Abstract
Morphological features of a collection of unknown-age wild kiwi (Apteryx mantelli) embryos from early development to point of hatch are described. Using these features, we assign developmental stages to each embryo and compare the progress of development to similar-staged ostrich (Struthio camelus) and chicken (Gallus gallus) embryos. Two ageing schemes for the kiwi embryos are developed by comparing measurements of their hindlimb segments, bills and crown–rump lengths with those of ostrich and chicken embryos at various stages of development. One of the 20 kiwi embryos was of known age. Both the ostrich model and the chicken model gave identical predictions for the marker and four other embryos. Developmental timing of some features differed between all three species, most markedly in the bill, with growth in the kiwi bill being relatively faster to achieve its larger relative and absolute size at hatch.
Introduction
Studies of avian embryo growth and development are not common, but exist for poultry (Buckner et al. Citation1950; Hamburger & Hamilton Citation1951; Daniel Citation1957; Fant Citation1957; Mun & Kosin Citation1960; Ancel et al. Citation1995), some waterfowl (Weller Citation1957; Cooper & Batt Citation1972; Caldwell & Snart Citation1974; Montgomery et al. Citation1978) and some seabirds (Ryder & Somppi Citation1977; Mahoney & Threlfall Citation1981). The series by Hamburger & Hamilton (Citation1951) of normal stages of development in the chicken act as a basis for most modern embryological comparisons. They divide the developmental process into 46 stages based on the presence or absence of morphological characteristics independent of incubation length and embryo size. All avian embryos pass through the same series of developmental events (Ricklefs & Starck Citation1998), but the exact chronology of these events can vary between species even in the earliest stages of development, suggesting that they are not as rigidly conserved as once thought (Richardson Citation1999; Lilja et al. Citation2001; Blom & Lilja Citation2005). Previous studies of morphological development of the embryo of the kiwi (Apteryx spp.) are limited to the observations of Parker (Citation1891, Citation1892).
Kiwi eggs are unusually large for a bird its size, weighing up to 25% of the female kiwi's body weight, or approximately 400 g (Reid & Williams Citation1975; Calder Citation1979). The incubation period is also exceptionally long, typically ranging from 74 to 84 days in the wild (Reid & Williams Citation1975) but it may extend to 91 days (Colbourne Citation2002). Artificially incubated eggs typically hatch at 78 days (Bassett Citation2012).
The kiwi's large egg size and long incubation period likely reflect benefits in retaining an ancestrally large egg in an environment devoid of mammalian predators (Calder Citation1979; Potter & McLennan Citation1992). The relatively recent impact of mustelid predation on kiwi chicks has caused a rapid decline in population size, making all kiwi species threatened and requiring active management (Heather & Robertson Citation2005; Holzapfel et al. Citation2008). Limited access to kiwi and their eggs and their status as taonga species (i.e. of cultural significance to the native Māori people [Holzapfel et al. Citation2008]) constrains the availability of specimens and precludes sacrificing healthy embryos for study of normal development. To learn more about the kiwi's embryological development with few available samples, a developmental analogue must be sought for initial comparison.
This study examined the development and growth of the morphological features of brown kiwi (Apteryx mantelli) embryos by comparison with two other precocial species: the chicken (Gallus gallus), which has a comparable adult body size to kiwi; and the ostrich (Struthio camelus), which is closely related phylogenetically to kiwi (Livezey & Zusi Citation2007). By using detailed descriptions of a collection of kiwi embryos of unknown age, we related developmental stage-defining features of all three species to establish an ageing scheme based on morphological measurements. Based on our analyses, an embryo's developmental stage can be used to estimate the date the egg was laid, the projected date of hatch, or when an embryo died, and to identify critical or high-risk stages of incubation.
Materials and methods
Twenty preserved embryos of wild brown kiwi (Apteryx mantelli) that had died during incubation were examined. Only one of the embryos (designated embryo I*) was of known age (38 days from date of laying with incubation starting immediately).
Developmental stages for each kiwi embryo were determined using Hamburger & Hamilton's (Citation1951) embryo staging methods for domestic chickens. Hamburger and Hamilton stages were also assigned to the ostrich embryo descriptions given in Gefen & Ar (Citation2001). Determination of the stages of development was based on external features only, such as presence of limb buds, limb segments, bill, feathers, nails and scales. The kiwi embryo was presumed to have completed the same portion of incubation as a chicken and as an ostrich at the same stage (which have incubation lengths of 21 days and 42 days, respectively). The age estimations given by these methods are referred to as the chicken and the ostrich models. Various incubation lengths have been reported for brown kiwi: 74–84 days (Reid & Williams Citation1975), 77–106 days (McLennan Citation1988), 75 days and 91 days (Colbourne Citation2002), and 65–75 days (Heather & Robertson Citation2005). Therefore, the models used both a 78-day and an 85-day incubation length to report an age range for each kiwi embryo. This range of incubation durations was chosen to avoid biasing age estimates by outliers.
Morphological measurements were taken with Vernier calipers (± 0.2 mm) or string and ruler (crown–rump length only). Limb segments were measured on the right hindlimb while it was flexed as much as possible. This was only possible for embryos F–T because of the lack of distinguishable limb segments in earlier embryos. Limb segment lengths included digit III, the longest (i.e. middle) toe (from the proximal phalangeal joint to the distal point of the nail), the tarsometatarsus (from the tarsometatarsal to the proximal phalangeal joint), the tibiotarsus (from the stifle to the tarsometatarsal joint), and the femur (from the greater trochanter of the femur to the stifle joint). Bill length was measured from the distal tip of the bill to the distal end of the cere at the midpoint of the cere's curve. Crown–rump length was measured in all embryos from the cere of the bill to the pygostyle.
Limb segment lengths for embryos F–L were linearly regressed against embryo age as predicted by both the chicken and the ostrich models, assuming both a 78-day (minimum) and an 85-day (maximum) incubation period. For bill-length measurements, a second-degree polynomial regression was fitted. The eight late-stage embryos (M–T) had reached a point in their incubation where they no longer had diagnostic features that could be used to allocate a stage beyond 40. In chickens, Hamburger & Hamilton (Citation1951) determined stages 40–45 by measuring toe and bill lengths. Here, we inserted the toe length of embryos M–T into the toe length regression equation produced using embryos F–L to create age estimations.
To validate our models, a mean bill measurement of 42.8 mm for a point-of-hatch wild kiwi chick (age 0–3 days, lengths 44.5 mm; 42.0 mm; 43.0 mm; 41.6 mm, John McLennan, unpublished data) was used in the bill-length regression line equations to calculate an estimation of incubation length.
Each embryo was photographed with a Fujifilm FinePix S200EXR digital camera using a tripod and light table.
Results
Embryo staging was most effectively determined using limb features, especially the toes. The presence of feathers or degree of joint bending were not useful criteria in this collection as many embryos were preserved in unnatural positions or had suffered varying degrees of damage ().
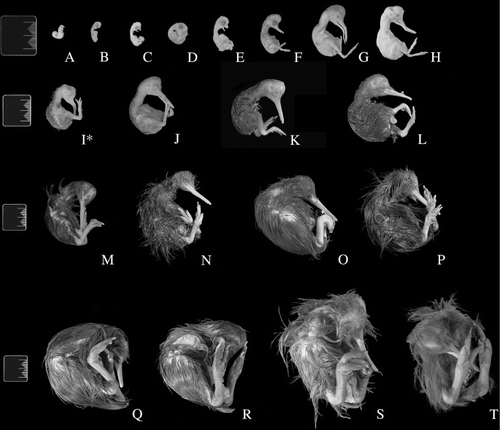
Table 1 Estimation of Hamburger and Hamilton (HH) stage for kiwi embryos.
Differences in timing of head and bill development between the kiwi and the chickens became apparent around Hamburger & Hamilton's (Citation1951) stage 28 (, ). Embryo C showed a more advanced mandible than the corresponding stage in the chicken. The appearance of feather germs and papillae in the kiwi lagged behind the chicken and did not become apparent until approximately stage 35 in embryo F. As our series is missing stages 32–34, it is possible that feather development occurs earlier. Eyelid development was not apparent to the extent of that in the ostrich and chicken in embryos E and F.
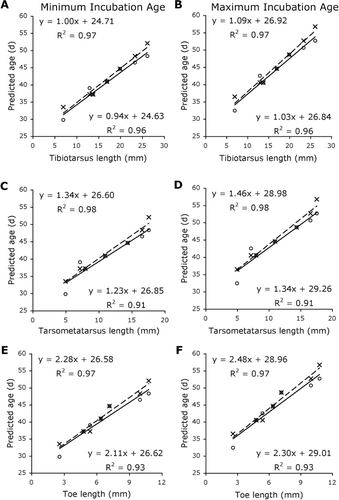
The best measurements for ageing kiwi embryos as determined by the ostrich model were crown–rump length and tarsometatarsus length (, ). In contrast, bill length and tibiotarsus length gave the best predictions in the chicken model (, ). The least useful measurement was femoral length (), possibly because of the difficulty in measuring this parameter in these specimens. In embryos A–J, both the ostrich and chicken models gave similar age predictions. An estimation of 40.9–44.5 days for the 38-day-old, known-age embryo (I*) was produced by both the ostrich and chicken models. In embryos K–Q, the ostrich model gave somewhat higher age estimations than the chicken model.
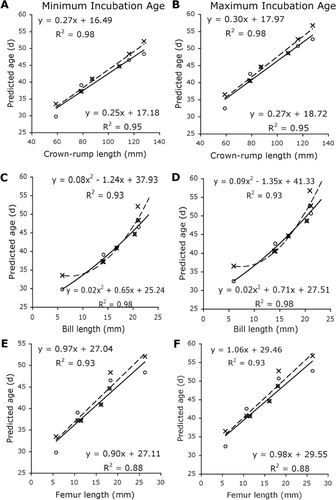
Bill growth differed from the other parameters measured and was best described by a second-degree polynomial equation. The regression equation obtained using the ostrich model indicated a greater bill growth rate than that generated using the chicken model. When both models were tested using 42.8 mm as a mean bill length for a wild kiwi at time of hatch, the ostrich model predicted a much longer incubation length of 131.4–148.4 days than the chicken model's prediction of an 89.7–94.5-day incubation length.
Discussion
This study of kiwi embryo development in relation to estimated age is a necessary first step towards a better understanding of kiwi embryology. Like Parker (Citation1891), we found that the length of the embryonic kiwi hindlimb increased rapidly and regularly in a linear fashion. Bill growth was well-described by a second-degree polynomial regression, with the ostrich model predicting a slower rate of growth than the chicken model. The bill growth rate predicted by the ostrich model for the kiwi is also similar to the rate found in ostrich embryos (Gefen & Ar Citation2001). Despite the ostrich being a much larger bird than the kiwi, the kiwi must attain a longer bill length relative to head size than the ostrich. Our data suggest that kiwi embryos achieve this by growing their bill at a similar rate to ostrich embryos but that they maintain this growth rate throughout their much longer incubation period, which has the potential to be twice as long as the 42-day incubation period for ostrich eggs.
Neither the chicken model nor the ostrich model gave a better estimate of incubation age for the known-age embryo, with both models over-estimating the age of this embryo by 2–7 days. In contrast, middle stages of development had similar age estimations for the kiwi embryos using both models. In later stages, the estimations diverged, with the ostrich model producing older estimated ages than the chicken model. This divergence may indicate that early embryo development follows similar patterns in the chicken and ostrich, but different patterns in late development. For the oldest embryo specimens, which were probably at or close to point of hatch, estimations agree well with the maximum incubation length used in this study. The marked difference between the two estimations of incubation length that was produced by using an average wild hatchling bill size may indicate that the ostrich model does not accurately describe kiwi bill growth throughout the incubation period. At this point, it is unclear whether the chicken, which is more similar to the kiwi in size, or the ostrich, which has a closer phylogenetic relationship, better predicts kiwi embryo age. However, good agreement between ages predicted by the ostrich and the chicken models supports the presented ageing scheme as a useful, but not exact, tool, and highlights the need for validation of this study with more known-age embryos.
The exact mechanism of an embryo's growth rate is not entirely clear, but may result from an antagonistic relationship between ‘supply’ organs such as the intestines and ‘demand’ organs such as the muscle or bone rather than the chick's postnatal developmental mode (Starck Citation1994; Lilja et al. Citation2001). The developmental mode is traditionally determined by a chick's physical characteristics, behaviour and level of self-sufficiency at hatch, where the most altricial birds hatch completely dependent upon their parents and the most precocial require little to no parental care (Nice Citation1962; Starck & Ricklefs Citation1998). A bird's precociality is not simply a measure of its postnatal growth rate, which is generally, but not exclusively, slower in precocial birds (Ricklefs Citation1968, Citation1973, Citation1979a,Citationb; Starck Citation1998), but rather a measure of its functional maturity at the time of hatching. In the case of a precocial species such as the kiwi, ostrich and chicken, the limbs must achieve a great deal of functional capability during the incubation period for the chick to walk and feed independently soon after it hatches.
The rate and timing of different embryonic tissues developing functional maturity differs from species to species (Blom & Lilja Citation2005) and possibly within species (Lilja et al. Citation2001) and is a consequence of selection for late ontogenetic characters (Richardson Citation1999). Gefen & Ar (Citation2001) observed temporal differences in several structures between ostrich and chicken embryos, much as we found differences between kiwi embryos and both ostrich and chicken embryos. The relatively rapid growth of the kiwi bill became apparent early in the embryo series, at Embryo C, and persisted throughout development to produce the large characteristic bill that allows for feeding and highly specialized prey detection (Cunningham et al. Citation2007; Martin et al. Citation2007). The fast embryonic growth leading to advanced function of the kiwi's limbs and bill during incubation may restrict the ability of those tissues to grow quickly during postnatal development (Kirkwood et al. Citation1989; Ricklefs et al. Citation1994; Starck Citation1994), as is evidenced by kiwi not reaching skeletal maturity until 4–6 years old (Beale Citation1991; Bourdon et al. Citation2009).
The obvious limitations of this study are the use of embryos of non-ideal quality that may obscure developmental landmarks, of unknown age and with uncertain cause of death, all of which make it possible that these embryos may not accurately represent normal development. The sample population is also small and skewed towards older embryos. The abundance of late-stage embryos is likely to be a consequence of the BNZ Operation Nest Egg™ programme where kiwi eggs are actively sought and collected from the wild in order to be hatched in captivity. Eggs are less likely to be found until the later stages and the more fragile early-stage embryos are more likely to be damaged or destroyed during collection or to have decayed beyond use by the time the egg is collected. The conservation status of kiwi does not allow for the sacrifice of healthy embryos. Validation of current embryo staging models is necessary, so as more specimens become available, they should be used for this purpose.
In conclusion, by providing detailed descriptions of a collection of kiwi embryos of unknown age, we have directly related the development stages and defining features of the chicken, the ostrich and the kiwi to establish an ageing scheme for brown kiwi embryos based on morphological measurements. This model is of use to conservation management in estimating the date of lay, the date when the embryo would have been expected to hatch, or when the embryo died. Our model may also be of use in identifying critical stages of incubation for kiwi embryo growth and development.
Acknowledgements
We would like to acknowledge the financial assistance provided by the Frank M. Chapman Memorial Foundation. We also thank the Department of Conservation for supplying the eggs used in this study.
References
- Ancel A, Liess S, Girard H 1995. Embryonic development of the domestic guinea fowl (Numida meleagris). Journal of Zoology, London 235: 621–634. 10.1111/j.1469-7998.1995.tb01773.x
- Bassett S 2012. Operation Nest Egg incubation and chick rearing best practice protocols. Wellington, New Zealand, Department of Conservation.
- Beale G 1991. The maturation of the skeleton of a kiwi (Apteryx australis mantelli) – a ten year radiological study. Journal of the Royal Society of New Zealand 21: 219–220. 10.1080/03036758.1991.10431408
- Blom J, Lilja C 2005. A comparative study of embryonic development of some bird species with different patterns of postnatal growth. Zoology 108: 81–95. 10.1016/j.zool.2005.02.001
- Bourdon E, Castanet J, de Ricqlès A, Scofield P, Tennyson A, Lamrous H et al. 2009. Bone growth marks reveal protracted growth in New Zealand kiwi (Aves, Apterygidae). Biology Letters 5: 639–642. 10.1098/rsbl.2009.0310
- Buckner CD, Jr. WMI, Harms A, Wachs HF, Wachs EF 1950. The comparative rates of growth and calcification of the femur, tibia and metatarsus bones of the male and female New Hampshire chicken having straight keel. Poultry Science 29: 332–335. 10.3382/ps.0290332
- Calder III WA 1979. The kiwi and egg design: evolution as a package deal. BioScience 29: 461–467. 10.2307/1307538
- Caldwell PJ, Snart AE 1974. A photographic index for aging mallard embryos. The Journal of Wildlife Management 38: 298–301. 10.2307/3800736
- Colbourne R 2002. Incubation behaviour and egg physiology of kiwi (Apteryx spp.) in natural habitats. New Zealand Journal of Ecology 26: 129–138.
- Cooper JA, Batt BDJ 1972. Criteria for aging giant Canada goose embryos. The Journal of Wildlife Management 36: 1267–1270. 10.2307/3799260
- Cunningham S, Castro I, Alley M 2007. A new prey-detection mechanism for kiwi (Apteryx spp.) suggests convergent evolution between paleognathous and neognathous birds. Journal of Anatomy 211: 493–503.
- Daniel JC, Jr. 1957. An embryological comparison of the domestic fowl and the red-winged blackbird. The Auk 74: 340–358. 10.2307/4081925
- Fant RJ 1957. Criteria for aging pheasant embryos. The Journal of Wildlife Management 21: 324–328. 10.2307/3796552
- Gefen E, Ar A 2001. Morphological description of the developing ostrich embryo: a tool for embryonic age estimation. Israel Journal of Zoology 47: 87–97. 10.1560/H2T8-1H2U-81H1-P5XY
- Hamburger V, Hamilton HL 1951. A series of normal stages in the development of the chick embryo. Journal of Morphology 88: 49–92. 10.1002/jmor.1050880104
- Heather B, Robertson H 2005. The field guide to the birds New Zealand. New York, NY, Oxford University Press. 1998 p.
- Holzapfel SA, Robertson HA, McLennan JA, Sporle W, Hackwell K, Impey M 2008. Kiwi (Apteryx spp.) recovery plan 2008–2018. Threatened species recovery plan 60. Wellington, New Zealand, Department of Conservation.
- Kirkwood J, Duigan P, Kember N, Bennet P, Price DJ 1989. The growth rate of the tarsometatarsus bone in birds. Journal of Zoology, London 217: 403–416. 10.1111/j.1469-7998.1989.tb02498.x
- Lilja C, Blom J, Marks HL 2001. A comparative study of embryonic development of Japanese quail selected for different patterns of postnatal growth. Zoology 104: 115–122. 10.1078/0944-2006-00016
- Livezey BC, Zusi RL 2007. Higher-order phylogeny of modern birds (Theropoda, Aves: Neornithes) based on comparative anatomy. II. Analysis and discussion. Zoological Journal of the Linnean Society 149: 1–95. 10.1111/j.1096-3642.2006.00293.x
- Mahoney SP, Threlfall W 1981. Notes on the eggs, embryos and chick growth of common guillemots Uria aalge in Newfoundland. Ibis 123: 211–218. 10.1111/j.1474-919X.1981.tb00928.x
- Martin GR, Wilson K-J, Wild JM, Parsons S, Kubke MF, Corfield J 2007. Kiwi forego vision in the guidance of their nocturnal activities. PLoS ONE 2: e198. 10.1371/journal.pone.0000198
- McLennan JA 1988. Breeding of North Island brown kiwi, Apteryx australis mantelli, in Hawkes Bay, New Zealand. New Zealand Journal of Ecology 11: 89–97.
- Montgomery RA, Burke CJ, Byers SM 1978. A field guide to the aging of wood duck embryos. The Journal of Wildlife Management 42: 432–437. 10.2307/3800287
- Mun AM, Kosin IL 1960. Developmental stages of the broad breasted bronze turkey embryo. Biological Bulletin 119: 90–97. 10.2307/1538937
- Nice MM 1962. Development of behavior in precocial birds. Transactions of the Linnean Society (NY) 8: 1–211.
- Parker TJ 1891. Observations on the anatomy and development of Apteryx. Philosophical Transactions of the Royal Society of London. B 182: 25–134. 10.1098/rstb.1891.0002
- Parker TJ 1892. Additional observations on the development of Apteryx. Philosophical Transactions of the Royal Society of London. B 183: 73–84. 10.1098/rstb.1892.0005
- Potter M, McLennan J 1992. Kiwi's egg size and moa. Nature 358: 548–548. 10.1038/358548b0
- Reid B, Williams GR 1975. VII. The Kiwi. In: Kuschel G ed. Biogeography and Ecology in New Zealand. The Hague, Auckland, Dr. W. Junk b.v. Publishers.
- Richardson MK 1999. Vertebrate evolution: the developmental origins of adult variation. BioEssays 21: 604–613. 10.1002/(SICI)1521-1878(199907)21:7%3C604::AID-BIES9%3E3.0.CO;2-U
- Ricklefs RE 1968. Patterns of growth in birds. Ibis 110: 419–451. 10.1111/j.1474-919X.1968.tb00058.x
- Ricklefs RE 1973. Patterns of growth in birds. II. Growth rate and mode of development. Ibis 115: 177–201. 10.1111/j.1474-919X.1973.tb02636.x
- Ricklefs RE 1979a. Patterns of growth in birds. V. A comparative study of development in the starling, common tern, and Japanese quail. The Auk 96: 10–30.
- Ricklefs, RE 1979b. Adaptation, constraint, and compromise in avian postnatal development. Biological Reviews 54: 269–290. 10.1111/j.1469-185X.1979.tb01013.x
- Ricklefs RE, Shea RE, Choi I-H 1994. Inverse relationship between functional maturity and exponential growth rate of avian skeletal muscle: a constraint on evolutionary response. Evolution 48: 1080–1088. 10.2307/2410368
- Ricklefs RE, Starck JM 1998. Embryonic growth and development. In: Starck JM, Ricklefs RE ed. Avian growth and development. Evolution within the altricial–precocial spectrum. New York, Oxford University Press. Pp. 31–58.
- Ryder JP, Somppi L 1977. Growth and development of known-age ring-billed gull embryos. The Wilson Bulletin 89: 243–252.
- Starck JM 1994. Quantitative design of the skeleton in bird hatchlings: does tissue compartmentalization limit posthatching growth rates? Journal of Morphology 222: 113–131. 10.1002/jmor.1052220202
- Starck JM 1998. Structural variants and invariants in avian embryonic and postnatal development. In: Starck JM, Ricklefs RE ed. Avian growth and development. Evolution within the altricial-precocial spectrum. New York, Oxford University Press. Pp. 59–88.
- Starck JM, Ricklefs RE 1998. Patterns of development: the altricial-precocial spectrum. In: Starck JM, Ricklefs RE ed. Avian growth and development. Evolution within the altricial–precocial spectrum. New York, Oxford University Press. Pp. 3–30.
- Weller MW 1957. Growth, weights, and plumages of the redhead, Aythya americana. The Wilson Bulletin 69: 4–38.
