Abstract
Macquaridrilus mcmurtrieae n. sp. is described from Campbell Island. This resembles the only other species in the genus, Macquaridrilus bennettae Jamieson, 1968, in most aspects, but shows significant differences in the anatomy of its genitalia. In particular, the spermathecal pores are dorsal rather than lateral, the spermathecae lack diverticulae, the ejaculatory duct is more stout and muscular, the vas deferens is shorter relative to other organs and the anterior prostate is compact rather than elongate. The presence of a cuticular sperm canal appears to be an apomorphy for the genus. The new species was collected from streams and tarns across the island.
http://zoobank.org/urn:lsid:zoobank.org:pub:652AF61D-CFB2-4D07-94C8-59E6FB549D5F
http://zoobank.org/urn:lsid:zoobank.org:act:984F2456-768D-48A1-87AD-4453768BAB8A
Introduction
In 1965 Professor Barrie Jamieson, from the University of Queensland (Brisbane), collected oligochaetes on Macquarie Island. Amongst the material in that collection of mostly terrestrial earthworms was an aquatic oligochaete, collected from a single pond by Isobel Bennett, with sufficiently unusual genital anatomy for Jamieson to establish the genus Macquaridrilus, within which he placed the new species Macquaridrilus bennettae (Jamieson Citation1968). This species was subsequently collected from Macquarie Island in 1978 (unpublished records from material held in South Australian Museum, collector Penny Greenslade) and 1992 (Marchant & Lillywhite Citation1994). Collections from Campbell Island, made by an expedition from the Australian Museum and borrowed by the senior author in 1992, included worms with very similar chaetae to those of M. bennettae, but these were not examined further as the focus at that time was on the Phreodrilidae (Pinder & Brinkhurst Citation1997). Twenty years later, new collections from Campbell Island also included specimens resembling M. bennettae and these are described herein as the new species Macquaridrilus mcmurtrieae. The latest collections were made during the 2010–2011 Campbell Island Bicentennial Expedition, a multidisciplinary expedition designed to uncover the island's human history, ecosystem processes and recovery following eradication of rodents and grazing mammals (www.campbellisland.org.nz).
Material and methods
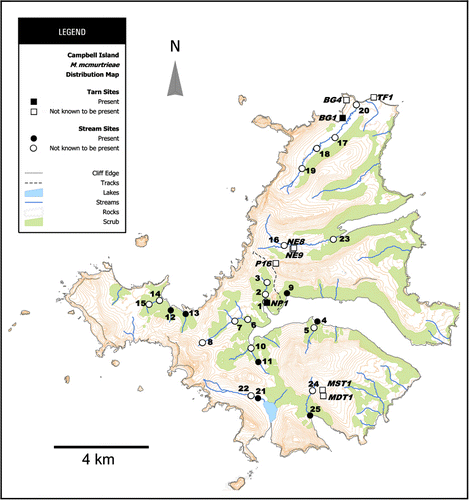
Specimens were collected by EOS Ecology as part of their survey of the aquatic macroinvertebrate communities of the island's streams and tarns in 2010–2011. A total of 25 stream sites and nine tarns were sampled () and photographs of representatives of these are shown in . At each stream site six Surber samples (0.25 m2, 500 µm mesh size, ) and two kick-net samples (each a composite of five ‘kicks’, 500 µm mesh size) were collected. At tarn sites four kick-net samples were taken (including benthic habitats and sweeps of the water column, 500 µm mesh size). All samples were preserved in the field in 4% formalin, except for one kick-net sample from each stream site, which was preserved in 95% ethanol. On their return to mainland New Zealand all formalin samples were washed and re-preserved in 70% isopropanol alcohol.

Oligochaetes were identified from 25 samples (from 12 stream sites and six tarns) out of the 218 samples collected. Thirteen stream sites and three tarns have not yet had any of their oligochaetes examined and no sites have had oligochaetes examined from all samples. All specimens were examined in ethanol and a selection of mature worms were stained in Grenacher's borax carmine, partially destained in 3% hydrochloric acid, dehydrated in isopropanol, cleared in Histoclear™ and then slide mounted in Permount™. Slide mounted specimens were either mounted whole or the genital segments were dissected. Drawings were made with the aid of a drawing tube. Measurements were made using an eyepiece graticule or from a calibrated measuring tool in the AutoMontage™ 5.01(A) software produced by Syncroscopy.
Habitats
Macquaridrilus mcmurtrieae is widespread on Campbell Island, where it has been recorded from seven stream reaches and two tarn sites to date out of 12 stream sites and six tarns from which oligochaetes were examined (). This is almost certainly an underestimate of its occurrence considering the proportion of samples examined to date (see Methods). The stream sites generally had coarse substrates dominated by bedrock and boulders (> 256 mm), large cobbles (128–256 mm) and small cobbles (64–128 mm). Pebbles (2–64 mm) dominated the substratum at one site (site 21: Kirk Stream). Water velocities were moderate (0.36–0.56 m/s), with channel morphology typically being some combination of pool, step pool, cascade, run and riffle. Thin mats of brown algae covered the substratum at all sites. Riparian vegetation was either dominated by Dracophyllum forest (e.g. ), tussocks (e.g. ) or a mixture of Dracophyllum, ferns and tussocks (e.g. ). Canopy cover varied widely from < 5% to 100%. The water of all streams was tannin stained (1.1–2.5 mg/L) with acidic to neutral pH (5.1–7.0) and specific conductivities that do not indicate any great saline influence (158–713 µS/cm). Spot measures of water temperature (7.4–10.8 °C) and dissolved oxygen (93.8%–98.9%) indicated cool, well-oxygenated conditions. Tarn NP1 () was in a protected cove and surrounded by Dracophyllum and tussocks, was approximately 8 m across and 14 m long and ≤ 0.8 m deep. It had steep sides and a peat substrate. Tarn BG1 () was located near the top of an exposed high cliff in the north of Campbell Island, with the adjacent vegetation being tussocks and megaherbs. It was approximately 10 m wide, 30 m long and ≤ 0.5 m deep, with a sand and rock substrate. NP1 was tannin stained (2.8 mg/L) while BG1 was not (0.1 mg/L) and both were acidic (pH 5.2 and 4.1 respectively). The specific conductivity of NP1 (574 µS/cm) was within the range of the stream sites, while BG1 (4810 µS/cm) was influenced by windblown salt spray.
Taxonomic account of the new species
Phylum
Annelida
Class
Clitellata
Family
Naididae Ehrenberg 1828
Genus
Macquaridrilus Jamieson 1968
Macquaridrilus mcmurtrieae n. sp.
(–)
Material examined
All collections by Shelley McMurtrie (SM) and Alex James (AJ) of EOS Ecology, or Colin Meurk (CM) of Landcare Research New Zealand. Type material has been deposited in Museum of New Zealand Te Papa Tongarewa in Wellington. All locations are on Campbell Island and all coordinates determined using the NZ Geodetic 2000 datum. Holotype: W.003476, Southeast Stream (site 25), Surber sample, mature specimen mounted whole on slide, 28 Jan 2011, 52°35.441′S, 169°10.313′E (SM and AJ). Paratypes: W.003483, Southeast Stream (site 25), Surber sample, 1 mature and 2 immature in ethanol, 28 Jan 2011, 52°35.441′S, 169°10.313′E (SM and AJ); W.003478 and W.003478/1, Garden Stream (site 11), Surber sample, 3 mature on two slides, 27 Dec 2010, 52°34.205′S, 169°08.339′E (SM and AJ); W.003479, Middle Bay Stream (site 12), Surber sample, 1 mature on slide in Permount, 29 Dec 2010, 52°34.205′S, 169°08.339′E (SM and AJ); W.003480, Kirk Stream (site 21), Surber sample, 1 on slide in Permount, 11 Jan 2011, 52°35.044′S, 169°08.329′E (SM and AJ); W.003482 (slide) and W.003482/1 (vial), Tucker Tarn (site NP1), kick-net sample in open water and macrophytes, 1 mature on slide (head and tail in Kaisers glycerin jelly and mid-body stained in Permount) and 2 mature in ethanol, 2 Feb 2011, 52°32.806′S, 169°08.644′E (SM); W.003477, W.003477/1, W.003477/2 and W.003477/3, Borchgrevink Bay Tarns (site BG1), kick-net sweep, 4 mature on three slides in Permount and 2 mature in ethanol, 31 Jan 2011, 52°28.541′S, 169°11.538′E (CM); W.003481 and W.003481/1, Capstan Cove Stream (Site 13), kick-net sweep, 2 on two slides, 31 Dec 2010, 52°33.094′S, 169°05.573′E (SM and AJ).
Additional material
All returned to EOS Ecology, Christchurch. Lookout Bay Stream (site 9), Surber sample, 1 in ethanol in mixed species vial, 22 Dec 2010, 52°32.609′S, 169°09.423′E (SM and AJ); Honey Falls Stream (site 4), Surber sample, 1 tail end only, 15 Oct 2010, 52°33.263′S, 169°10.581′E (SM and AJ).
Description
All features are as observed for specimens killed in formalin or ethanol and then preserved in alcohol.
Dimensions
Length of whole worms 5.5 to 10 mm, width at VI to 0.3 to 0.35 mm, width at XI of mature worms 0.35 to 0.45 to mm, number of segments 32 to 38.
External features
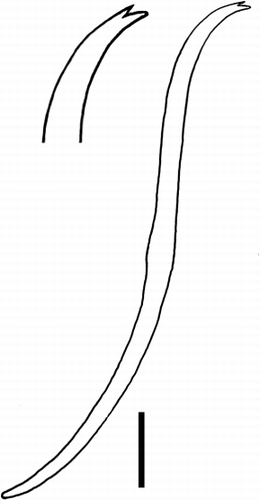
Prostomium bluntly conical in dorsal/ventral view, sometimes more squarish in lateral view. Anterior chaetae bifid crotchets with upper teeth much shorter than lower, posterior chaetae with upper tooth minute or absent, all chaetae sigmoid and with indistinct nodulus approximately medial. Anterior chaetae 60 to 70 µm long (measured as a straight line between base and tip) and 3 µm wide in II (at nodulus), increasing in length in subsequent few segments, so that chaetae along most of body 85 to 105 µm long and 4 µm wide at nodulus. Number of chaetae 3 to 7 (mostly 4 or 5) per bundle. Clitellum from 10/11 to about the level of the chaetae on XII, with about three-quarters of the cells being glandular and the rest hyaline. Crescent-shaped ventro-lateral male pores (exterior opening of the bursae) on postero-lateral edges of a broad ventral depression which runs the length of XI. Opening of bursae with particularly large and protruding epithelial cells on the lateral walls. Female pores within intersegmental furrow 11/12. Spermathecal pores above the line of the dorsal chaetae and about two-thirds of the distance between 9/10 and 10/11.
Internal somatic features
Pharynx in III with numerous muscular connections to body wall in II–V. Pharyngeal gland development variable but primarily ventro-lateral to lateral in clumps from IV to posterior of septa 5/6. No coelomocytes observed. A layer of chloragogue cells covering gut from VI. Oesophagus expanded and with taller lining cells in VIII, IX and sometimes X. Post-clitellar intestine intrasegmentally constricted (at about a third of the distance from anterior to posterior of the segment), as well as at each septa. In most of the specimens examined there are parasitic ciliates partially embedded in the walls of the fore-gut, especially in segments VIII to X. Paired nephridia present in VII to IX with lateral pores anterior to ventro-lateral chaetae, then again in most segments between XV and XVIII.
Genitalia
Small antero-ventral testes anteriorly in X. Ovaries larger than testes and present anteriorly in XI. Egg sacs extending posterior as far as XIV. Sperm sacs not observed but a few sperm morulae sometimes present in coelom of IX and X.
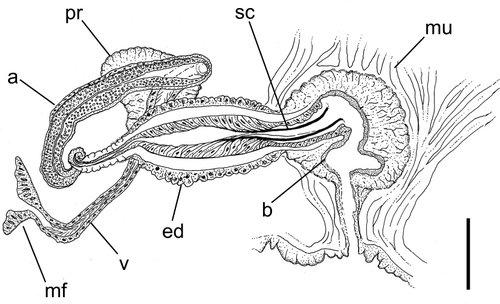
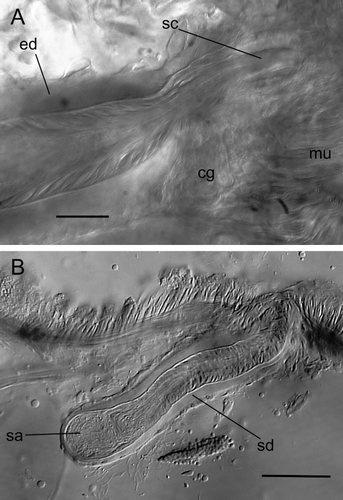
Sperm funnels 50–65 µm across at the flare (with cilia projecting forward by up to 40 µm), narrowing to 20–30 µm behind the flare, then narrowing further to 10 µm by the time it becomes the ental vas deferens. The ciliated vas expanding to the width of the atrium as it passes (or passes through) the prostate to join the atrium. Atrium 170–190 × 20–30 µm, consisting of a thin layer of peritoneal cells around a strongly vesiculated epithelial lining with cell walls often not visible, around a narrow ill-defined lumen. A compact prostate gland appears to be broadly attached to the ental portion of the atrium, although the precise nature of the connection(s) could not be determined. Atrium joining the ental end of an ejaculatory duct. Entally, the latter is a duct of about 80 µm in length and 15–20 µm in width, with well developed circular muscle and epithelial cells around a narrow lumen. This duct then expands into a spindle-shaped organ 180–240 µm long and 55–70 µm wide with a thick (12–15 µm) layer of spiral muscle fibres between a layer of peritoneal cells and epithelial cells with cuticular walls: the basements of the latter forming a well-defined line inside of the muscle layer. Epithelial cells directed posteriad and strongly elongated and tapered at their extremity, especially from the middle to ectal part of the ejaculatory duct, so that their apical walls combine to form a cuticular tube (‘sperm canal’ sensu Jamieson Citation1968) with medial width of 13–16 µm that projects into the anterio-medial wall of the dorsal chamber of the bursae. The epithelial lining cells of the ectal-most part of the ejaculatory duct are not elongated and are continuous with the epithelial tissue of the bursa to which it connects. There are muscle fibres encircling the region on the ejaculatory duct where it enters the bursa. The nature of the ectal end of the sperm canal cannot be discerned in the type material.
The internal structure of the bursa is difficult to discern from dissected or whole-mounted worms, but it appears to be at least two-chambered (or at least with the walls strongly folded inwards to form constrictions). It has a layer of epithelial cells surrounded by multiple layers of muscle fibres differing in their orientation. Additionally, there are muscle fibres that run in parallel from the ventral to dorso-lateral body wall and anchor the entire bursa to the lateral body wall. The upper half of the bursa is covered by diffuse accessory copulatory gland cells (sensu Erséus & Jamieson Citation1981) underlying the muscle fibres. In some of the examined specimens the bursa are protruded from the body a distance of about half of the body width. Within this protruded bursa, the ectal part of the muscular ejaculatory duct can be seen, though it is not clear how close the cuticular sperm canal then is to the opening of the bursa.
Spermathecae short (115–155 µm × 35 µm), hardly differentiated along their length other than a constriction at the pore and thinner epithelium at its ental extremity. Each spermatheca entirely covered by a thick (5–6 µm) layer of longitudinal muscle covered by a single layer of low peritoneal cells. Glandular cells are clustered around the base of the duct at the pore. For the ectal two-thirds to three-quarters of the spermathecae (this part being equivalent to a duct) the epithelial layer is broad, leaving only a small lumen. The epithelium becomes much thinner in the ental portion (the ampulla). Sperm in the ampulla is loose and occupies the entire lumen and there are often sperm lying along the lumen of the duct. The spermathecae lack a diverticulum.
Etymology
Named for Shelley McMurtrie, from EOS Ecology, New Zealand; leader of the freshwater team on the 2010–2011 Campbell Island Bicentennial Expedition.
Discussion
With its elongate atria, each with a prostate gland and well developed muscular ejaculatory ducts terminating in a cuticular sperm canal which protrudes into copulatory bursae covered by glandular cells, the new species appears to be most closely related to M. bennettae Jamieson Citation1968. The general arrangement and histology of the genitalia of the Campbell Island worms is very similar to that of M. bennettae, but there are sufficient differences in the genitalia to be certain that they are a separate species. In particular, the prostate runs along the length of the atrium in M. bennettae, whereas it is compact and ovoid to triangular in M. mcmurtrieae and certainly not present all along the atria. The ejaculatory duct is much broader in relation to its length in M. mcmurtrieae. The most conspicuous difference is the much simpler spermathecae. Whereas M. bennettae has a small diverticulum joining the spermathecal duct near its base, this is lacking in M. mcmurtrieae. Furthermore, the spermathecal duct is much narrower than the ampullae in M. bennettae and leads to lateral pores, whereas in M. mcmurtrieae the ampulla is hardly wider than the duct and the latter leads to dorsal pores.
Placing this new species in Macquaridrilus requires two modifications to the definition of the genus as defined by Jamieson (Citation1968): The spermathecae can possess (bennettae) or lack (mcmurtrieae) diverticulae and have pores that are lateral (bennettae) or dorsal (mcmurtrieae).
Erséus & Jamieson (Citation1981) described two Australasian genera Heronidrilus and Macquaridriloides and allied these with Macquaridrilus on the basis of similarities in the genitalia. Erséus (Citation1990) later synonymised Macquaridriloides and Heronidrilus and suggested that Macquaridrilus bennettae could belong in the same group. There is certainly little to distinguish Macquaridrilus from Heronidrilus other than that Macquaridrilus species possess the cuticular sperm canals and have prostate tissue that is more restricted in its covering of the atria. Both characters may be synapomorphies for the two species irrespective of what larger group they belong to.
Jamieson (Citation1968) suggested that M. bennettae might only recently have reached Macquarie Island, probably via other southern landmasses such as Campbell Island. This was on the basis that: (1) it was a freshwater species; (2) it appeared at that time to be related to freshwater genera such as Tubifex and Limnodrilus; (3) it seemed to have a restricted distribution on Macquarie Island (it is now known to be widespread; Marchant & Lillywhite Citation1994); and (4) there may not have been time since retreat of Pleistocene glaciers to have allowed speciation from a colonising ancestor (the island is now known to have been partly ice-free during the last glacial maximum; Selkirk et al. Citation1990). Canvassed dispersal mechanisms were rafting and avian transport.
Subsequent discovery of the clearly allied marine worms mentioned above led Erséus & Jamieson (Citation1981) to suggest that M. bennettae may have had a marine ancestry. There are certainly other tubificoid Naididae in the subantarctic/antarctic marine benthos, including Torodrilus Cook, 1970, which Erséus & Rota (Citation1996) suggested may also be related to Heronidrilus. The Macquarie Island and Campbell Island Macquaridrilus may, therefore, have had a common marine ancestor that colonised the limnic habitats of each island. Marchant & Lillywhite (Citation1994) noted that multiple invasions of subantarctic island freshwaters are known for some other marine lineages, such as some janirid isopods (Wilson & Wagele Citation1994) and planarians (Ball & Hay Citation1977). A hybrid hypothesis is that a marine ancestor of these Macquaridrilus species made a single invasion of freshwater and descendants have then island hopped and speciated. For some other oligochaetes, non-marine dispersal between subantarctic islands seems much more likely. Astacopsidrilus campbellianus Benham, 1909 belongs to a family (Phreodrilidae) that is almost entirely restricted to freshwater but occurs on both Campbell Island and Macquarie Island (Pinder & Brinkhurst Citation1997). Similarly, the cosmopolitan naidid Nais elinguis Müller, 1774, which also occurs on both islands (Marchant & Lillywhite Citation1994 and unpublished data), often occurs in brackish water but rarely in marine waters. There is growing evidence for avian transport of aquatic invertebrates (Green & Figuerola Citation2005; Green et al. Citation2008) and given the large numbers of seabirds using oceanic islands this method of dispersal for oligochaetes seems likely.
Acknowledgements
Thanks to Shelley McMurtrie and Amber Sinton at EOS Ecology for making the Campbell Island worms available to AMP and for providing funds for their identification. Thanks also to Professor Christer Erséus of the University of Gothenburg in Sweden for attempting to obtain molecular genetic information that would have helped understand the phylogenetic affinities of this species. The Campbell Island Bicentennial Expedition was made possible by the 50° South Trust (www.50south.org.nz), established to further research and education on New Zealand's subantarctic islands. Processing of the benthic aquatic invertebrate samples was supported by EOS Ecology and a Terrestrial and Freshwater Biodiversity Information System grant (TFBIS 278).
References
- Ball IR, Hay DR 1977. The taxonomy and ecology of a new monocelid flatworm from Macquarie Island (Platyhelminthes, Turbellaria). Bijdragen tot de Dierkunde 47: 205–214.
- Erséus C 1990. Marine Oligochaeta of Hong Kong. In: Morton B ed. The marine flora and fauna of Hong Kong and Southern China. Hong Kong, Hong Kong University Press. pp. 259–334.
- Erséus C, Jamieson BGM 1981. Two new genera of marine Tubificidae (Oligochaeta) from Australia's Great Barrier Reef. Zoologica Scripta 10: 105–110. 10.1111/j.1463-6409.1981.tb00489.x
- Erséus C, Rota E 1996. Tubificidae (Oligochaeta) from the Ross Sea (Antarctica), with descriptions of one new genus and two new species. Polar Biology 16: 491–496. 10.1007/BF02329068
- Green AJ, Figuerola J 2005. Recent advances in the study of long-distance dispersal of aquatic invertebrates via birds. Diversity & Distributions 11: 149–156. 10.1111/j.1366-9516.2005.00147.x
- Green AJ, Jenkins KM, Bell D, Morris PJ, Kingsford RT 2008. The potential role of waterbirds in dispersing invertebrates and plants in arid Australia. Freshwater Biology 53: 380–392.
- Jamieson BGM 1968. Macquaridrilus: a new genus of Tubificidae (Oligochaeta) from Macquarie Island. University of Queensland Papers, Department of Zoology 3: 55–69.
- Marchant R, Lillywhite P 1994. A survey of the stream invertebrate communities on Macquarie Island. Australian Journal of Marine and Freshwater Research 45: 471–481. 10.1071/MF9940471
- Pinder AM, Brinkhurst RO 1997. A review of the Phreodrilidae (Annelida: Oligochaeta: Tubificida) of Australia. Invertebrate Taxonomy 11: 443–523. 10.1071/IT95025
- Selkirk PM, Seppelt RD, Selkirk DR 1990. Subantarctic Macquarie Island: environment and biology. Cambridge, Cambridge University Press. 285 p.
- Wilson GDF, Wagele JW 1994. Review of the family Janiridae (Crustacea: Isopoda: Asellota). Invertebrate Systematics 8: 683–747. 10.1071/IT9940683