.5% MgCl2 in distilled water before fixation in Bouin's fluid. The specimens, for histology, were embedded in paraffin following the Peterfi method (Pantin Citation1968), sectioned at 7 µm, and stained using the Para-pak® (Meridian Biosciences, Inc., Cincinnati, OH, USA) trichrome method.
The ratio of epidermal thickness to body diameter in the brain and intestinal regions was calculated as the index E, following the method of Kajihara (Citation2006). The classification in all taxonomic levels has been taken from Kajihara (Citation2007).
Family Tubulanidae Bürger Citation1904
Carinellidae McIntosh Citation1874: 137.
Tubulanidae Bürger Citation1904: 10; Bürger Citation1897–1907: 401, 402, 405.
Genus Tubulanus Renier Citation1804
Tubulanus Renier 1804: 20.
Carinella Johnston Citation1833: 232; synonymized by Bürger (Citation1904).
Type species
Tubulanus polymorphus Renier 1804 by monotypic designation.
Diagnosis
The following diagnosis was given by Gibson (Citation1994), Gibson and Sundberg (Citation1999), Cantell (Citation2001) and Ritger and Norenburg (Citation2006).
Length at the onset of sexual maturation 2–10 cm; coloured, usually with a conspicuous pattern; head furrows present; body-wall musculature consisting of outer and inner circular and middle longitudinal layers; muscle crosses present between outer and inner circular muscles in some species; cerebral sensory organs consisting of ciliated pits or ciliated canals in the epidermis; side organs present in foregut region in some species; cephalic region with nerve layer; two nerves in rhynchodaeal epithelium; proboscis nerves present; brain and lateral nerves situated between epidermal basement membrane and outer circular muscles; epidermal basement membrane usually thick; buccal nerves present and paired; rhynchocoel wall with circular muscles only; proboscis musculature with inner longitudinal and outer circular layers; circular muscle layer in rear end of rhynchocoelic or in nephridial regions strongly developed; gland-like anterior part of excretory system entering lateral blood vessels; blood vascular system without mid-dorsal vessel; eyes absent; sexes separate.
A complete discussion of the nomenclatural history of the family and the genus is provided by Kajihara (Citation2006, Citation2007) and Ritger and Norenburg (Citation2006), respectively.
Tubulanus mawsoni (Wheeler Citation1940) comb. nov. (–, Appendix 1)
Diagnosis
Tubulanus without a conspicuous colour pattern; blood-pink head; cream-coloured body with a pinkish tinge; body cylindrical in section; head not wider than trunk; epidermal basement membrane thick; dorsal and ventral muscle crosses present between outer and inner circular muscles; diagonal muscles absent; cerebral sensory organs consisting of ciliated pits; side organs absent; with nerve layer; neurochords into lateral nerve cords; muscle plate present between rhynchocoel and gut; intestine without lateral diverticula; excretory system present; rhynchocoel extending almost throughout the body.
Type locality
Antarctica (67°03′ S, 74°29′ E), at 437 m depth.
Material examined
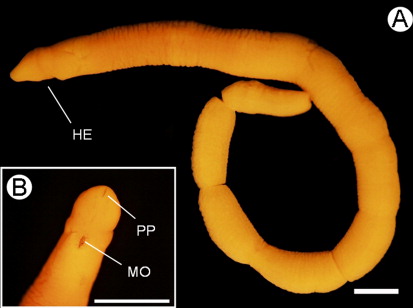
Sexually immature female (), 18 mm long and 1 mm maximum width, February 2003, NA coll.; 7-µm transverse serial sections through the anterior portion of the body to the posterior tip of the body, 39 slides in total (MNCN 5.01/16 to MNCN 5.01/54).
Sexually mature male, 24 mm long and 1.2 mm maximum width, February 2003, NA coll.; 7-µm transverse serial sections through anterior body to foregut region (10 slides) and posterior tip (eight slides), 7-µm longitudinal serial sections in the middle of the body (10 slides) and unsectioned portions of intestinal region and a fragment of everted proboscis, 28 slides in total (MNCN 5.01/55 to MNCN 5.01/82).
The material is deposited at the Museo Nacional de Ciencias Naturales (MNCN), Madrid.
Collecting locality
Off the west Peter I Island, Bellingshausen Sea (Antarctica) (68°56′47″ S, 90°35′13″ W), at 128 m depth.
External features
The anaesthetized living specimens ranged from 2 to 2.5 cm long and 1 to 1.5 mm wide. The cephalic lobe is rounded and distinct, narrower than the trunk. Two pairs of shallow ventral cephalic furrows are present. The mouth is a small compressed slit at the rear of the head situated ventrally (). The body is circular in section while the posterior region is slightly compressed dorsoventrally in the sexually mature specimen. The posterior end of the body presents a slightly pointed tip. The ground colour is cream with a pinkish tinge; head is blood pink with cephalic margins translucent white. In the original description the colour is ‘yellowish-brown at the ends, but brownish-purple in the middle’, but is based in fixed material (Wheeler Citation1940, p. 235) without any note about the colour of the living specimen.
Body wall, musculature and parenchyma
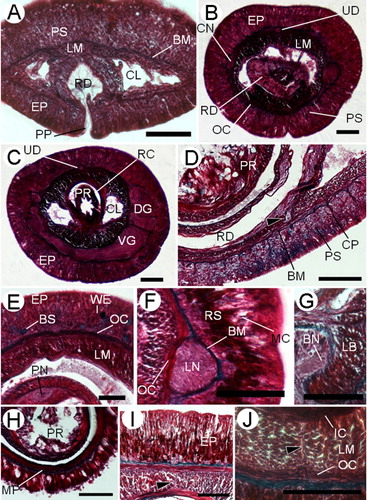
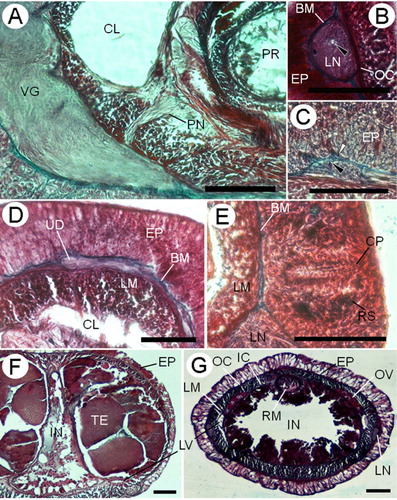
The epidermal thickness varies along the body length. It is thicker in the cephalic (88–131 µm) and medial (72–138 µm) regions than in the posterior region (59–71 µm). In the pre-cerebral region, the ciliated epidermis is pseudostratified; the dorsal side is twice the thickness of the ventral. A well-developed basal stratum (58–82 µm) of pink-stained serous glandular cells () is present in dorsal epidermal tissue and it extends and acquires more prominence in front of the brain (). Behind the ventral cerebral commissure, this cellular stratum is poorly developed dorsally and reaches its maximum height ventrally. Posterior to this, the stratum decreases abruptly. At the level of the ventral cerebral commissure, mucous cells with unstained cytoplasm () appear. In the buccal region, cells with blue-staining mucus and weakly red-stained cells are present (). In the foregut region, the proximal half of the epidermis shows a band of serous or bacillary deep-red-stained secretory cells with small granules; the upper half of the epidermis in this region is dominated by mucous cells (). In the intestinal region, the mucous glandular cells occupy the greater part of the height of the epidermis at the expense of the deep-red-stained cells. Towards the back, the epidermis becomes progressively a monostratified tissue. In the posterior intestinal region the mucous cells are nearly the only glandular cell type observed. E(b)=0.13; E(i)=0.09.
In the preserved specimens the fixation band typical of several Tubulanus species is not observed. However, in serial transverse sections our specimens exhibit differences in cell type distribution (, ; in anteroposterior sequence), which agrees with the observation by Ritger and Norenburg (Citation2006; ) observation. In accordance with what these authors presented, the fixation band is observable in formalin- and ethanol-fixed specimens. The material examined in this paper is preserved in Bouin's fluid.
The epidermal basement membrane is up to 6.2 µm thick. Connective tissue processes extend from the basement membrane into the epidermis (), forming a mesh-like network in the pre-cerebral region. A very thin neural layer is observed between the body-wall outer circular muscle layer and the basement membrane.
The body wall comprises outer and inner circular muscle layers and middle longitudinal ones. The body-wall outer circular muscle layer appears immediately anterior to the rhynchopore and acquires width progressively. The body-wall longitudinal musculature is formed at the level of the rhynchodaeum and is always well-developed throughout the body (). In the pre-cerebral region the blood lacunae are pierced by some dorsoventral fibres that connect the dorsal and ventral sides of the outer circular muscle layer ().
In front of the cerebral commissure some horizontal transverse muscle fibres (3–4 µm) derived from the outer circular muscle layer occur in the dorsal part of the longitudinal muscle layer (). These horizontal transverse muscles disappear posteriorly to the buccal cavity.
In the mouth region, the ventral portion of the longitudinal body wall branches off ventrally to both sides of the buccal cavity (). Also, in this region the inner circular musculature is formed and surrounds the rhynchocoel and the gut.
Between the rhynchocoel and the foregut wall, there is a thin muscle plate that is one fibre thick; it extends back almost the posterior part of the rhynchocoel (). No dorsoventral musculature is found post-cerebrally. In the foregut and intestinal regions, dorsal () and ventral () muscle crosses are observed between the two body-wall circular muscle layers in foregut and intestinal regions. The muscle crosses are formed by a scarce number of muscle fibres and are in close connection with the nervous connections between the upper and lower mid-dorsal nerves (). The ventral muscle cross only is visible in several sections of the female specimen and in almost the whole mature male, which could be related with the corporal size.
Parenchymatous connective tissues are poorly developed and present between the gonadal sacs in the mature specimen.
Proboscis apparatus
The proboscis pore opens subterminally in the ventral tip of the head (). The rhynchodaeum forms a tubular chamber lined by slender epithelial cells with long cilia, up to 18.8 µm, and numerous serous, pink-stained cells in its anterior portion. This is a pseudostratified epithelium and its thickness increases back to 125 µm tall. In the posterior rhynchodaeal region, cilia are scarcer and glandular cells are more vacuolated (). The rhynchodaeal serous glands are connected with epidermal similar cells (), as occurs in other tubulanids (Hylbom Citation1957; Senz Citation1994). The rhynchodaeum is enclosed by a thin circular muscle layer (two to four fibres thick).
The rhynchocoel extends throughout almost the entire length of the animal. The proboscis sheath comprises an epithelium and a circular muscle layer (11–16 µm) in contact with the body-wall inner circular muscle layer. In the posterior end of the rhynchocoel, near the retractor muscle insertion, the epithelium and rhynchocoel wall are separated by a deeply staining basal membrane.
The proboscis insertion is situated in front of the mouth at the level of the ventral cerebral commissure (). Few fibres from the ventral side of the body-wall longitudinal musculature contribute to the proboscis insertion. There are two proboscis nerves that originate from each end of the ventral cerebral commissure and penetrate into the proboscis.
The proboscis () is composed of an outer glandular epithelium (70–78 µm), an outer circular muscle layer (6–10 µm), an inner longitudinal muscle layer (19–25 µm) and a thin endothelium. The two proboscis nerves are situated between the glandular epithelium and the outer circular muscle layer (). In the mature male a thin basal membrane is observed between inner longitudinal musculature and endothelium. A bundle of longitudinal retractor muscles (30–66 µm) extends from the end of the proboscis to the proboscis sheath musculature. The proboscis does not exhibit any significant regional histological differentiation.
Digestive system
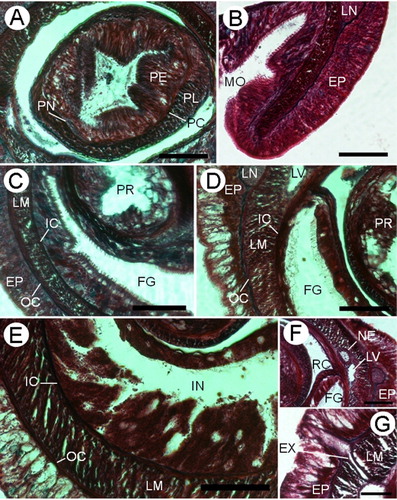
The mouth is a small oval slit that opens just behind the brain (). The buccal cavity is lined by ciliated and glandular cells of several types: serous pink, red and blue-staining with small granules and mucous glandular cells are present (). The foregut epithelium is ciliated and is divided into two different histological regions. The principal elements of the anterior region () are bacillary red and violet-staining gland cells. Mucous glandular cells are present in the anterior region but only in the ventral surface, while at the rear all kinds of gland cells are observed in both surfaces. In the posterior region and anteriad to nephridia, the columnar epithelium bears long cilia and serous red-staining gland cells that are the main constituent (). In the junction between foregut and intestine, the epithelium decreases in thickness. In the intestinal region the epithelium increases in thickness and is deeply folded (). In the posterior tip of the body a thin basal membrane appears between the gut and the body-wall inner circular muscle layer. There are no lateral intestinal diverticula or pouches. The intestine of the mature male specimen is reduced and compressed laterally in the gonadal region (). Behind the rhynchocoel, the gut occupies most of the inner space of the body ().
Blood system
In the head a pair of cephalic blood lacunae lies inside the body-wall outer circular musculature and meet anteriorly above the rhynchodaeal opening. Throughout their length, these lacunae are irregularly subdivided by dorsoventral muscle bundles () that flank the rhynchodaeum and rhynchocoel. Behind the ventral cerebral commissure (), both lacunae reduce their lumen and give rise to the lateral blood vessels (). Cephalic lacunae and lateral blood vessels are lined by a thin endothelium and some circular muscle fibres, surrounded by a scarce amount of parenchymatous tissue. The internal surface of the circular fibres of the lateral blood vessels is in contact with the inner circular muscle layer. In the sexually mature male specimen, these lateral vessels are displaced by gonadal sacs to a ventral position, losing contact with the body-wall inner circular muscle ().
Excretory system
The nephridial excretory system consists of a single pair of main ducts (52–93 µm in diameter) located medial to the longitudinal musculature in the foregut region and close to lateral blood vessels (). In the anterior region, nephridial glands are observed as protrusions in the lateral blood vessels. The nephridia have some branches near the posterior end of the excretory system, which are observed in the sexually mature male specimen and in accordance with the observation of Wheeler (Citation1940, ). The nephridial ducts run forward 623–756 µm and open dorsoventrally by a single excretory pore on either side. The efferent duct is about 12–18 µm in diameter ().
Nervous system
The cerebral ganglia and lateral nerve cords are situated between the epidermal basement membrane and the body-wall outer circular muscle layer (, ). The cerebral ganglia (, ) are well developed and connected by dorsal and ventral commissures, 20–26 µm and 55–70 µm thick, respectively. The dorsal and ventral ganglia are fused and separate only for a short distance in the posterior part of the brain. The ventral ganglia posteriorly extend as the lateral nerve cords (), which possess a single neurochord () asymmetrically distributed into the fibrous core of the lateral nerve cords. An inner neurilemma is present, but an outer neurilemma is not observed. Only in the larger specimen the neurilemma seems distinct from the basement membrane ().
A pair of buccal nerves () arises as a single trunk from the mid-ventral region of the ventral ganglia and runs at the base of the buccal epithelium alongside the mouth and the foregut region; each nerve up to 56 µm in diameter. Each nerve gives rise to a dorsal branch near the middle of the mouth. The buccal nerves disappear in the proximal foregut region.
The slender dorsal commissure gives rise to an upper mid-dorsal nerve () that lies in the base of the epidermis. Only to the anterior to the cerebral region this nerve is conspicuously covered by the basement membrane. It extends to the rear of the rhynchocoel and connects with the lower mid-dorsal nerve.
Several short but distinct cephalic peripheral nerves (24 µm in diameter) run anteriorly from the brain and extend between the epidermis and the basement membrane, forming a cephalic neural layer ().
Sense organs
There are no eyes. The cerebral sensory organs are located just behind the ventral ganglia (). They are two simple ciliated pits, approximately 91 µm deep and 22 µm wide, lined with slightly differentiated epithelium with small reddish glandular cells. There are no statoliths. No evidence of lateral sensory organs was observed.
Reproductive system
Sexes are separate. One specimen is a nearly sexually mature male collected in February (austral summer). The testes are confined to the intestinal region () where gonadal sacs are in a lateral position on each side of the body, above the lateral blood vessels and compressing the gut. The outer surface of the testis sacs is lined by some muscle fibres.
In the immature female specimen, the oogonia are found in the parenchyma, located on the dorsolateral sides of the intestinal region above the lateral blood vessels ().
Discussion
Morphological and histological characters indicate that the two Antarctic palaeonemerteans belong to C. mawsoni Wheeler Citation1940. This author based the description of this species on an incomplete specimen with no more than the stump of the proboscis at its anterior attachment. This species is not described in detail, lacking key data for external and internal characters. Therefore, Sundberg and Hylbom (Citation1994) excluded this species from their cladistic analysis of the class Palaeonemertea. The search for the holotype in the Australian Museum and in the Natural History Museum, London, was unsuccessful and this material is considered lost and not available to study. Despite this, Wheeler's illustrations are very detailed and the identity of the specimens from histological slides is convincing.
In the current paper the species is redescribed using the proposed checklist of the character-matrix (Appendix 1) by Sundberg et al. (Citation2009) to avoid ambiguities in morphological species and genus delimitation. The generic status of this species is controversial: Hylbom (Citation1957), Müller (Citation1965), Friedrich (Citation1970), Kulikova (Citation1984) and Sundberg and Hylbom (Citation1994) have questioned its generic placement. Hylbom (Citation1957) considered this species as belonging to the genus Tubulanus because ‘C. mawsoni is probably a Tubulanus species with the nervous system situated between the basement membrane and the outer circular muscle layer and with the lateral blood vessels and the nephridial canals situated outside the inner circular muscle layer.’ Nevertheless, this second character used by Hylbom to transfer C. mawsoni to Tubulanus is present in C. johnstoni Senz 2000. Although most of the Tubulanus species have a conspicuous body colour pattern, this character is not shared by several species, including T. mawsoni. Other features place this species in the genus Tubulanus: the arrangement of the body-wall muscle layers and the nervous system, the structure of the cerebral sensory organs, the basement membrane, the rhynchocoel wall, the rhynchodaeal and buccal nerves. The presence of muscle crosses between circular muscle layers and the proboscis nerves, considered by Sundberg and Hylbom (Citation1994) as possible synapomorphies for the genus Tubulanus, supports the inclusion of this species into Tubulanus.
As in most of the nemertean genera, several species allocated to Tubulanus are considered not well-described species (see Sundberg and Hylbom Citation1994). In addition, the character ‘intestine with shallow lateral diverticula’ is not present in some of the species assigned to Tubulanus, as occurs in T. hylbomi Gibson and Sundberg Citation1999, T. longivasculus Gibson and Sundberg Citation1999, T. riceae Ritger and Norenburg Citation2006, nor in T. mawsoni. Similarly, no body-wall diagonal muscles are reported in several species, including T. hylbomi, T. longivasculus and T. mawsoni, but these may be difficult to detect. Although the Tubulanus diagnosis specifies ‘nervous system with neither neurochords nor neurochord cells’, neurochord cells are present in T. riceae, whereas in the current redescribed species a single neurochordal processes in the lateral nerves is observed. To avoid ambiguities, these three characters are deleted in the diagnosis for the genus Tubulanus provided here. Both cladistic and molecular phylogenetic studies (Sundberg and Hylbom Citation1994; Andrade et al. Citation2012, respectively) point to a possible paraphyletic status of the genus Tubulanus. All these facts lead to the need to carry out more taxonomic and phylogenetic studies so as to clarify the status of this genus and to avoid the inconsistencies between the genus diagnosis and the species descriptions.
To date, the only two species of palaeonemerteans recorded for Antarctica are C. antarctica and C. mawsoni (Dawson Citation1957, Citation1957). But as C. antarctica is considered nomem dubium (Sundberg and Hylbom Citation1994), T. mawsoni (Wheeler Citation1940) comb. nov. is the only valid palaeonemertean species recorded for Antarctic waters. However, molecular survey of Antarctic planktonic larvae, Mahon et al. (Citation2010) found 19 distinct Antarctic lineages of hoplonemerteans and heteronemerteans not previously genetically characterized and probably representing undescribed taxa. The absence of molecular data for Antarctic palaeonemerteans in the study of Mahon et al. (Citation2010) may reflect scarcity of planktonic larvae or that they were not sampled. Future morphological and molecular studies may reveal greater species richness of palaeonemerteans in Antarctic waters.
Acknowledgements
The Bentar-03 cruise was supported by the Antarctic Programme REN2001 1074/ANT of the Spanish Government. We are grateful to Dr Ana Ramos, the leader of Bentart's projects and to our colleagues from Bentart cruise 2003. Thanks are due to the crew and UTM technicians of RV Hespérides. We greatly appreciate Helen Stoddart (Australian Museum) and Emma Sherlock (The Natural History Museum, London) for their help in the search of Wheeler's material. We would like to thank C. Lastra for assistance and linguistic review of the text of an earlier draft of the manuscript. Thanks to three anonymous reviewers for their helpful comments and suggestions, which substantially improved the manuscript. Javier Cristobo of the Spanish Oceanographic Institute provided the map in .
References
- Andrade SCS, Strand M, Schwartz M, Chen H, Kajihara H, von Döhren J, Sun S, Junoy J, Thiel M, Norenburg JL, Turbeville JM, Giribet G, Sundberg P 2012. Disentangling ribbon worm relationships: multi-locus analysis supports traditional classification of the phylum Nemertea. Cladistics 28: 141–159. 10.1111/j.1096-0031.2011.00376.x
- Baylis HA 1915. Nemertinea, British Antarctic Terra Nova Expedition 1910. Zoology 2: 113–134.
- Bürger O 1904. Nemertinen. Resultats du voyage du S. Y. Belgica en 1897–1899. Zoologie: 1–10.
- Bürger O 1897–1907. Nemertini (Schnurwürmer). InBronn HG,Winter'sche Verlagshandlung CF. Klassen und Ordnungen des Tier-Reichs, wissenschaftlich dargestellt in Wort und Bild, Vol. 4 Suppl. Leipzig, Germany, CF Winter. Pp. 1–542.
- Cantell CE 2001. On the anatomy and taxonomy of Tubulanus lutescens n. sp. (Nemertini) from the west coast of Sweden. Ophelia 54: 213–221. 10.1080/00785236.2001.10409467
- Clarke A, Johnston NM 2003. Antarctic marine benthic diversity. Oceanography and Marine Biology: an Annual Review 41: 47–114.
- Dawson EW 1957. Checklist of Marine Nemertines from Antarctica. Antarctic Research Committee 22: 1–10.
- Dawson EW 1969. Nemertea. Antarctic Map Folio Series 11: 18–21.
- Fernández-Álvarez FÁ, Anadón N 2012. Oligodendrorhynchus hesperides gen. et sp. n. (Heteronemertea) from the Bellingshausen Sea. Polish Polar Research 33: 81–98.
- Friedrich H 1970. Nemertinen aus Chile. Sarsia 40: 1–80.
- Gibson R 1985. Antartic nemerteans: heteronemertea—description of new taxa, reappraisal of the systematic status of existing species and a key to heteronemerteans recorded south of latitude 50°S. Journal of the Linnean Society of London (Zoology) 83: 95–227. 10.1111/j.1096-3642.1985.tb01176.x
- Gibson R 1994. In: Nemerteans: keys and notes for identification of the species. Shrewsbury, UK, The Linnean Society of London and The Estuarine and Coastal Sciences Association, Field Studies Council, 224 pp.
- Gibson R, Sundberg P 1999. Six new species of palaeonemerteans (Nemertea) from Hong Kong. Zoological Journal of the Linnean Society 125: 151–196. 10.1111/j.1096-3642.1999.tb00590.x
- Hylbom R 1957. Studies on palaeonemerteans of the Gullmar Fiord area (West Coast of Sweden). Arkiv för Zoologi 10: 539–582.
- Johnston G 1833. Illustrations in British zoology. Magazine of Natural History 6: 232–235.
- Joubin L 1910. Nemertinea. National Antarctic Expedition, 1901–1904. 5: 1–15.
- Kajihara H 2006. Four palaeonemerteans (Nemertea: Anopla) from a tidal flat in middle Honshu, Japan. Zootaxa 1163: 1–47.
- Kajihara H 2007. A taxonomic catalogue of Japanese nemerteans (phylum Nemertea). Zoological Science. 24(4): 287–326. 10.2108/zsj.24.287
- Kimura K 1982. Geological and geophysical survey in the Bellingshausen Basin, off Antarctica. Antarctic Record 75: 12–24.
- Kulikova VI 1984. Novyi vid nemertiny roda Carinina (Palaeonemertini, Tubulanidae) iz belogo morya. Zoologicheskii zhurnal 63: 1092–1095.
- Mahon AR, Thornhill DJ, Norenburg JL, Halanych KM 2010. DNA uncovers Antarctic nemertean biodiversity and exposes a decades-old cold case of asymmetric inventory. Polar Biology 33: 193–202. 10.1007/s00300-009-0696-0
- McIntosh WC 1874. In: A monograph of the British Annelids Part I: the Nemerteans. London, UK, Ray Society, 214 p.
- Müller GI 1965. Carinina heterosoma n. sp. si citeva consideratii asupra genului Carinina (Vermes, Palaeonemertini). Hidrobiologia 6: 243–257.
- Pantin CFA 1968. In: Técnicas microscópicas para zoólogos. León, Spain, Editorial Academia León. 97 pp.
- Renier SA 1804. In: Prospetto Della Classe dei Vermi, nominati e ordinati secondo il sistema di bosc. Padua, Italy. 38 p. xv–xxvii. [An uncompleted work issued in 1804.].
- Ritger RK, Norenburg JL 2006. Tubulanus riceae new species (Nemertea: Anopla: Palaeonemertea: Tubulanidae), from South Florida, Belize and Panama. Journal of Natural History 40: 931–942. 10.1080/00222930600833867
- Saíz JI, Anadón N, Cristobo FJ, García-Álvarez O, García-Castrillo G, López E, Palacín C, Troncoso JS, Ramos A. in press. Enhancement of the benthic communities around an isolated island in the Antarctic Ocean. Acta Oceanologica Sinica.
- Saíz JI, García FJ, Manjón-Cabeza ME, Parapar J, Peña-Cantero A, Saucède T, Troncoso JS, Ramos A 2008. Community structure and spatial distribution of benthic fauna in the Bellingshausen Sea (West Antarctica). Polar Biology 31: 735–743. 10.1007/s00300-008-0414-3
- Senz W 1994. On the occurrence the cephalic gland in the epidermis and rhynchodaeal wall in nemerteans. Bonner Zoologische Beiträge 45: 79–86.
- Sundberg P, Chernyshev AV, Kajihara H, Kanneby T, Strand M 2009. Character-matrix based descriptions of two new nemertean (Nemertea) species. Zoological Journal of the Linnean Society 157: 264–294. 10.1111/j.1096-3642.2008.00514.x
- Sundberg P, Hylbom R 1994. Phylogeny of the nemertean subclass Palaeonemertea (Anopla, Nemertea). Cladistics 10: 347–402. 10.1111/j.1096-0031.1994.tb00185.x
- Uz S de la, Anadón N, Crandall FB 2010. Redescription of Valdivianemertes valdiviae (Bürger, 1909) (Nemertea: Hoplonemertea: Cratenemertidae) and designation of neotype. Journal of Natural History44:37: 2425–2451.
- Wheeler JFG 1940. Nemerteans of Kerguelen and the southern ocean. B. A. N. Z. Antarctic Research Expedition Report, Series B 4: 233–256.