Abstract
Here we report on the presence of the deep-sea blind lobster Stereomastis suhmi in the Southwestern Atlantic. The species identification was based on morphological traits and confirmed with genetic barcoding. The specimen analysed shows little difference from previous descriptions. This work extends the range of S. suhmi in America, where it was previously limited to the Pacific coasts off Chile, to the mouth of the Rio de la Plata, Argentina, and confirms its circumpolar presence with molecular data. A review of all available records shows that the overall distribution of the species is well correlated with the presence of Antarctic Intermediate Waters suggesting that the species habitat is restricted to cold and relatively low-salinity/oxygen-rich waters.
Introduction
Polychelidan lobsters are noticeable among reptant decapods in having chelae on almost all pereopods (always on pereopods 1–4, but sometimes also on pereopod 5). The most recent phylogenetic analyses depict polychelidan lobsters as basal in the Reptantia, recognising a separate infra-ordinal status for the polychelidans (Ahyong Citation2009; Bracken-Grissom et al. Citation2014) with four families, from which only one, the Polychelidae Wood-Mason, 1875, is extant. Although evolved from shallow-water forms, extant Polychelidae have strongly reduced eyes, are confined to deep water, and are therefore known as ‘deep-sea blind lobsters’. Modern polychelids display burrowing habits (Gore Citation1984) and are scavengers and detritus feeders, although they appear to be also ambush predators, particularly of small epibenthic crustaceans (Cartes & Abello Citation1992). To date, Polychelidae contains six genera and 38 species worldwide (Chan Citation2010), of which only four species in three genera have been recorded from the southwestern Atlantic, all from Brazilian waters: Pentacheles laevis Bate Citation1878; Pentacheles validus A. Milne Edwards 1880; Polycheles typhlops Heller 1862 (Dall’Occo & Tavares Citation2004); and Stereomastis sculpta Smith 1880 (Ramos-Porto et al. Citation2000; mentioned as [Polycheles sculptus] in Dall’Occo & Tavares Citation2004).
Recently the RV Puerto Deseado, belonging to CONICET, started a series of expeditions extensively surveying the Argentinian marine fauna, particularly deep-sea areas that remained largely unexplored. As part of this programme, during September 2013 the campaign ‘Talud III’ was carried out with the specific aim of exploring the Mar del Plata Canyon, located off Mar del Plata (around 37.5°S, 54°W), Argentina. This submarine canyon is a perpendicular cut of the continental slope starting at 500-m depth that becomes V-shaped between 1200 and 3700 m, and finally opens to connect the continental shelf with the abyssal plains (Violante et al. Citation2010). Here we report the first record of the deep-sea blind lobster Stereomastis suhmi (Bate Citation1878), in the southwestern Atlantic, and discuss this new finding in the framework of the general distribution of the species and the related oceanographic features.
Materials and methods
During the ‘Talud III’ cruises of the RV Puerto Deseado, a total of 28 hauls were performed (16 with an otter bottom trawl net with 6-m headrope, 10-mm mesh size at the cod end; and 12 with a small epibenthic dredge with 0.6-m horizontal opening, 5-mm mesh size) between 774 m and 3320 m depth. The specimen of S. suhmi was collected with the bottom trawl, photographed immediately after it came on board and preserved in 96% ethanol. The carapace length (CL) was measured to the nearest 0.1 mm dorsally along the midline, from the base of the rostrum to the posterior margin of the carapace.
Species identification was based on morphology, following the keys and terminology in Bate’s original description (Bate Citation1878) and Galil (Citation2000) and Ahyong (Citation2009). To corroborate the species identification, a tissue sample was obtained from the abdomen of the specimen and used for molecular analysis of a partial fragment of the barcode gene cytochrome c oxidase subunit I (COI). DNA extraction, polymerase chain reaction amplification, polymerase chain reaction product purification and sequencing were conducted following the protocols of the Canadian Centre for DNA Barcoding (CCDB protocols http://ccdb.ca/resources.php; see also Ivanova et al. Citation2006), using the universal primers HCO2198 (5′-TAA ACT TCA GGG TGA CCA AAA AAT CA-3′), and LCO1490 (5′-GGT CAA CAA ATC ATA AAG ATA TTGG-3′). Identification was performed using standard nucleotide BLAST® (http://blast.ncbi.nlm.nih.gov/Blast.cgi) to search in the Barcode of Life (BOLD, http://www.boldsystems.org/) and GenBank (www.ncbi.nlm.nih.gov/GenBank) databases. The voucher specimen was deposited at the Museo Argentino de Ciencias Naturales ‘Bernardino Rivadavia’, Argentina under the catalogue number MACN-In 39629. The COI sequence obtained is available on GenBank under accession number KM503116, as well as in the ‘Native and exotic invertebrate of Mar del Plata harbour’ project (code INVMP) on the BOLD system.
Results
Material examined
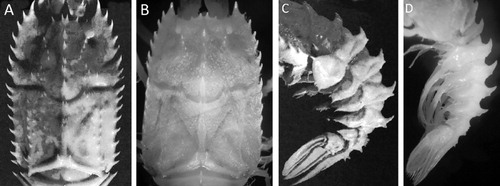
One male S. suhmi (), CL 15.7 mm, (right chela is missing), Mar del Plata Canyon, continental slope of Argentina, southwest Atlantic, 38°072′S, 54°123′W, 1800 m depth, 7 September 2013, MACN-In 39629.
Morphological diagnosis and molecular identification
Carapace distinctly longer than wide; dorsal orbital notch U-shaped; median carina on abdominal tergites 2–5 with a short, upright posterior tooth in addition to strong antrorse spine; inner angle of dorsal orbital sinus spinose (–). Examined but not shown in figures: epipod of pereopods 1–5 vestigial; dactylus and pollex of pereopods straight; outer proximal margin of basal antennular segment with two spines; ischium and merus of pereopod 2 articulated.
The 658 nucleotides of COI sequence obtained from our specimen showed 99% similarity with the mitochondrial COI sequence of an S. suhmi collected by RV Tangaora in Campbell Plateau, New Zealand (48°45′S, 172°036′E; 700 m depth) and published in GenBank (accession number: KF828018.1). It also matched (100% similarity) a sequence of another specimen of S. suhmi available on the BOLD system, also from southeast of New Zealand, confirming that the Argentine specimen and the previously sequenced specimens are conspecific.
Remarks
This specimen shows little difference with Bate’s (Citation1878) original description of S. suhmi and further descriptions elsewhere (Galil Citation2000; Ahyong Citation2009). In general all spines seem blunt and smaller than in previous descriptions (Griffin & Stoddart Citation1995; Galil Citation2000) and particularly the spines of the anterolateral angle of the carapace and those posterior to the post-cervical incision, appear to be more upwardly curved (see ). A similar pattern was described in at least one individual from off southern Africa (figure 9 in Calman Citation1925). Spines in the branchial carina are less conspicuous, low and smooth than previous descriptions. In all other respects this specimen is a typical S. suhmi. There are two rostral spines plus a smaller spine on the internal orbital angle. The orbital sinus is smooth, subtriangular and deep; there is no spine at the external orbital angle. The spinal formula of the lateral edge of the carapace is 5:2:8.
Discussion
The present work extends the range of S. suhmi in America, from the Pacific coasts off Chile and archipelago of Tierra del Fuego (Bate Citation1878; Arntz et al. Citation1999; Boschi & Gavio Citation2005), to the southwestern Atlantic, reaching north to almost the mouth of the Rio de la Plata (36°S). Hence, the species is expected to be present along the entire Magellanic Biogeographic Province, which encompasses the coasts from northern Chiloé Island on the eastern Pacific to the southwestern Atlantic waters, off the continent at 43°S, stretching north to reach 35°S () (Boschi & Gavio Citation2005).
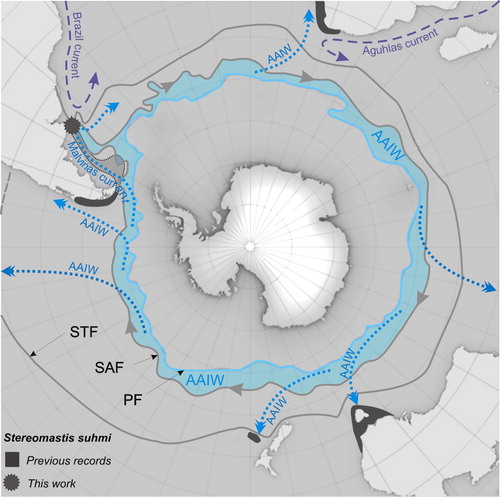
Stereomastis suhmi was recorded at depths ranging from 200 to 2200 m in southeastern Atlantic Ocean (western coast of South Africa), western Pacific Ocean (south-eastern Australia and New Zealand), and south-eastern Pacific Ocean (south-western coast of South America, west coast of Patagonia of Chile) (Calman Citation1925; Griffin & Stoddart Citation1995; Galil Citation2000; Ahyong Citation2012). To date the southernmost record of S. suhmi (larvae or adults) has been from the Pacific portion of the Magellanic region, south of the Beagle Channel around 2100 m depth on the continental slope (Arntz et al. Citation1999; Thatje et al. Citation2003). Although it was suggested that adult S. suhmi may occur in the deep of the Drake Passage and even further south (Thatje & Arntz Citation2004), recent polychelid lobsters are virtually absent south of the Polar front (Gorny Citation1999; Thatje & Arntz Citation2004; Griffiths et al. Citation2013). The only exception to date is a record of five mesopelagic larvae of S. suhmi found close to South Georgia Island, between 400 and 800 m depth (Tiefenbacher Citation1994).
Current records of S. suhmi fall mostly within the so-called antiboreal or transitional region (Gorny Citation1999), which is located between the Subtropical and Polar fronts (Orsi et al. Citation1995; also known as the Subtropical and Antarctic Convergences respectively) depicting a rather circumpolar distribution (). Nevertheless, circumstantial records of adult S. suhmi far north of the Subtropical front in waters off West Africa (in the Cameroon volcanic line at c.3°S–3°E; Ocean Biogeographic Information System challenge this idea. (Specimen collected by the South Atlantic MAR-ECO 2009 cruise EurOBIS. The record is published in the Ocean Biogeographic Information System with an error in the collection depth, which is actually 997 m. Both the species ID and collection depth were confirmed by Dr Irene A. Cardoso in a personal communication to NEF.) The virtual absence of the species in areas where one would expect it to be based solely on a continuous circumpolar distribution (e.g. southeastearn Africa) also challenges this idea.
Because the species is clearly limited to deep bottoms, an explanation of its current geographic distribution requires taking into account the circulation pattern of the deep water masses, which has proved to be the main explanatory factor for the diversity and biomass of deep-sea decapods (e.g. Cartes et al. Citation2014 and references therein). At the depth range and areas inhabited by adult S. suhmi and their larvae (Thatje et al. Citation2003) the predominant oceanographic feature is the presence of the cold and relative low-salinity/oxygen-rich Antarctic Intermediate Water (AAIW) formed in the Antarctic Convergence zone, where it sinks and is distributed into all ocean basins, to lastly spread northward, filling most of the world’s ocean at intermediate depths (c.1000 m) (Talley Citation1999). Branches of AAIW that flow towards the equator, carry cold and relatively fresh subantarctic water that can be identified in intermediate waters as far north as 20°N, with trace amounts as far as 60°N (Talley Citation1999). The new record for S. suhmi reported here occurred precisely within the northern limit of the Malvinas Current, which transports subantarctic waters northwards and extends all the way to the sea-floor (Boschi & Gavio Citation2005). Similarly, the record of S. suhmi close to the equator off West Africa, may be explained also by the presence of a branch of AAIW that stretches northeast along the African slope, reaching as far north as the Canary Archipelago (28°N) (Machín & Pelegrí Citation2009). In both cases many hauls were performed with different sampling gear but only one specimen was collected. The seeming rarity of the species at these two locations should not be surprising considering that both records are at the limits of AAIW branches, i.e. at the periphery of the supposed species optimal distribution.
On the other hand, the absence of S. suhmi in well-explored, deep-sea areas (where other Polychelidae are commonly found) also supports a species distribution influenced by the presence of AAIW. For example, explorations in the southern continental slope of Brazil found many polychelidan species other than S. suhmi (Ramos-Porto et al. Citation2000; Dall’Occo & Tavares Citation2004), indicating that the species does not extend north beyond the influence of the Malvinas Current. In turn, the absence of S. suhmi off East Africa supports the association of S. suhmi with cold AAIW because the Agulhas Current blocks the eastward penetration of AAIW from the Atlantic (Piola & Georgi Citation1982). In summary, the current distribution of S. suhmi appears strongly correlated with the presence of intermediate subantarctic waters, suggesting that the species is restricted to cold and relative low-salinity/oxygen-rich waters.
Associate Editor: Dr Jonathon Banks.
Acknowledgements
We want to acknowledge the crew of the RV Puerto Deseado and colleagues for their assistance during the cruise. We are grateful for the support provided by the Government of Canada through Genome Canada and the Ontario Genomics Institute to the International Barcode of Life Project. This funding enabled the Canadian Centre for DNA Barcoding (University of Guelph) to carry out the sequence analysis on our specimen. We also thank the Ontario Ministry of Economic Development and Innovation for funding the ongoing development of BOLD. The present work was supported by PIP 830/ 2012 (CONICET—Consejo Nacional de Investigaciones Científicas y Técnicas de Argentina) and EXA 618/12 (UNMdP—Universidad Nacional de Mar del Plata, Argentina). NEF was also supported by CONICET through a postdoctoral fellowship. Lastly, we want to thank the two anonymous referees for their helpful suggestions and comments.
References
- Ahyong ST 2009. The polychelidan lobsters: phylogeny and systematics. In: Martin JW, Crandall KA, Felder DL eds. Decapod crustacean phylogenetics. Boca Raton, FL, CRC Press. Pp. 369–396.
- Ahyong ST 2012. Polychelid lobsters (Decapoda : Polychelida) off Central Queensland, with a summary of Australian and New Zealand distributions. Memoirs of the Queensland Museum - Nature 56: 1–7.
- Arntz W, Gorny M, Soto R 1999. Species composition and distribution of decapod crustaceans in the waters off Patagonia and Tierra del Fuego, South America. Scientia Marina 63: 303–314.
- Bate CS 1878. On the Willemoesia group of Crustacea. Annals and Magazine of Natural History 5: 273–283.
- Boschi E, Gavio M 2005. On the distribution of decapod crustaceans from the Magellan Biogeographic Province and the Antarctic region. Scientia Marina 69: 195–200. 10.3989/scimar.2005.69s2195
- Bracken-Grissom HD, Ahyong ST, Wilkinson RD, Feldmann RM, Schweitzer CE, Breinholt JW et al. 2014. The emergence of lobsters: phylogenetic relationships, morphological evolution and divergence time comparisons of an ancient group (decapoda: achelata, astacidea, glypheidea, polychelida). Systematic Biology 63: 457–79.
- Calman WT 1925. On macrurous decapod Crustacea collected in South African waters by the S.S. ‘Pickle.’ Reports of the Fisheries and Marine Biological Surveys of South Africa 4: 1–26.
- Cartes J, Abello P 1992. Comparative feeding habits of polychelid lobsters in the Western Mediterranean deep-sea communities. Marine Ecology Progress Series 84: 139–150. 10.3354/meps084139
- Cartes J E, Papiol V, Frutos I, Macpherson E, González-Pola C, Punzón A et al. 2014. Distribution and biogeographic trends of decapod assemblages from Galicia Bank (NE Atlantic) at depths between 700 and 1800m, with connexions to regional water masses. Deep Sea Research Part II: Topical Studies in Oceanography 106: 165–178. 10.1016/j.dsr2.2013.09.034
- Chan T 2010. Annotated checklist of the world’s marine lobsters (Crustacea: Decapoda: Astacidea, Glypheidea, Achelata, Polychelida). The Raffles Bulletin of Zoology 23: 153–181.
- Dall’Occo PL, Tavares M 2004. New and additional records of deep-water blind lobsters from Brazil (Decapoda, Polychelidae). Nauplius 12: 143–149.
- Galil BS 2000. Crustacea Decapoda: review of the genera and species of the family Polychelidae Wood-Mason, 1874. Mémoires du Muséum national d’Histoire naturelle 21: 285–387.
- Gore RH 1984. Abyssal lobsters, genus Willemoesia (Palinura, Polychelidae), from the Venezuela Basin, Caribbean Sea. Proceedings of the Academy of Natural Sciences of Philadelphia 136: 1–11.
- Gorny M 1999. On the biogeography and ecology of the Southern Ocean decapod fauna. Scientia Marina 63: 367–382. 10.3989/scimar.1999.63s1367
- Griffin DJG, Stoddart HE 1995. Deep-water decapod Crustacea from eastern Australia: lobsters of the families Nephropidae, Palinuridae, Polychelidae and Scyllaridae. Records of the Australian Museum 47: 231–263. 10.3853/j.0067-1975.47.1995.239
- Griffiths HJ, Whittle RJ, Roberts SJ, Belcher M, Linse K, Archambault P 2013. Antarctic crabs: invasion or endurance? PLoS One 8: e66981.
- Ivanova NV, Dewaard JR, Hebert PDN 2006. An inexpensive, automation-friendly protocol for recovering high-quality DNA. Molecular Ecology Notes 6: 998–1002. 10.1111/j.1471-8286.2006.01428.x
- Machín F, Pelegrí JL 2009. Northward penetration of Antarctic intermediate water off northwest Africa. Journal of Physical Oceanography 39: 512–535.
- Orsi AH, Whitworth III T , Nowlin WD Jr. 1995. On the meridional extent and fronts of the Antarctic circumpolar current. Deep Sea Research Part I: Oceanographic Research Papers 42: 641–673. 10.1016/0967-0637(95)00021-W
- Piola AR, Georgi DT 1982. Circumpolar properties of Antarctic intermediate water and Subantarctic Mode Water. Deep Sea Research Part A. Oceanographic Research Papers 29: 687–711. 10.1016/0198-0149(82)90002-4
- Ramos-Porto M, Viana GFS, Silva KCA, Cintra IHA, Coelho PA 2000. Stereomastis sculpta (Smith, 1880) (Decapoda: Polycheloidea: Polychelidae) in Brazilian waters. Nauplius 8: 249–251.
- Talley L 1999. Some aspects of ocean heat transport by the shallow, intermediate and deep overturning circulations. In: Clark PU, Webb RS, Keigwin LD eds. Mechanisms of global climate change at millennial time scales. Geophysical Monograph Series. Washington, DC, American Geophysical Union. Pp. 1–22.
- Thatje S, Arntz WE 2004. Antarctic reptant decapods: more than a myth? Polar Biology 27: 195–201.
- Thatje S, Schnack-Schiel S, Arntz W 2003. Developmental trade-offs in Subantarctic meroplankton communities and the enigma of low decapod diversity in high southern latitudes. Marine Ecology Progress Series 260: 195–207. 10.3354/meps260195
- Tiefenbacher L 1994. Decapode Crustaceen aus westantarktischen Gewässern gesammelt von der R.V. “John Biscoe”, Reise 11. Spixiana 17: 13–19.
- Violante RA, Paterlini CM, Costa IP, Hernández- Molina FJ, Segovia LM, Cavallotto JL et al. 2010. Sismoestratigrafía y evolución geomorfológica del talud continental adyacente al litoral del este bonaerense, Argentina. Latin American Journal of Sedimentology and Basin Analysis 17: 33–62. 10.3354/meps260195

