Abstract
Measuring hatching success in birds is important for assessing population viability, but nests are often cryptic and may be abandoned if birds are disturbed during nesting. We evaluated the suitability of Wildtech New Zealand Ltd's Chick Timer™ radio telemetry software for monitoring hatching success in little spotted kiwi (Apteryx owenii). The software provided accurate reports of activity levels and times of nest emergence by adult birds. Eighty four per cent of incubation attempts and 70% of first hatching events were detected, with no false indications of incubation or hatching. Our results suggest that this technology will facilitate studies of hatching success in little spotted kiwi and other large birds with cryptic or inaccessible nests. A ground truthing study such as this one, however, is required before full implementation of the software in any species.
Introduction
Understanding the ecology of a species is crucial to its conservation, but collecting ecological and life history data can be time consuming and challenging. In particular, an accurate measure of reproductive success is a necessary parameter for population modelling, including population viability analyses (Allendorf & Luikart Citation2007). Reproductive success is also widely used as a fitness indicator in inbreeding depression studies (Lacy et al. Citation1996; Brekke et al. Citation2010; Billing et al. Citation2012). In birds, hatching success is a frequently used proxy for reproductive output (Berg et al. Citation1992; Sebei et al. Citation2009; Brown et al. Citation2013). Measuring hatching success in the wild can be problematic due to the need to locate and monitor individuals over large geographic areas and relatively long time periods (Mayfield Citation1975). In species that are cryptic or nocturnal, locating a nest and determining its success can be especially difficult (Newman et al. Citation2009; Zangmeister et al. Citation2009). Measuring hatching success typically involves direct observation, making it time intensive and potentially disruptive for the individuals being monitored, which can lead to nest abandonment (Blackmer et al. Citation2004).
Radio telemetry can facilitate studies of reproductive success (Aldridge & Brigham Citation2001; Nguyen et al. Citation2003; Ziesemann et al. Citation2011). Increasingly sophisticated radio tag technology has been developed, and it is now possible to use radio tags to measure heart rate, temperature (Signer et al. Citation2010) and, crucially, motion (Acquarone et al. Citation2001). New Zealand-based wildlife technology company Wildtech Ltd has adapted motion sensor technology for use in its Chick Timer™ software.Footnote1 This software can be loaded into radio tags that are fitted to kiwi (Apteryx spp.) to monitor their reproduction remotely, as well as recording data on their activity patterns (SF1, SF2). Kiwi are cryptic and nocturnal ratites that nest in sometimes fragile, subterranean cavities. Some species of kiwi camouflage the entrance to their nest cavities (Heather & Robertson Citation2005) and are known to abandon incubation in the event of excessive human disturbance (R. Colbourne and H. Robertson, New Zealand Department of Conservation (DOC), pers. comm. June 2012). Thus, early detection of nests and minimising disturbance when monitoring kiwi nesting is vital.
To date, Chick Timer™ software has been used primarily to enable egg and chick collection for captive husbandry and reintroduction programmes for kiwi (Colbourne et al. Citation2005; Holzapfel et al. Citation2008), but not for scientific studies of hatching success in wild populations. The Chick Timer™ program notifies the user when a bird is incubating, which makes it easier and safer for researchers investigating hatching success in the field to locate nests for the first time, and permit remote data collection from anywhere within range of the tag. This enables information to be collected on activity and nest emergence times of incubating parents, nest abandonments, and hatching events; together, this information can facilitate nest monitoring while minimising disturbance. These advantages make Chick Timer™ software an attractive tool for measuring hatching success in kiwi and other cryptic and/or nocturnal species with a tendency to abandon nests when disturbed directly.
Apteryx owenii, the little spotted kiwi (LSK), is the second rarest kiwi species, currently numbering approximately 1700 birds (H. Robertson, DOC, pers. comm. June 2012). The species is restricted to eight isolated populations, and all individuals are descended from, at most, five birds translocated to Kapiti Island in the early 1900s (Ramstad et al. Citation2013). This bottleneck has resulted in extremely low genetic diversity in LSK (Miller et al. Citation2011; Ramstad et al. Citation2013) and understanding the impacts of this has been identified as a priority for their management (Holzapfel et al. Citation2008). Monitoring reproductive success in LSK is crucial to understanding their future viability.
Previous estimates of hatching success in LSK involved fitting individual males (the sole incubator in this species) with standard VHF radio tags, following them until a nest could be identified and then checking that nest during the night at regular intervals for the duration of incubation to ascertain whether or not an egg was present and whether or not it had hatched (Jolly Citation1989). This approach is thought to have led to nest failure in several cases due to a predatory endemic bird species, the weka (Gallirallus australis), being drawn to increased human activity around nest sites (Colbourne Citation1992) and an increased risk of abandonment by incubating males due to disturbance (R. Colbourne, DOC, pers. comm. June 2012). Despite its wide use in the other four species of kiwi, no variant of Chick Timer™ software has ever been used in LSK.
In this study, we trialled the North Island brown kiwi (NiB) version of the Chick Timer™ program as a tool for measuring hatching success in LSK. We specifically asked: 1. how accurate are the data outputs provided by NiB Chick Timer™ software for LSK; and 2. how reliable are the hatching success measurements facilitated by NiB Chick Timer™ software?
Materials and methods
Study site
Zealandia is a 225 ha mainland island sanctuary in Wellington, New Zealand consisting of native podocarp and introduced pine forest. The sanctuary is surrounded by a 2.2 m high fence that is impervious to introduced mammalian predators, which are the primary agents of decline in LSK. Forty LSK were introduced to Zealandia between 2000 and 2001 and the current population is c. 120 individuals (H. Robertson, DOC, pers. comm. June 2012).
Radio tags and software
A total of eight male LSK in Zealandia were fitted with V2L 152AVHF leg band radio tags (Sirtrack, Havelock North, New Zealand). Tags weighed 11 g and were specially designed to provide maximum battery life while remaining within the acceptable size and weight limits for an LSK tag (Robertson & Colbourne Citation2003). Birds were initially located using trained kiwi tracking dogs. All kiwi capture, handling and tagging was conducted according to the New Zealand Department of Conservation's Kiwi (Apteryx spp.) Best Practice Manual (Robertson & Colbourne Citation2003).
Tags were preloaded with NiB Chick Timer™ V3.5 for LSK (Wildtech Ltd, Havelock North, New Zealand) (SF1, SF2). Pulse output schemes were modified for use in LSK tags to provide maximum battery life, but the decision-making algorithms within the software were not changed. Birds were located and tag data collected in Zealandia using a Telonics TR4 receiver (Telonics, Mesa, Arizona, USA) and Yagi folding antenna (Kiwitrack, Havelock North, New Zealand).
Ground-truthing data
Four types of output were selected to test the accuracy of the NiB Chick Timer™ software in LSK: minutes of activity; emergence time; occurrence of incubation; and occurrence of hatching.
Activity
Activity recorded by the NiB Chick Timer™ software forms the basis of incubation status, hatching, emergence time and the trigger of the desertion alert as reported by the program (SF1) and therefore it was crucial to test the accuracy of this metric. Bushnell Trophy Cam – Model 11-9436c trail cameras (Bushnell, Kansas City, USA) were positioned between 1 and 3 m from nest entrances for five birds during November 2011–January 2012 (LSK nesting season) and used to record emergence and return times, allowing time away from the nest to be calculated. Tag data were collected for these five birds for five to 13 days per bird and video footage was downloaded from the cameras once a week. The corresponding video data and tag output data were then compared.
Emergence time
The time a bird actually exited the burrow or nest was monitored in two discrete time periods. During July 2011 (austral winter—outside of main LSK nesting season), four male birds fitted with NiB Chick Timer™ programmed tags were located every day for two weeks using radio telemetry. When the bird was found sleeping in a suitable burrow (i.e. one where the entrance could be seen clearly by an observer at night), an observer would return to that burrow before dusk, wait for the bird to emerge and record the time of emergence. The same bird was then tracked again the following day and the tag output recorded so that the corresponding time of emergence estimated by the software was also logged. Emergence time was recorded in this way five times for each of the four birds.
A second set of observed exit times was collected for four additional birds as well as one of the previous birds, using the trail cameras set up at nest entrances as detailed above. NiB Chick Timer™ data were collected on five to 14 days per bird from the radio tags on these birds and then matched up with the corresponding camera data.
Incubation and hatching signals
Each tag signal of incubation initiation (n = 13) and hatching (n = 15) was verified by direct observation. Suspected nests were visited once the male had exited for the night and a Taupe infra-red burrowscope (Sextant Technologies, Wellington, New Zealand) was used to confirm the presence of an egg. To verify hatching events, nests were staked out from before dusk as soon as possible after the hatching event was indicated by the software so that either the emergence of a chick could be observed or, once the male had left, the nest could be scoped to assess chick or egg presence or absence.
Analysis
Analyses were carried out and figures produced in R (R Development Core Team Citation2013). The alpha value for all statistical tests was set at 0.05. All data was tested for normality using a Shapiro-Wilks test.
Results
Activity
Tag-recorded activity values were paired with direct observations of birds entering and exiting the nest five to 14 times per bird (). The average difference in activity recorded by the software and that observed on the cameras ranged from 2–17 min between birds with the average difference across birds being 3 min (). Significant differences between tag and camera data were observed in two of the five birds, but only five software outputs spread across three different tags showed an error of more than 30 min (). The majority of differences were positive (), indicating that the software was more likely to report longer periods of activity than those observed based on camera-recorded nest exit and return times.
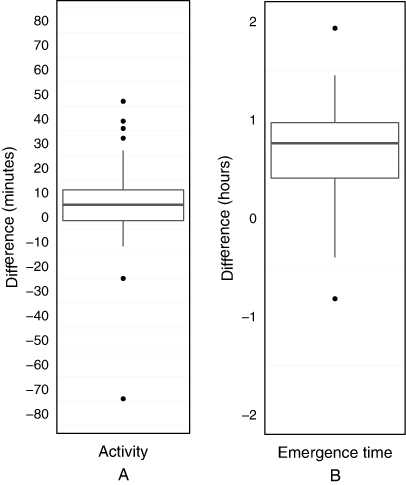
Table 1 Mean activity and emergence times recorded by NiB Chick Timer™ software and collected via direct observation for male LSK in Zealandia Sanctuary.
Emergence time
Paired data for emergence times recorded from tags and direct observations of bird emergence time varied from five to 14 observations per bird (). The software consistently recorded an earlier emergence time than that actually observed () and significant differences between tag data and that from direct observation were recorded in all birds except bird 1 (). The mean difference between recorded and observed emergence time for each tag was never more than 1 h and the majority (83%) of software outputs fell within an error of 1 h from the directly observed time ().
Predicting incubation and hatching
The NiB Chick Timer™ software detected 85% (11/13) of incubation attempts made by male LSK. The software also registered 80% (4/5) of hatches in single egg nests, 60% (3/5) of first hatches in two-egg nests and 60% (3/5) of second egg hatches in two-egg nests. In two cases, the tag missed the first hatching event in a clutch and recorded the second. Throughout the 2011–2012 field season, there were no false indications of incubation or hatching by the tag software.
Discussion
Hatching success is an important measure of reproductive success across bird species, but measuring hatching success in the wild remains problematic (Blackmer et al. Citation2004; Carey Citation2009, Citation2011). In this study, we tested the accuracy of the NiB Chick Timer™ software for measuring hatching success in LSK. We found the program to be an effective and highly suitable tool, although there are some caveats to its wider implementation.
Activity
All data outputs from Chick Timer™ software are based on the activity recorded by the tag; thus it is crucial that activity is measured accurately. Our results suggest the activity measured and reported by the software is very close to the birds' actual activity. Significant differences between tag activity reports and direct observation data were found in two birds. However, the majority of differences were less than 30 min and the largest difference for any tag on any night was 74 min. As the difference in activity levels between an incubating and non-incubating LSK male is, on average, c. 300 min per night (H. Taylor, unpubl. data), this level of error would not lead the software to give a false indication of incubation or, conversely, to miss an incubation event.
Reporting a longer period of activity than that observed could be due to birds moving around inside the nest before exit and/or after their return. The degree to which this occurs would depend on individual behaviour and space available within individual nests, leading to differences in accuracy between birds. Conversely, reporting shorter periods of activity than that indicated by nest exit and return times could be caused by the birds resting while away from the nest, reflecting the limitations of emergence and re-entry times as a proxy for activity levels.
Emergence time
In kiwi, emergence time is frequently used to plan researcher arrival time at burrows and nests for the purpose of catching males and chicks (especially during nesting season when emergence times can be erratic). As a result, Chick Timer™ software is designed to record an emergence time slightly earlier than that actually occurring, to avoid missing a bird exiting its burrow. In addition, Chick Timer™ software is only able to count time since emergence in hour intervals, leading to imprecise estimates. Thus, the statistically significant differences seen between tag-reported and directly observed emergence times across all but one bird in this study is accounted for as an artefact of the software design. As no one tag showed a mean difference of more than an hour, and estimates were almost always earlier than the real emergence time, the differences are, again, statistically, but not practically significant.
Incubation events
There were two instances where the software failed to register an incubation event. In both cases, the bird did not show the decreased activity expected when LSK are incubating, and the software was unable to distinguish their behaviour prior to and during nesting. This illustrates the importance of variation in individual behaviour with regards to the efficacy of software such as Chick Timer™. It can never be a complete replacement for verifying the incubation status of individuals because it is based on the average behavioural pattern for a given species. Both the birds that showed ‘abnormal’ activity levels during incubation went on to hatch a chick. Any ‘rules’ created for this kind of software for a given species are better described as guidelines and each time a tool like this is deployed in a new species (even within other kiwi species), ground-truthing studies such as this one will be important to establish the limitations of the software and to inform adjustments to its algorithms for that species where possible. In any study of hatching success, great care should be taken around birds that may be nesting, even if equipment such as a Chick Timer™ tag does not indicate incubation.
Hatching events
While the Chick Timer™ software reliably detected hatches in single-egg nests, and could register at least one hatch in a two-egg nest, it was unable to detect multiple hatch events in the same nest. LSK are commonly cited as incubating one egg at a time, with rare instances of two-egg clutches (Heather & Robertson Citation2005). Thus, the NiB version of the Chick Timer™ software was thought to be the most suitable for use in LSK as it is designed for detection of one hatching event. However, we have found higher instances of two-egg clutches in LSK than was previously reported (H. Taylor, unpubl. data). Modification of the software to detect more than one hatching event would be useful for future studies of LSK and other species with multiple eggs per clutch.
Applications and challenges
Chick Timer™ software provides a disturbance minimising monitoring tool that allows remote data collection. As such, it is also useful for field sites that cannot be accessed for direct observation on a regular basis. Bird species on islands that can be circumnavigated by boat can be monitored without researchers regularly landing on the island and, in larger areas, aerial monitoring technology can be fitted to light aircraft or helicopters to facilitate data collection and remote monitoring of large numbers of individuals (Wilks & Bramley Citation2010).
The main limitation currently for the broader use of Chick Timer™ software is its dependence on a two-stage radio transmitter, the smallest of which currently available weighs 11 g. For birds smaller than LSK (especially those that fly), the tags required for Chick Timer™ software would currently be too heavy. Tag technology is constantly developing, however, and lighter two-stage transmitters may soon be available. For larger species, Chick Timer™ software is highly adaptable in terms of the type and amount of data that can be collected for an individual, making it suitable for use outside of kiwi. It has already been adapted to monitor mating and incubation in another threatened New Zealand endemic bird, the kākāpō (Strigops habroptila) (J. Wilks, Wildtech New Zealand Ltd, pers. comm. November 2013) and could be used in other ratites, larger species of fowl, raptors and seabirds.
The data provided by the Chick Timer™ software is designed to be an approximation to guide researchers in their activities rather than to completely replace direct observation. Statistics such as the start of incubation or the date of hatch, for example, are usually accurate to within a few days rather than the exact date (J. Wilks, Wildtech New Zealand Ltd, pers. comm. November 2013). This could result in failures very early in incubation being missed, especially in species with shorter incubation periods, but this can be compensated for by using measures of hatching success such as the Mayfield method, which account for nest detectability (Mayfield Citation1975). Chick Timer™ software can save researchers' and managers' time, allowing a larger number of individuals to be monitored for hatching success. The resultant capacity to increase sample size and statistical power, reduce disturbance and conduct studies in remote or relatively inaccessible locations makes Chick Timer™ software an attractive option for researchers and conservation managers. We encourage the trialling of Chick Timer™ software in other bird species to enable collection of accurate hatching success data across diverse taxa.
Supplementary files
Supplementary file 1: How NiB Chick Timer™ tags record activity.
Supplementary file 2: Decoding a NiB Chick Timer™ tag output.
How NiB Chick Timer[TM] tags record activity.
Download PDF (38.5 KB)Decoding a NiB Chick Timer[TM] tag output.
Download PDF (36 KB)Acknowledgements
The authors wish to acknowledge the assistance and advice of John Wilks, Hugh Robertson and Rogan Colbourne throughout this study; Raewyn Epsom and the staff of Zealandia for access to kiwi and permission to use the sanctuary as a study site; Tristam Price for camera operation and maintenance; Judy Briggs, Erin Daldry, Andrew Digby, Karen Koopu, Judi Miller and Linton Miller for their assistance with observations and monitoring of LSK and two anonymous reviewers for their helpful comments on the manuscript. This study was supported by funding from the Allan Wilson Centre for Molecular Ecology and Evolution, Victoria University of Wellington, the New Zealand Ministry for Business, Innovation and Employment and Kaitiaki o Kapiti Trust.
Handling and sampling of kiwi was carried out under research permits from the New Zealand Department of Conservation (WE/31248/FAU) and Zealandia Sanctuary with approval of the Victoria University of Wellington Animal Ethics Committee, Waiorua Bay Trust, Kaitiaki o Kapiti Trust, and Port Nicholson Block Settlement Trust.
Notes
1. Chick Timer™ software is a commercial product that was not designed by the authors. Researchers interested in using Chick Timer™ software in their own studies should contact John Wilks at Wildtech at [email protected]
References
- Acquarone C, Cucco M, Malacarne G 2001. Daily and seasonal activity of moorhens studied by motion-sensitive transmitters. Waterbirds 24: 1–7. 10.2307/1522236
- Aldridge CL, Brigham RM 2001. Nesting and reproductive activities of greater sage-grouse in a declining northern fringe population. Condor 103: 537–543. 10.1650/0010-5422(2001)103[0537:NARAOG]2.0.CO;2
- Allendorf FW, Luikart G 2007. Conservation and the genetics of populations. Malden, Blackwell Publishing.
- Berg A, Lindberg T, Kallebrink KG 1992. Hatching success of lapwings on farmland: differences between habitats and colonies of different sizes. Journal of Animal Ecology 61: 469–476. 10.2307/5337
- Billing AM, Lee AM, Skjelseth S, Borg AA, Hale MC, Slate J et al. 2012. Evidence of inbreeding depression but not inbreeding avoidance in a natural house sparrow population. Molecular Ecology 21: 1487–1499. 10.1111/j.1365-294X.2012.05490.x
- Blackmer AL, Ackerman JT, Nevitt GA 2004. Effects of investigator disturbance on hatching success and nest-site fidelity in a long-lived seabird, Leach's storm-petrel. Biological Conservation 116: 141–148. 10.1016/S0006-3207(03)00185-X
- Brekke P, Bennett PM, Wang J, Pettorelli N, Ewen JG 2010. Sensitive males: inbreeding depression in an endangered bird. Proceedings of the Royal Society B 277: 3677–3684. 10.1098/rspb.2010.1144
- Brown JL, Steenhof K, Kochert MN, Bond L 2013. Estimating raptor nesting success: old and new approaches. Journal of Wildlife Management 77: 1067–1074. 10.1002/jwmg.566
- Carey MJ 2009. The effects of investigator disturbance on procellariiform seabirds: a review. New Zealand Journal of Zoology 36: 367–377. 10.1080/03014220909510161
- Carey MJ 2011. Investigator disturbance reduces reproductive success in short-tailed shearwaters Puffinus tenuirostris. Ibis 153: 363–372. 10.1111/j.1474-919X.2011.01109.x
- Colbourne R 1992. Little spotted kiwi (Apteryx owenii): recruitment and behaviour of juveniles on Kapiti Island, New Zealand. Journal of the Royal Society of New Zealand 22: 321–328. 10.1080/03036758.1992.10420825
- Colbourne R, Bassett S, Billing T, McCormick H, McLennan J, Nelson A et al. 2005. The development of Operation Nest Egg as a tool in the conservation management of kiwi. Science for Conservation 259. Wellington, Department of Conservation.
- Heather B, Robertson H 2005. The field guide to the birds of New Zealand. Auckland, Penguin.
- Holzapfel S, Robertson HA, McLennan JA, Sporle W, Hackwell K, Impey M 2008. Kiwi (Apteryx spp.) recovery plan 2008–2018. Threatened Species Recovery Plan 60. Wellington, Department of Conservation.
- Jolly JN 1989. A field study on the breeding biology of the little spotted kiwi Apteryx-owenii with emphasis on the causes of nest failures. Journal of the Royal Society of New Zealand 19: 433–448. 10.1080/03036758.1989.10421846
- Lacy RC, Alaks G, Walsh A 1996. Hierarchical analysis of inbreeding depression in Peromyscus polionotus. Evolution 50: 2187–2200. 10.2307/2410690
- Mayfield HF 1975. Suggestions for calculating nest success. Wilson Bulletin 87: 456–466.
- Miller HC, Bowker-Wright G, Kharkrang M, Ramstad K 2011. Characterisation of class II B MHC genes from a ratite bird, the little spotted kiwi (Apteryx owenii). Immunogenetics 63: 223–233. 10.1007/s00251-010-0503-7
- Newman J, Fletcher D, Moller H, Bragg C, Scott D, McKechnie S 2009. Estimates of productivity and detection probabilities of breeding attempts in the sooty shearwater (Puffinus griseus), a burrow-nesting petrel. Wildlife Research 36: 159–168. 10.1071/WR06074
- Nguyen LP, Hamr J, Parker GH 2003. Survival and reproduction of wild turkey hens in central Ontario. Wilson Bulletin 115: 131–139. 10.1676/02-123
- R Development Core Team 2013. R: a language and environment for statistical computing. Vienna, Austria, R Foundation for Statistical Computing.
- Ramstad KM, Colbourne RM, Robertson HA, Allendorf FW, Daugherty CH 2013. Genetic consequences of a century of protection: serial founder events and survival of the little spotted kiwi (Apteryx owenii). Proceedings of the Royal Society B 280: 20130576. 10.1098/rspb.2013.0576
- Robertson H, Colbourne R 2003. Kiwi (Apteryx spp.) best practice manual. Wellington, Department of Conservation.
- Sebei SK, Bergaoui R, Ben Hamouda M, Cooper RG 2009. Wild ostrich (Struthio camelus australis) reproduction in Orbata, a nature reserve in Tunisia. Tropical Animal Health and Production 41: 1427–1438. 10.1007/s11250-009-9331-x
- Signer C, Ruf T, Schober F, Fluch G, Paumann T, Arnold W 2010. A versatile telemetry system for continuous measurement of heart rate, body temperature and locomotor activity in free-ranging ruminants. Methods in Ecology and Evolution 1: 75–85. 10.1111/j.2041-210X.2009.00010.x
- Wilks J, Bramley A 2010. Sky ranger. In: National kiwi hui (South Island). Wellington, Department of Conservation. Pp. 13–14. http://www.kiwisforkiwi.org/wp-content/uploads/2012/09/2010_National_Kiwi_Hui_Proceedings.pdf ( accessed 22 January 2014).
- Zangmeister JL, Haussmann MF, Cerchiara J, Mauck RA 2009. Incubation failure and nest abandonment by Leach's storm-petrels detected using PIT tags and temperature loggers. Journal of Field Ornithology 80: 373–379. 10.1111/j.1557-9263.2009.00243.x
- Ziesemann B, Brunton DH, Castro IC 2011. Nesting success and breeding ecology in a high-density population of brown kiwi (Apteryx mantelli). Emu 111: 148–154. 10.1071/MU10011
