Abstract
New Zealand geckos face a range of threats yet, for most species, basic biological information necessary for conservation management is lacking. The southern North Island forest gecko (Mokopirirakau ‘Southern North Island’) is an arboreal gecko about which little has been published. We used systematic searches and radio telemetry at Otari-Wilton's Bush in Wellington to investigate its habitat use and movement patterns. Nearly 30 hours of searching in 2011 and 2012 resulted in 47 gecko sightings at a rate of 1.58 geckos per person hour. Six geckos were radio-tracked for 7–13 days in January 2012. Geckos displayed diurnal and nocturnal activity, but moved greater distances in the middle of the day. They moved on average 9.5 m in 24 hours. Geckos were mainly arboreal and usually located on the trunks and branches of trees and shrubs, and used retreats irregularly. This exposed behaviour may increase the vulnerability of this species to predation by introduced mammalian predators.
Introduction
Effective species conservation requires basic understanding of biology. This is often lacking for arboreal vertebrates in general (Kays & Allison Citation2001), and reptiles in particular because their cryptic habits make them difficult to study (Ellinger et al. Citation2001; Fitzgerald et al. Citation2002; Neilson et al. Citation2004). Geckos that are small, arboreal and nocturnal are particularly difficult to monitor (Bell Citation2009). New Zealand has 36 species of endemic Diplodactylid geckos (Nielsen et al. Citation2011; Nielsen's taxonomy is used throughout this paper), few of which have been studied in detail. Knowledge of arboreal gecko habitat use and behaviour is needed to optimise sampling techniques, accurately evaluate population trends and identify responses to management interventions (Hare & Cree Citation2005; Hare et al. Citation2007; Hoare et al. Citation2007; Bell Citation2009; Hoare et al. Citation2013).
The arrival of humans in New Zealand about 800 years ago has had a detrimental effect on indigenous geckos. Habitat destruction and the introduction of mammals, especially rats, have caused the extinction of Hoplodactylus delcourti and range contractions of many other gecko species (Towns & Daugherty Citation1994). Since the mid-1980s rats have been systematically eliminated from many offshore islands with the aim of restoring indigenous island systems (Towns & Broome Citation2003). This has been beneficial for island lizard communities and, in some cases, geckos have been translocated to these islands (Towns & Daugherty Citation1994; Van Winkel Citation2008; Adams Citation2009). However, geckos on the mainland continue to face a range of problems including introduced predators, habitat loss, fragmentation of populations and, for the more attractive species, illegal collection. Predator control on the mainland must deal with a range of predators, their complex relationships and ongoing reinvasion. Some operations result in mesopredator release and competitor release of exotic mammals that may have devastating effects on local gecko populations (Zavaleta et al. Citation2001; Caut et al. Citation2007).
Mokopirirakau is a widespread New Zealand gecko genus that consists of four species and six putative undescribed species including M. ‘Southern North Island’ (Nielsen et al. Citation2011). Mokopirirakau ‘Southern North Island’ is an arboreal, nocturnal gecko that is confirmed in the south of the North Island but its northern limits are unresolved (Jewell Citation2011). Under the New Zealand Threat Classification System M. ‘Southern North Island’ is ranked as At Risk because of predicted decline. However, it is acknowledged that currently a lack of data hamper this assessment (Hitchmough et al. Citation2013). A better understanding of M. ‘Southern North Island’ would contribute to: 1. assessment of threat status; 2. an enhanced understanding of threats; 3. planning conservation management; and 4. developing appropriate monitoring techniques for the species.
The objectives of this study were to investigate the habitat use and movement patterns of M. ‘Southern North Island’ at Otari-Wilton's Bush, Wellington. Basic information on morphology and reproductive condition of the population is also reported, as size is correlated with extinction risk for New Zealand lizards (Tingley et al. Citation2013) and reproductive output is correlated with resilience to extrinsic threats like predation (Cree Citation1994).
Materials and methods
Our study was conducted at Otari-Wilton's Bush which is a Scenic Reserve located in the western suburbs of Wellington. It is situated on the steep hill slopes of the Kaiwharawhara Stream which flows northeast towards the Ngaio Gorge. The reserve encompasses about 100 ha and ranges between 70–200 m above sea level. Otari-Wilton's Bush has about 5 ha of cultivated native plant collections and about 96 ha of regenerating or mature native forest. It has one of the few mature podocarp northern rātā forest remnants left on the Wellington peninsula (Wellington City Council Citation2007). There is intensive predator control at Otari-Wilton's Bush. In 1993–94 an initial knock down of mammalian predators was carried out and since then a network of bait stations containing brodifacoum and trapping stations with DOC 200 traps have been set up. Monitoring of rodents and mustelids began in 2007. In 2011 there were 87 bait stations and 25 DOC 200 traps. Between January 2007 and February 2012 the average tracking rate was 7.4 ± 5.2% (n = 14, range 2%–18%) for rats and 12.7 ± 7.5% (n = 14, range 5% 29%) for mice (Moylan & Crisp Citation2012). Mustelids and cats have been observed in the study area.
Between 20 February 2011 and 1 October 2012 we undertook 20 timed searches at Otari-Wilton's Bush. Twelve daytime searches took place between 1030 h and 1800 h, and eight nighttime searches took place between 1900 h and 2315 h. The searches averaged 89 minutes (range 30–150 minutes). The total search effort was 29.8 search hours. Binoculars with a lamp mounted on them were used at night to detect eye-shine, while during the day geckos were found basking or in retreats. Whenever a gecko was located a range of variables were collected including: the time of observation; substrate type; plant species (if on a plant); and height above the ground. If the animal was captured, a range of morphometric measurements were collected; however, if capture was not possible it was only assigned an age classification (see below for morphometric information).
A radio telemetry study to investigate habitat use and movement patterns of a small number of M. ‘Southern North Island’ was conducted from 11–23 January 2012. Radio transmitters (BD-2 transmitters weighing 0.65 g; Holohil Systems Ltd, Ontario, Canada) were attached to geckos using the backpack method (Salmon Citation2002; Hare et al. Citation2007; Hoare et al. Citation2007), or were attached to the base of the tail of animals caught in rock crevices when a backpack mount may have interfered with the gecko's use of a retreat. The harnesses were made of hypoallergenic, breathable, surgical tape (Nexcare flexible clear tape). Two reflector dots were attached to the backpack, or to the body of the animal, with a layer of clear tape to assist with locating animals at night. The weight of the transmitter and tape for the backpack/tail-mount was ≤ 7.5% of the geckos' body weight for seven geckos (geckos weighed ≥ 14.25 g) and ≤ 10% of one individual's body weight (gecko weighed 10 g). Studies of other arboreal lizards indicate that transmitters weighing more than 7.5% of body weight can affect climbing performance (Knapp & Abarca Citation2009), so gecko G2 may have been negatively affected by its transmitter and harness. Before being released all geckos were checked to see if they were able to move freely and adjustments were made to harnesses accordingly. On several occasions during the study the tape that held the transmitters in place started to peel off, so we recaptured the geckos and made quick repairs. This interference may have affected the geckos' behaviour.
Radio-tracking was conducted four times a day (beginning at 0200, 1000, 1400 and 2200 h) and on the last day of tracking geckos were located every 2 hours. At the beginning of each search session the temperature was recorded. Tagged geckos were located using a Telonics telemetry receiver and a Sirtrack antenna. After obtaining an approximate location of the gecko with triangulation, we homed in to see an emerged gecko or to gain an accurate fix on a gecko hidden in a retreat. Horizontal and vertical movement and direction from previous locations were recorded. It was difficult to get accurate fixes on animals that were not visible high in trees; however, it was possible to ascertain minimum height off the ground. At each gecko location substrate type (including plant species), canopy species and height were also recorded.
The following morphometric measurements were recorded for all animals captured during the timed searches and the radio telemetry study: age (adult, juvenile); sex; snout-vent-length (SVL); vent-tail-length (VTL); tail re-growth; and mass (using a Pesola spring balance). Animals were classified as adults if they had a SVL ≥ 60 mm. Males of this size had a noticeable swelling under the base of the tail, which is a sign of sexual maturity. Females were palpated to identify whether or not they were gravid and, if so, how many embryos were being carried. Only geckos with transmitters attached were individually identified. However, individuals from the timed searches were determined from the location of capture and by comparing measurements and other identifying marks.
Data were analysed in the statistical program SAS Enterprise 4.3. Student's t-tests were used to compare morphometric data (e.g. SVL, VTL and mass) of males and females. The assumptions of normality, equal variance and independence were met. Statistical significance was assumed at α = 0.05. Statistical comparison was not used for the radio telemetry study as the number of tagged geckos was so small. Instead, descriptive statistics were used and are presented as means ± SD. Results from two of the eight geckos tracked are excluded because they were tracked for less than 24 hours. Results from the remaining six geckos were used to investigate vertical habitat use and substrate preference. Because of the necessity for precise locational data, only data from three male geckos were used to determine emergence behaviour and movement patterns.
Results
Timed searches
During timed searches 47 M. ‘Southern North Island’ geckos (40 adults, seven juveniles) were observed at a rate of 1.58 geckos sighted per hour. The 33 geckos located during the day were found at a rate of 1.95 geckos per hour (range 0–6 geckos per hour). The 14 geckos found during the night were found at a rate of 1.09 geckos per hour (range 0–2.4 geckos per hour). Geckos were observed at similar heights off the ground during day and night searches (day 4.6 ± 6.1 m [range 0–15 m]; night 5.3 ± 5.3 m [range 0.4–16 m]). Geckos were either located on rock walls/rock (34%) or vegetation (66%), and all individuals located on vegetation were either on branches or trunks. We observed geckos using nine plant species, with most observations (45%) on tawa (Beilschmieda tawa). Individuals were usually seen on their own (79% of observations).
Radio telemetry study
Eight adult geckos (five males and three pregnant females) were captured for the study (). The six geckos that were tracked for more than 24 hours had an average of 42.2 ± 8.0 fixes during the main study periods and were sighted about 30% of the time (12.5 ± 3.3 occasions). Most geckos (83.3%) were in locations with tall trees (height range 7–13 m tall). Geckos that lived in the forest were usually high in the trees (86.1 ± 13.7% of the time spent > 3 m off the ground, n = 3), whereas geckos that lived on the edge of forest/scrub spent less time in the canopy and more time on the ground (19.9 ± 1.2% of the time spent > 3 m and 31.9 ± 18.2% of the time spent < 0.5 m off the ground, n = 2). One gecko, which lived in a rock garden where trees were less than 3 m tall, was usually observed on or close to the ground (92% of the time < 0.5 m off the ground).
Table 1 Morphometric details and radio tracking information about the eight adult Mokopirirakau ‘Southern North Island’ geckos tracked at Otari-Wilton's Bush between 11–23 January 2012.
Most geckos were usually found on vegetation (88.0 ± 13.7% of the time, n = 5), but one gecko was only found on vegetation 24% of the time. While the geckos were detected on vegetation on 169 occasions, it was only possible to determine what part of the vegetation they were using on 84 occasions. Of those observed, geckos were located on trunks or branches (77.4% of observations), twigs or foliage (17.7% of observations) and at the base of mountain flax (Phormium cookianum) and Astelia sp. (6.0% of observations) (). Geckos were found on 23 plant species ranging from large trees to prostrate vines (). Gecko fixes indicated that geckos were among vines on some trees, but we could not confirm whether geckos were on the vines or the trees so just recorded presence on the tree species. Most of the geckos (66.6%) displayed strong site fidelity for particular trees, with more than 80% of observations on single large trees (tawa, for two of the geckos; black beech [Nothofagus solandri solandri] and celery pine [Phyllocladus trichomanoides] for the other two).
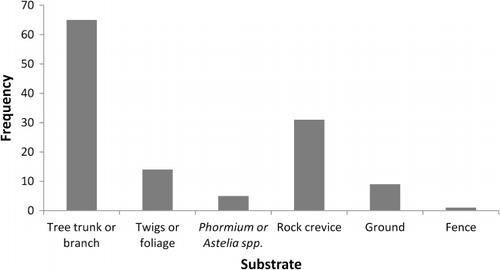
Table 2 The number of observations (n = 170) of six Mokopirirakau ‘Southern North Island’ geckos on different plant species (n = 23) during the radio telemetry study at Otari-Wilton's Bush between 11–23 January 2012.
Geckos were observed using a range of retreat sites including stone piles, rock crevices, the bases of plants, tree hollows and dead branches/wood piles on the forest floor. Geckos used an average of 3.8 ± 3.0 retreat sites (range 0–8 sites) with an average height above ground of a retreat being 0.58 ± 0.96 m (range 0–3.1 m, n = 23). Most retreat sites were only visited once. However, a branch hollow was used on six separate occasions (22 observations in total) by an individual. Retreats were not always used. Geckos were observed on exposed trunks in cool conditions and signals from one gecko indicated that it was often on the outside of the tree among dense vines and not using retreats.
Emerged geckos were observed regularly between 2200–0400 h and 1000–1600 h. On the last day of the study some geckos were emerged outside of the core research times (at 0450, 0510, 0550, 0700, 0715, 0910 and 2030 h). Ambient temperatures at the beginning of night searches (2200 and 0200 h) ranged from 7.8–16.3 °C, while temperatures at the beginning of day sessions (1000 and 1400 h) ranged from 15.6–19.6 °C. Emerged geckos were observed at the lowest and highest temperatures during both day and night sessions. Emergence observations for three male geckos were similar by day and at night (day 32 ± 6.6%, night 33 ± 14%).
On average three male geckos moved 9.5 m in 24 hour periods and the average hourly movement was only 0.4 ± 0.2 m (). The maximum total distance moved by an individual gecko was 103 m in 7 days. The greatest distance moved per hour was 5.1 m when a gecko moved 13.2 m between 0040–0315 h. Geckos moved between the two daytime fixes (1000 and 1400 h) 52% of the time, but only moved between the two nighttime fixes (2200 and 0200 h) 27% of the time. On average geckos moved greater distances between 1000 and 1400 h (0.7 ± 0.8 m) ().
Table 3 Distances moved by three male Mokopirirakau ‘Southern North Island’ geckos from Otari-Wilton's Bush during January 2012.
Only once during the radio telemetry study was a tagged individual observed with another gecko. However, tagged individuals used the same crevices as other geckos, but at different times. Adult males, females and juveniles were all seen in the same vicinity. Geckos were never observed feeding; however, they do eat fruit because one animal defecated a large amount of orange fruit, which was probably either kohia (Passiflora tetranda) or kawakawa (Piper excelsum).
Morphometric analysis
Nineteen geckos were captured (). Although the largest female had a SVL 8 mm longer than the largest male and most females (67%) were gravid, there was no significant difference in the SVL (t = 1.76, d.f. = 14, P = 0.101) or the mass (t = 1.53, d.f. = 9, P = 0.153) of males and females. Most adult females (83%) and 40% of adult males had experienced tail loss, but there was no significant difference in male and female tail lengths (t =−1.05, d.f. = 14, P = 0.313). All four adult females caught in January were gravid with two embryos, equating to an annual reproductive output of 2.0 offspring/female/year. Variation in the dorsal patterning of individual M. ‘Southern North Island’ was discernable in two areas: 1. dark blotches between the neck and pelvis varied in shape and size; and 2. the V-shaped mark on the head (between the eyes) and the dark patch on the top of the head created different patterns ().
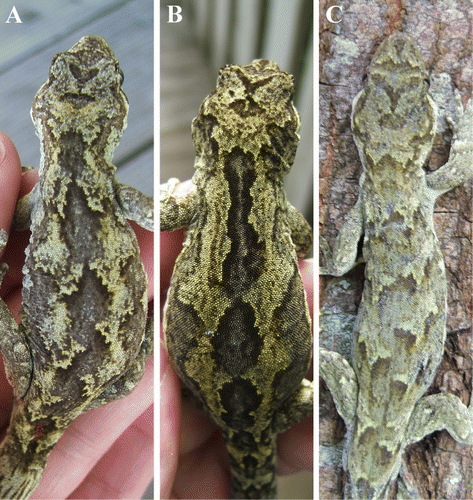
Table 4 Morphometric measurements (mean ± SD) of 19 individual Mokopirirakau ‘Southern North Island’ geckos from Otari-Wilton's Bush. Figures in parentheses are sample sizes and range.
Discussion
This study provides basic biological information about M. ‘Southern North Island’ and advances our knowledge of the activity period, movement patterns and habitat use of these geckos.
The observation rate indicates that a relatively healthy population of M. ‘Southern North Island’ exists at Otari-Wilton's Bush, but it is difficult to ascertain natural abundance levels of this species. Before the eradication of kiore (Rattus exulans) and Norway rats (R. norvegicus) on Kapiti Island only one M. ‘Southern North Island’ was found in forest habitat (0.01 geckos per person hour; Whitaker Citation1995). Seventeen years after rats were eradicated, only three geckos were found in forest and regenerating scrub (0.06 geckos per person hour) and one in a large area of flax (B. Barr, North Tec; R. Romijn, Greater Wellington Regional Council; and C. Knox, Ecogecko Consultants, unpubl. data), suggesting a slow recovery of forest geckos on the island. In contrast, at Zealandia Karori Sanctuary 3 years after the eradication of all mammals (with the exception of the house mouse, Mus musculus) 13 M. ‘Southern North Island’ were located at a rate of 0.42 geckos per person hour (A.H. Whitaker, Whitaker Consultants Ltd, unpubl. data). Surveys of other Mokopirirakau species also suggests that the current predator control regime at Otari-Wilton's Bush is providing benefits for arboreal forest geckos. In areas without predator control, M. ‘Southern Forest’ were located in the Catlins at a rate of 0.6 gecko sightings per person hour in optimal weather conditions (Hoare et al. 2013) and M. granulatus were located in Opuiaki Forest at a rate of 0.25 geckos per person hour (A.H. Whitaker, Whitaker Consultants Ltd, unpubl. data).
Mokopirirakau ‘Southern North Island’ is reported to be a nocturnal gecko that sun basks (Jewell Citation2011). Diurnal thermoregulation is beneficial to lizards because it results in an increased metabolic rate, which is thought to facilitate physiological processes such as digestion and reproduction (Hare et al. Citation2006). In this study, however, geckos were observed to spend the same amount of time emerged by day as they did by night, and the average distance moved during the day was greater than at night. This may indicate the geckos were actively foraging and not just thermoregulating during the day. Our data necessitates reevaluating the assumptions about the activity phase of Mokopirirakau geckos. Other supposedly nocturnal geckos in New Zealand also display diurnal activity, for instance a population of Dactylocnemis ‘Poor Knights’ was observed foraging during the day amongst a seabird colony (Jewell Citation2011).
Pianka (Citation1966) classified lizards as being either active foragers or sit-and-wait predators. Bauer (Citation2007), however, suggested that diplodactylid geckos do not fit neatly into Pianka's dichotomy and actually have three modes of foraging: 1. sit-and-wait foraging where geckos wait by their retreats to capture food as it passes by; 2. slow cruise foraging where geckos have nearly continuous movement searching for food, punctuated by brief pauses; and 3. serial ambushing where geckos use several ambush sites to capture food as it passes by. According to the classifications of both Pianka and Bauer M. ‘Southern North Island’ is clearly not a sit-and-wait predator. Geckos moved a mean of 9.5 m in 24 hours, averaging nearly half a metre per hour, with the longest individual distance being 103 m in 7 days. These distances moved are significantly longer than those of the diurnal sit-and-wait predator Naultinus manukanus. They only moved 0.59 m in 24 hours, averaging 0.05 m an hour, with the longest individual distance being 4.0 m in 5 days (Hare et al. Citation2007). The actual foraging mode of M. ‘Southern North Island’ has still to be determined. Based on our observations we hypothesise that geckos are serial ambushers as they were usually stationary when located and did not regularly move between fixes.
The majority of tagged geckos displayed strong short-term site fidelity, which has also been observed in M. ‘Southern Forest’ (Hoare et al. Citation2013). Many New Zealand geckos display strong site fidelity and have been caught in the same location more than 10 years apart (e.g. Toropuku stephensi, Hare & Cree Citation2005; Woodworthia maculata, Anastasiadis & Whitaker Citation1987; Hoplodactylus duvaucelii, Thompson et al. Citation1992; and W. brunnea, Lettink & Whitaker Citation2006). Currently no M. ‘Southern North Island’ populations are permanently marked so it is not possible to determine longevity or long-term site fidelity. However, this species has distinctive dorsal makings and therefore it is possible to create a photo library of individual animals. Photo identification from dorsal markings has been used successfully as a method of permanent identification for Naultinus gemmeus (Knox et al. Citation2013) and M. ‘Southern Forest’ (Hoare et al. Citation2013).
Mokopirirakau ‘Southern North Island’ geckos are mainly arboreal and are usually found on vegetation. When using vegetation, most individuals were mainly observed on the trunks and branches of shrubs and trees, unlike arboreal Naultinus geckos which prefer foliage and stems (Hare et al. Citation2007). Some M. ‘Southern North Island’ geckos were often found on the ground or among lower levels of vegetation. This behaviour may be possible at Otari-Wilton's Bush because of the effective predator control which has kept rats below 5% tracking rate since August 2010 (Moylan & Crisp Citation2012). Other gecko species are known to change their habitat use when introduced mammals are eliminated. An island population of H. duvaucelii spent more time in relatively open habitats, used the ground more often and chose retreat sites nearer to the ground after rats were eradicated (Hoare et al. Citation2007).
As has been observed in other studies, M. ‘Southern North Island’ made use of a variety of retreat sites (Bell Citation2009). Only solitary M. ‘Southern North Island’ were observed in retreats whereas other gecko species are known to form small groups (H. duvaucelii; Christmas Citation1995), or large aggregations (W. maculata; Hare & Hoare Citation2005) in retreats. However, M. ‘Southern North Island’ are not totally reliant on shelter in retreats. Some stay in the foliage of dense vines and others just remain emerged on tree trunks. These characteristics have been observed in other New Zealand geckos, for instance M. ‘Southern Forest’ does not consistently rely on retreats (Hoare et al. Citation2013) and N. manukanus appears to stop and rest overnight wherever on a plant its daily activity has ceased (Hare et al. Citation2007).
Mokopirirakau ‘Southern North Island’ may be particularly susceptible to predation by introduced mammals. Ship rats (R. rattus) and stoats (Mustela ermina) are adept climbers that are present throughout the forests in the lower North Island (Innes Citation2005; King & Murphy Citation2005). By spending long periods on trunks and branches, M. ‘Southern North Island’ are directly in the path of foraging mammals. This may make them more vulnerable than Naultinus species that sit on narrow branches and foliage, which are more inaccessible to mammals (Hare et al. Citation2007).
This study confirms that M. ‘Southern North Island’ eat fruit, as do several other New Zealand geckos (Whitaker Citation1987). However, M. ‘Southern North Island’ were not observed drinking nectar even though Metrosideros excelsa, M. carminea, and Phormium cookianum were in flower and in close proximity to geckos (R. Romijn, pers. obs). This was surprising as several New Zealand geckos are known to travel long distances to drink nectar (Whitaker Citation1987) and will exclude other geckos from this valuable resource (Eifler Citation1995).
At Otari-Wilton's Bush M. ‘Southern North Island’ reaches a maximum SVL of 96 mm, which has extended the known maximum size of this species by 11 mm (Jewell Citation2011). The largest female was 8 mm longer than the largest male, which may indicate sexual dimorphism, with females being larger than males. Such dimorphism has been observed in other New Zealand geckos; for example, female N. manukanus are on average 5 mm longer than males (Hare et al. Citation2007). As others have noted, the tail length of M. ‘Southern North Island’ is longer than its SVL (Jewell Citation2011), and it appears that the ratio of unbroken tail to SVL increases as animals grow older. Sub-adults have tails up to 10% longer than SVL, whereas adults' tails can be up to 33% longer.
New Zealand geckos have a low reproductive output of 0.62–2.0 offspring/female/year, which is due to phylogenetic constraints, viviparous reproductive mode and cool summers (Cree Citation1994; Hare & Cree Citation2005). Based on a small sample size, it appears M. ‘Southern North Island’ have the highest reproductive rate possible for New Zealand geckos as all females captured in January were pregnant with two embryos, resulting in a potential reproductive output of 2.0 offspring/female/year. Therefore, there is a relatively high potential for populations to recover following predator eradication or sustained suppression.
Conclusions
Our observations demonstrate that M. ‘Southern North Island’ is equally active during the day and at night during summer, and that individuals move nearly 10 m per 24 hour period. This species is mainly arboreal and was usually located on trunks and branches of trees. While they do use the full range of vertical structures available to them, animals in large trees may stay in the canopy for several days. Retreats are used, but not regularly. Individuals display strong site fidelity and may spend most of their time on a single tree. Mokopirirakau ‘Southern North Island’ may be vulnerable to introduced arboreal predators because they use the same habitat. The frequent use of exposed sites by geckos, and infrequent use of refuges, suggests that predator control operations at Otari-Wilton's Bush are having a positive effect on the gecko population.
Acknowledgements
We thank Les Moran (Department of Conservation; DOC), Amelia Romijn and Samantha Romijn for field assistance. We are grateful for the support of Rewi Elliot from Wellington City Council for allowing us to undertake the study at Otari-Wilton's Bush. Tony Whitaker, Ben Barr and Carey Knox shared their unpublished data with us. David Burlace (DOC) formatted figures for publication. We thank Tony Whitaker, Don Newman, Grant Norbury and an anonymous reviewer for helpful comments on the manuscript. This work was partly funded by DOC Science Investigation 4231, and approved by the VUW Animal Ethics Committee (Permit 2009 R12).
References
- Adams L 2009. Lizard action plan for Poneke area, Wellington conservancy, 2009–2014. Wellington, New Zealand, Department of Conservation. 25 p.
- Anastasiadis JM, Whitaker AH 1987. Longevity of free-living Hoplodactylus maculatus (Reptilia: Gekkonidae). New Zealand Journal of Ecology 10: 141–142.
- Bauer AM 2007. The foraging biology of the Gekkota: life in the middle. In: Reilly SM, McBrayer LB, Miles DB eds. Lizard Ecology. Cambridge, Cambridge University Press. Pp. 371–404.
- Bell TP 2009. A novel technique for monitoring highly cryptic lizard species in forests. Herpetological Conservation and Biology 4: 415–425.
- Caut S, Casanovas JG, Virgos E, Lozano J, Witmer GW, Courchamp F 2007. Rats dying for mice: modeling the competitor release effect. Austral Ecology 32: 858–868. 10.1111/j.1442-9993.2007.01770.x
- Christmas E 1995. Interactions between Duvaucel's gecko (Hoplodactylus duvaucelii) and kiore (Rattus exulans). Unpublished MSc thesis. Dunedin, New Zealand, University of Otago. 64 p.
- Cree A 1994. Low annual reproductive output in female reptiles from New Zealand. New Zealand Journal of Zoology 21: 351–372. 10.1080/03014223.1994.9518005
- Eifler DA 1995. Patterns of plant visitation by nectar feeding lizards. Oecologia 101: 228–233. 10.1007/BF00317288
- Ellinger N, Schlatte G, Jerome N, Hödl W 2001. Habitat use and activity patterns of the neotropical arboreal lizard Tropidurus ( = Uracentron) azureus werneri (Tropiduridae). Journal of Herpetology 35: 395–402. 10.2307/1565957
- Fitzgerald M, Shine R, Lemckert F 2002. Spatial ecology of arboreal snakes (Hoplocephalus stephensii, Elapidae) in an eastern Australian forest. Austral Ecology 27: 537–545. 10.1046/j.1442-9993.2002.01214.x
- Hare KM, Cree A 2005. Natural history of Hoplodactylus stephensi (Reptilia: Gekkonidae) on Stephens Island, Cook Strait, New Zealand. New Zealand Journal of Ecology 29: 137–142.
- Hare KM, Hoare JM 2005. Hoplodactylus macultaus (Common gecko) aggregations. Herpetological Review 36: 179.
- Hare KM, Hoare JM, Hitchmough RA 2007. Investigating natural population dynamics of Naultinus manukanus to inform conservation management of New Zealand's cryptic diurnal geckos. Journal of Herpetology 14: 81–93. 10.1670/0022-1511(2007)41[81:INPDON]2.0.CO;2
- Hare KM, Pledger S, Thompson MB, Miller JH, Daugherty CH 2006. Daily patterns of metabolic rate among New Zealand lizards (Reptilia: Lacertilia: Diplodactylidae and Scincidae). Physiological and Biochemical Zoology 79: 745–753. 10.1086/504618
- Hitchmough RA, Hoare JM, Jamieson H, Newman D, Tocher MD, Anderson PJ, et al. 2010. Conservation status of New Zealand reptiles, 2009. New Zealand Journal of Zoology 37: 203–224. 10.1080/03014223.2010.496487
- Hitchmough R, Anderson P, Barr B, Monks J, Lettink M, Reardon J, et al. 2013. Conservation status of New Zealand reptiles, 2012. New Zealand Threat Classification Series 2. Wellington, New Zealand, Department of Conservation. 14 p.
- Hoare JM, Pledger S, Nelson NJ, Daugherty C 2007. Avoiding aliens: behavioural plasticity in habitat use enables large, nocturnal geckos to survive pacific rat invasions. Biological Conservation 136: 510–519. 10.1016/j.biocon.2006.12.022
- Hoare JM, Melgren P, Chavel EE 2013. Habitat use by southern forest geckos (Mokopirirakau ‘Southern Forest’) in the Catlins, Southland, New Zealand. New Zealand Journal of Zoology 40: 129–136. 10.1080/03014223.2012.707663
- Innes JG. 2005. Ship rat. In: King CM ed. The handbook of New Zealand mammals. Auckland, Oxford University Press. Pp. 187–203.
- Jewell T 2011. A photographic guide to reptiles and amphibians of New Zealand. Revised edition. Auckland, New Holland Publishers (NZ) Ltd. 143 p.
- Kays R, Allison A 2001. Arboreal tropical forest vertebrates: current knowledge and research trends. Plant Ecology 153: 109–120. 10.1023/A:1017585622940
- King CM, Murphy EC. 2005. Stoat. In: King CM ed. The handbook of New Zealand mammals. Auckland, Oxford University Press. Pp. 261–287.
- Knapp CR, Abarca JG 2009. Effects of radio transmitter burdening on locomotor ability and survival of iguana hatchlings. Herpetologica 65: 363–372. 10.1655/09-003.1
- Knox CD, Cree A, Seddon PJ 2013. Accurate identification of individual geckos (Naultinus gemmeus) through dorsal pattern differentiation. New Zealand Journal of Ecology 37: 60–66.
- Lettink M, Whitaker AH 2006. Hoplodactylus maculatus (common gecko) longevity. Herpetological Review 37: 223–224.
- Moylan S, Crisp P 2012. Rodent and small mammal tracking tunnel monitor: August–September 2012. Wellington, New Zealand, Greater Wellington Regional Council. 42 p.
- Neilson K, Duganzich D, Goetz B, Waas JR 2004. Improving search strategies for the cryptic New Zealand striped skink (Oligosoma striatum) through behavioural contrasts with the brown skink (Oligosoma zelandicum). New Zealand Journal of Ecology 28: 267–278.
- Nielsen SV, Bauer AM, Jackman TR, Hitchmough RA, Daugherty CH 2011. New Zealand geckos (Diplodactylidae): cryptic diversity in a post-Gondwanan lineage with trans-Tasman affinities. Molecular Phylogenetics and Evolution 59: 1–22. 10.1016/j.ympev.2010.12.007
- Pianka ER 1966. Covexity, desert lizards, and spatial heterogeneity. Ecology 47: 1055–1059. 10.2307/1935656
- Salmon NM 2002. Telemetric studies of the geckos Hoplodactylus maculatus and Naultinus gemmeus. Unpublished MSc thesis. Dunedin, New Zealand, University of Otago.
- Thompson MB, Daugherty CH, Cree A, French DC, Gillingham JC, Barwick RE 1992. Status and longevity of the tuatara, Sphenodon guntheri, and Duvaucel's gecko, Hoplodactylus duvaucelii, on North Brother Island, New Zealand. Journal of the Royal Society of New Zealand 22: 123–130. 10.1080/03036758.1992.10420810
- Tingley R, Hitchmough RA, Chapple DG 2013. Life-history traits and extrinsic threats determine extinction risk in New Zealand lizards. Biological Conservation 165: 62–68. 10.1016/j.biocon.2013.05.028
- Towns DR, Daugherty CH 1994. Patterns of range contractions and extinctions in the New Zealand herpetofauna following human colonisation. New Zealand Journal of Zoology 21: 325–339. 10.1080/03014223.1994.9518003
- Towns DR, Broome KG 2003. From small Maria to massive Campbell: forty years of rat eradications from New Zealand Islands. New Zealand Journal of Zoology 30: 377–398. 10.1080/03014223.2003.9518348
- Wellington City Council 2007. Otari-Wilton's bush native botanic garden management plan. Unpublished report. Wellington City Council, Wellington, New Zealand.
- Whitaker AH 1987. The roles of lizards in New Zealand plant reproductive strategies. New Zealand Journal of Botany 25: 315–328. 10.1080/0028825X.1987.10410078
- Whitaker AH 1995. The lizard fauna of Kapiti Island: an assessment prior to rat eradication. Unpublished report, Wellington Conservancy, New Zealand Department of Conservation, Wellington, New Zealand. 30 p.
- Zavaleta ES, Hobbs RJ, Mooney HA 2001. Viewing invasive species removal in whole-ecosystem context. Trends in Ecology and Evolution 16: 454–459. 10.1016/S0169-5347(01)02194-2
- Van Winkel D 2008. Efficiency of techniques for post translocation monitoring of the Duvaucel's gecko (Hoplodactylus duvaucelii) and evidence of native avian predation on lizards. Unpublished MSc thesis. Auckland, New Zealand, Massey University.
