Abstract
Repellents used to reduce by-kill of birds during pest control must not compromise acceptance by target species. Two repellents combined, anthraquinone (AQ; 0.4 g kg−1) and d-pulegone (DP; 1.0) did not reduce the palatability of blue-coloured carrot baits to laboratory rats (Rattus norvegicus); nor did DP (2.0). Green-coloured carrot baits coated with AQ, DP or AQ + DP were taken from bait stations by wild possums (Trichosurus vulpecula) and rats. Toxic (1080) bait coated with AQ (0.4) and peanut oil (0.1) had reduced palatability but was accepted by laboratory rats. However, laboratory rats did not consume enough baits coated with AQ and bacon, peanut butter, cinnamon or DP to be killed. Anthraquinone (0.4 or 0.8) plus cinnamon and DP (0.5) did not affect palatability or lethality to captive ship rats (R. rattus) or possums. Anthraquinone and DP as surface coatings on baits are therefore acceptable to possums and possibly rats, at concentrations that deter some bird species.
Introduction
The Australian brushtail possum (Trichosurus vulpecula), an introduced omnivorous marsupial, is a serious conservation and agricultural pest in New Zealand (Montague Citation2000). Predation on native fauna and competition for food by ship rats (Rattus rattus) and Norway rats (R. norvegicus) also has negative impacts (Innes Citation2005a,b). These pests are often controlled by aerial distribution of carrot or cereal baits containing sodium fluoroacetate (compound 1080). However, the baits are also accessible to non-target species, including native birds (Spurr Citation2000). The North Island robin (Petroica longipes) is particularly at risk from consumption of mammalian pest baits in New Zealand forests because it is a ground-feeding insectivore (Brown Citation1997; Powlesland et al. Citation1999). The risk to native fauna is unacceptable to some members of the public (Fitzgerald et al. Citation2000). The standard procedure in New Zealand to reduce consumption of baits by birds is to eliminate small bait fragments, and to dye the baits green as is required by law. Green is considered to be a colour that is avoided by some bird species (Caithness & Williams Citation1971), and may make baits less conspicuous against vegetation (Schmidt et al. Citation2004). Green-dyed baits are acceptable to possums (Morgan Citation1990; Day & Matthews Citation1999). While rats have rudimentary colour vision (Jacobs et al. Citation2001), and therefore might have the ability to discriminate between differently coloured foods, dyed baits are acceptable if they are odourless and tasteless (Marsh Citation1985). Green-dyed baits are commonly used for rat control (Veitch & Clout Citation2002).
Cinnamon oil, used as an olfactory lure for possums over short distances (Morgan et al. Citation1995) and to mask the taste of 1080 (Morgan Citation1990), is thought to act as a primary repellent to some birds, including the North Island kākā (Nestor meridionalis) (Udy & Pracy Citation1981; Hickling Citation1997). Despite the precautionary measures of using lures and colouring baits, ground-feeding species remain at risk (Powlesland et al. Citation2000; Veltman & Westbrooke Citation2011). Day et al. (Citation2003) found that North Island robins pecked at cinnamon-flavoured baits and Orr-Walker et al. (Citation2012) concluded that cinnamon oil is not an effective repellent for kea (Nestor notabilis) at standard concentrations used in bait manufacture.
Chemical repellents can reduce the consumption of foods and baits by birds. Anthraquinone (AQ) is a secondary repellent, inducing a learned taste aversion to treated foods (Avery et al. Citation1997). It is used to deter birds from feeding on crops (Avery et al. Citation1998; Werner et al. Citation2011a, Citation2014) as well as preventing non-target birds from eating toxic baits (Werner et al. Citation2011b). D-pulegone (DP) is a mint derivative that is both a primary repellent, acting as a sensory irritant, and a secondary repellent, inducing post-ingestional malaise (Mason Citation1990; Avery et al. Citation1996). Its efficacy as a bird repellent has been demonstrated on starlings (Sturnus vulgaris) (Mason Citation1990), red-winged blackbirds (Agelaius phoeniceus) (Avery et al. Citation1996) and cowbirds (Molothrus ater) (Belant et al. Citation1997). Individually, AQ and DP reduced feeding by pigeons (Columba livia) on maize seed in aviaries, but did not completely protect seedlings from damage by pigeons (Esther et al. Citation2013). In New Zealand, combinations of these compounds (0.4–1 g kg−1 AQ and 0.5–2 g kg−1 DP) as surface coatings can be effective at deterring house sparrows (Passer domesticus) from eating wheat and North Island robins from eating mammalian pest baits (Day et al. Citation2003, Citation2012; Clapperton et al. Citation2012, Citation2014). Captive kea reduced consumption of cereal pellets when they contained both AQ and DP (Orr-Walker et al. Citation2012).
Secondary repellents work most effectively when paired with a distinctive sensory cue (Nelms & Avery Citation1997). Previous studies have shown that colour is an effective cue in establishment of food aversions in sparrows and North Island robins (Clapperton et al. Citation2012, Citation2014). As colour can also act as a primary repellent and, because both sparrows and North Island robins have been shown to find the colour blue more repellent than green (Hartley et al. Citation1999; Clapperton et al. Citation2012), blue was selected as the colour for further testing of bird repellents for mammalian baits in this study.
To be of use in protecting non-target species, bird repellents also need to be acceptable to the target species. Birds and mammals have different sensitivities to taste stimuli and irritants (Kare Citation1970; Beauchamp & Mason Citation1991; Mason et al. Citation1991), but there is also variation among mammalian species. For example, the bird repellent cinnamamide was acceptable to possums (Spurr & Porter Citation1998), but it was unacceptable to Norway rats or mice (Mus domesticus) at a concentration effective at deterring rock doves (Columba livia) (Crocker et al. Citation1993; Gurney et al. Citation1996; Watkins et al. Citation1998; Spurr et al. Citation2001). Meanwhile, methyl anthranilate and ortho-aminoacetophenone are repellent to mice (Nolte et al. Citation1993) but are tolerated by rats (Spurr et al. Citation2001).
Since our experiments have been conducted, Cowan et al. (Citation2014) have extended our work to assess the response of captive wild possums and rats to AQ and DP incorporated into cereal baits. They referred to our unpublished experiments and we now document the results of those experiments in this paper. We aimed to test whether concentrations of AQ and DP, which have been shown to adequately deter sparrows and North Island robins from feeding, do not compromise uptake by mammalian pests.
Cowan et al. (Citation2014) queried whether baits containing AQ would be acceptable to rats. Accordingly, we also report experiments that attempted to minimise the deterrent effects of the taste or smell of bird repellents through the addition of lures that may make the baits more attractive to the target pests. For example, rats are attracted to animal and vegetable fats including peanut and bacon (Taylor & Thomas Citation1989; Clapperton Citation2006). Peanut butter is the standard bait used in rat traps in pest control operations in New Zealand (Cunningham & Moors Citation1996). Peanut oil did not reduce the repellency of AQ to sparrows in a previous study (Clapperton et al. Citation2012).
Repellents also need to be cost effective. While DP is an effective primary repellent, it is likely to add substantially to the cost of a poisoning programme (Avery et al. Citation1996). Pennyroyal oil, from which DP is derived, might offer a cheaper mint option. In combination with 0.4 g kg−1 AQ, 5 g kg−1 pennyroyal oil reduced consumption of wheat by sparrows to below 40% (Clapperton et al. Citation2012). It was also nearly as effective as pulegone at deterring red-winged blackbirds from consuming rice seed (Avery et al. Citation1996).
Here we report the results of experiments designed to assess the acceptance and palatability to possums and rats of blue- or green-coloured pest control baits containing AQ and DP (or pennyroyal oil), with or without other additives attractive to the pest species. We also assess whether acceptance is sufficiently high to ensure poison efficacy.
This study comprised a series of experiments that were part of a more extensive research project developing bird repellent strategies. While a wide range of chemical repellents were being tested on sparrows in 1999 (Day et al. Citation2012), we started concurrent trials on acceptance of these compounds by the pest mammals. The initial experiment on laboratory rats began in December 1999. It assessed palatability of DP at 2 g kg–1 and combinations of DP (1–3 g kg−1) and AQ (0.4–1.2 g kg–1). It was followed by two field experiments in May 2000, targeting wild possums and rats, to determine whether it was worth further refining the AQ–DP repellent combinations (0.4 or 0.8 g kg−1 AQ and 1 or 2 g kg−1 DP). Here we used green-dyed baits, as required by law, when they can be accessed by the public. This led to a series of four captive animal efficacy experiments, which were conducted between 2002 and 2004. These experiments were conducted following trials on the responses of sparrows and native species to bird repellents (Day et al. Citation2003; Clapperton et al. Citation2012; B.K. Clapperton et al., unpubl. data), so the choices of treatments were in part dictated by the results of those bird trials, as well as the previous palatability and field acceptance experiments. As well as testing the two concentrations of AQ (0.4 and 0.8 g kg−1), we also tested the addition of higher and lower doses of DP (0.5 and 0.01 g kg−1) and the alternative mint extract, pennyroyal oil (0.5 g kg−1). We also tested the addition of flavours thought to be attractive to rats (Meehan Citation1984; Taylor & Thomas Citation1989), including cinnamon, peanut and bacon.
Methods
Ethics approval
Experimental protocols were approved by an independent animal ethics committee (Ruakura Animal Ethics Committee approvals 3928, 4362, 4668 and4687).
Experiment 1: Bait palatability (laboratory rats)
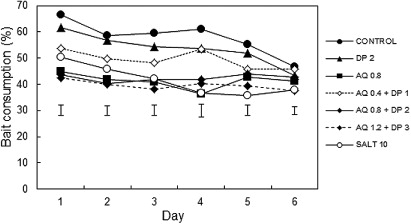
Experiment 1 was conducted on laboratory rats (60 Sprague-Dawley adult males, body weight 185–295 g) bred and housed at the Ruakura small animal colony (SAC). Only male rats were available at the time of the experiment; however, preliminary trials found that males and females respond similarly to carrot treated with either AQ or DP (T.D. Day and B.K. Clapperton, unpubl. data). The seven bait treatments tested are listed in . The bird repellent compounds included AQ in the form of the commercially available formulation AvexTM (containing 42% 9,10-anthraquinone, CAS No. 84-65-1; Loveland Industries Inc., Loveland, CO, USA) and d-Pulegone (CAS No. 89-82-7, 85% technical grade, Aldrich Chemical Company Inc., USA). The concentrations of DP cited here are for the full product, thus 2 g kg−1 DP used here is equivalent to the 0.17% DP used by Cowan et al. (Citation2014). The cinnamon oil was from Bush Boake Allen Ltd (Auckland, New Zealand). Baits were coloured green for control and blue for bird repellent. Salt baits were used as a negative control, as food containing high salt concentrations is known to be unpalatable to possums (T.D. Day, unpubl. data).
Table 1 Mean (±SE) amount of bait consumed by the Norway rats (n = 60) in the palatability experiment averaged over 6 day replicates.
The treatments were prepared as solutions in water and dye (0.01% wt/wt royal blue permanent colour powder H6406, International Flavours and Fragrances, Auckland, or 0.01% wt/wt special green V200A dye, Bayer NZ Ltd, Auckland) and applied to freshly cut carrot slices at appropriate concentrations.
The laboratory rats were tested individually in their home cages. The rats were maintained on the AgResearch SAC cereal-based pelleted rodent diet and water, and randomly allocated to a treatment group. They were given a measured quantity of pellets on the 3 days prior to the start of the experiment and throughout the experiment, to ensure that the pellet diet was available ad libitum at all times. On 6 consecutive days, 50 g of freshly prepared carrot bait (c. 3–4 carrot slices) was provided. During the trial, the food was placed in piles on either side of the cage. Position of pellets and carrot bait was alternated on experimental days. The amount of carrot bait eaten by each rat was determined by weighing the remaining bait at the end of each 24 h period. Bait weight change from desiccation or water absorption was not calculated as the experiment was conducted under stable laboratory-controlled temperature and humidity conditions and there was no evidence to suggest different carrot bait formulations would have desiccated or rehydrated at different rates. Data are expressed as the percentage of the total food consumption per day. Each treatment had a sample size of eight rats. The means across the 6 days were analysed using a one-way ANOVA with Tukey's multiple comparison test. Changes over time were analysed with a repeated measures analysis and with one-way ANOVA of the change over the 6 days, day 1–day 6.
Experiment 2: Field acceptance of baits (possums and rats)
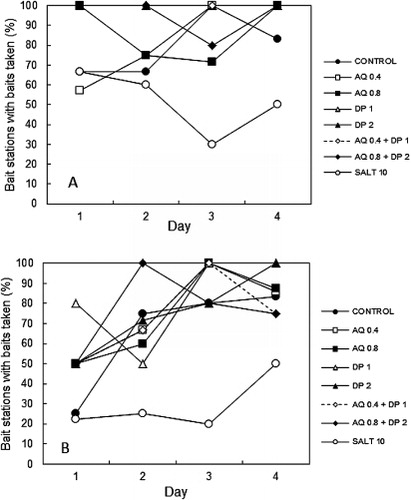
Field experiments were conducted on possums (Experiment 2.1) and rats (Experiment 2.2) at Whatawhata, Waikato, New Zealand, over 4 days in the austral autumn. Bait stations were established in a bush–pasture margin, and in forest habitats. The possum-specific bait station comprised a 450 mm high wire stake with the bait in a perforated paper bag attached to the top of the wire. A plastic disc attached to the stake below the bait prevented rodents climbing the wire. The rat-specific bait station comprised a 60 mm diameter, 300 mm long, PVC tube, pinned to the ground by a holding stake, with one bait placed in the centre. The bait stations enabled us to determine the response of possums or rats to bait without other species interfering with the baits. Plasticine was used on both types of bait station to record the presence (e.g. footprints, claw marks, bite marks) and species of animals present at the bait station, even when baits had not been touched. Additionally, fresh faecal material around or in the bait station was used to determine when animals had been present. We did not distinguish between signs of ship rats and Norway rats. Both species inhabit pasture margins, while the ship rat is the more common species in New Zealand forests and rural areas (Innes Citation2005a,b; Morgan et al. Citation2006). The eight bait treatments compared are listed in . The treatments were applied to freshly sliced carrot baits each morning before being placed in the field during the day. This mimics the procedure used in field poisoning operations, when the repellent is added to the toxic solution, as reported by Clapperton et al. (Citation2014). The treatments were prepared as solutions in water and green dye (0.01% wt/wt special green V200A dye, Bayer NZ Ltd, Auckland) at appropriate concentrations. For example, for 0.8 g kg−1 AQ, a 2% solution of Avex™ (0.42% active ingredient) was prepared and added to the carrot bait at a 1:10 ratio. Salt baits were used as a negative control.
Table 2 Percentage of bait stations with bait taken over 4 nights in the field acceptance experiments.
Eighty-eight bait stations for each species (11 replicates per treatment) were set out on four transects, with the eight treatments randomly allocated along each transect line. Each transect line (22 bait stations per line; four possum transects and four rat transects in total) and individual bait stations along each line were a minimum of 200 m apart, to reduce the probability of multiple bait station encounters by individual possums or rats. Each possum and rat station was baited with one 4 g non-toxic repellent or control treated carrot bait for 4 nights, with single, freshly made baits placed in each bait station each day to maintain consistent freshness. The bait stations were assessed every day to determine bait take, and animal sign and video observations were collected in the evenings from eight randomly selected stations per night to observe animals interacting with the bait stations. The stations were categorised as: ‘not touched’ or ‘disturbed by another species’, ‘investigated with < ½ the bait eaten’ or ‘bait taken with ≥ ½ the bait eaten’. None of the possum stations was disturbed by other species. For the rat stations, 9.9% of the data were excluded because of interference by possums and mice. For each station we calculated the number of nights it was visited by rats and the number of those times the bait was eaten. We analysed the proportion of visits on which the bait was eaten using a logistic regression allowing for extra-binomial variation. The effects included in the model were transect (found to have no effect and so then discarded), and bait treatment.
Experiment 3: Bait acceptability (possums and rats)
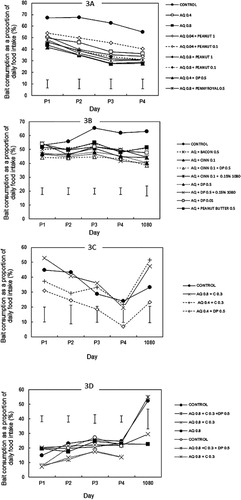
Four experiments (3.1–3.4) were conducted on rats and possums to determine both the palatability and efficacy of toxic baits containing various formulations of bird repellent and rodent attractant materials (). Experiment 3.1 used laboratory rats to assess two concentrations of AQ, with or without DP or pennyroyal oil, or two concentrations of peanut oil. Experiment 3.2 tested further rat attractants (cinnamon, bacon and peanut butter) combined with the lower rate of AQ on laboratory rats. It also compared two concentrations of DP, and the effect of two different rates of 1080. Experiment 3.3 assessed the two AQ concentrations and the combination of AQ and DP or cinnamon on captive ship rats. AQ with or without DP and/or cinnamon was tested on possums in Experiment 3.4.
Table 3 Bait consumed as a percentage of total food consumption per day by possums and rats in the efficacy Experiments 3.1–3.4 averaged over 4 day replicates, and the outcome of the toxic bait presentation.
The AQ, DP and cinnamon oil were sourced as for Experiment 1. The pennyroyal oil and peanut oil were from Bronson & Jacobs Pty Ltd (Auckland, New Zealand). The bacon and peanut butter flavours were from Bush Boake Allen Ltd (Auckland, New Zealand). The test materials were formulated in water with the addition if necessary of monopropylene glycol and surface coated onto carrot baits (as described for the field acceptance experiments) or 30 g RS5 cereal bait (Animal Control Products Ltd, Wanganui, New Zealand) in three treatment groups in Experiment 3.4. RS5 baits are commonly used in possum control operations in New Zealand (Cowan et al. Citation2014).
Experiments 3.1 and 3.2 were conducted on laboratory Sprague-Dawley rats held in individual cages at the AgResearch SAC at Ruakura (90 males, initial mean body weight 341 ± 42 g in Experiment 3.1, 50 male, 50 female, mean body weight 318 ± 84 g in Experiment 3.2). They were fed as described for Experiment 1. The ship rats tested in Experiment 3.3 (19 male, 10 female, three unknown sex, mean body weight 145 ± 26 g, weighed at the end of experiment) were live trapped from bush habitat near Maungatautari, Waikato, and individually housed in the Animal Behaviour and Welfare Centre animal house in Ruakura. They were acclimatised for at least 1 month prior to the first bait presentation, fed on mash and supplementary apples, and water ad libitum. The sex and weight of the ship rats was not determined until the end of the experiment as the rats were not handled so as to minimise stress.
The possums tested in Experiment 3.4 (43 male, 27 female, mean body weight 2505 ± 448 g) were wild caught from bush/pasture margin habitat at Whatawhata. They were acclimatised to captive conditions in individual cages at the AgResearch possum quarantine facility in Ruakura for at least 1 month with food mash made from a cereal-based, possum-specific diet (NRM New Zealand Ltd, Hornby, Canterbury, New Zealand) and water, supplemented with apple and water ad libitum. The mash was placed in one of two feeding bowls positioned on either side of each cage, and the position alternated every day. No subjects had previously been exposed to bird repellents. The ship rats and possums were fed alternately from the two feeding bowls throughout the acclimatisation period. The allocation of the laboratory rats and possums to treatments was balanced for food intake levels, body weight and sex.
The procedure for the efficacy experiments was similar to that used in Experiment 1. Individuals were given the choice between 90% ration of their normal mash diet and 50 g of non-toxic bait: (1) on the floor of the cages for the laboratory rats (Experiments 3.1 and 3.2); and (2) in the second feeding tray for the ship rats and possums (Experiments 3.3 and 3.4) on 2 consecutive days per week for 2 weeks (to mimic non-toxic pre-feeding and allow for the establishment of a taste aversion). One week later, a single toxic 1080 bait was presented (in a randomly selected feeding position) on one occasion for 24 h.
Bait and mash consumption was measured daily for all individuals in all experiments. On the toxic bait test day, the behaviour of the animals was monitored at least hourly for 12 h for signs of toxicosis. Any animals in extremis or unconscious were euthanised. The condition of the surviving animals was checked the following morning. Bait weight loss or gain from water evaporation or absorption was assessed only during Experiment 3.3, by placing a weighed sample of each type of bait in an unoccupied cage and reweighing after 24 h. Data were adjusted for mean weight change per treatment.
To minimise the number of individuals exposed to non-lethal toxicosis, in accordance with our animal ethics approval, only the rats in the four treatment groups with the highest mean bait consumption scores were offered toxic bait in Experiment 3.1. All rats in Experiment 3.2 received toxic baits. The ship rats (Experiment 3.3) in all four treatment groups received toxic baits because pre-feed consumption was satisfactory. None of the possums in the cereal bait treatment groups in Experiment 3.4 received the toxic treatments because their consumption of non-toxic bait was not sufficient to justify the continuation of the experiment on ethical grounds, but all four of the carrot treatment groups proceeded to toxic bait presentation.
Data on bait consumption are presented as percentages of total food consumption, and arcsine-transformed data were analysed using a treatment × sex ANOVA of mean bait consumption on each experimental day and averaged over all 4 non-toxic bait presentation days. Comparisons between treatments were analysed using Tukey's multiple comparison tests. Changes over time were analysed with a repeated measures analysis and with a treatment × sex ANOVA of the change over the treatment days. We compared percentages killed using the likelihood ratio (G statistic) chi-square test.
Results
Experiment 1: Bait palatability (laboratory rats)
There were significant differences in mean carrot bait consumption over the 6 days amongst the treatments in Experiment 1 (F6, 49 = 14.05, P < 0.001). Baits containing either 0.8 or 1.2 g kg−1 AQ with or without DP were no more palatable than the salt baits, based on the percentage of bait consumed (). Baits containing DP alone or together with the lowest AQ concentration (0.4 g kg−1) were as palatable as baits without repellent. Although four out of seven of the treatments showed a significant linear decline in consumption over time (), this included the untreated control baits as well as the salt negative control and the two high dose AQ–DP combinations. The repeated analysis showed no significant treatment by day interaction so there was no indication of a learned aversion to the bird repellent compounds.
Experiment 2: Field acceptance of baits (possums and rats)
Experiment 2.1 AQ and/or DP (possums)
shows that the proportion of investigated stations from which bait had been removed by possums in Experiment 2.1 was similar for all repellent-treated baits and for the positive control. Significantly fewer of the negative (salt) control stations had bait removed compared with all the other treatments (F7,58 = 6.05, P < 0.001). shows the percentage of bait stations from which baits were eaten each day; bait removal from the negative control was notably low on days 3 and 4 compared with the other treatments. The consumption of DP baits was consistently high across all days. Removal of baits from stations containing AQ and combined AQ plus DP did not consistently increase or decrease over the 4 days (). Preliminary observations using video cameras and time-lapse VCRs confirmed that possums readily consumed the baits in the immediate vicinity of the stations and were not seen to take them away from the bait station area.
Experiment 2.2 AQ and/or DP (rats)
There were no significant differences in bait take among treatments in Experiment 2.2 (F7, 61 = 1.64, P = 0.141) including controls, from the rat stations although numerically fewer salt baits were taken (, ). Removal of DP-treated baits from rat stations increased over the 4 days while the combined repellents did not show any consistent temporal pattern (). Some baits were removed from the stations but we do not know if these baits were consumed.
Experiment 3: Bait acceptability (possums and rats)
Experiment 3.1 AQ concentration, addition of DP, pennyroyal, rat attractants (laboratory rats)
For the Norway rats exposed to non-toxic carrot baits, the consumption of all treatments with added repellents was less than for the control (F8, 72 = 17.78, P ≤ 0.001). The consumption of baits containing low concentrations of both AQ (0.4 g kg−1) and peanut oil (0.1 g kg−1) was higher than AQ (0.4 g kg−1) baits combined with either DP or pennyroyal (0.5 g kg−1), and also than AQ (0.8 g kg−1) baits alone or with added high (1 g kg−1) or low-concentration peanut oil.
Bait consumption declined significantly over the 4 non-toxic bait days (F3, 221 = 39.31, P ≤ 0.001) and there was no significant treatment × day interaction ().
Twenty-nine of the 36 (80.5%) Norway rats presented with toxic baits were killed, including the entire control (green plus cinnamon) group, all of the 0.4 g kg−1 AQ plus 0.1 g kg−1 peanut oil, six of nine rats in the 0.4 kg kg−1 AQ group and five of nine rats in the 0.4 g kg−1 AQ plus 1 g kg−1 peanut oil group (). Although these differences were significant overall (G = 11.65, d.f. = 3, P = 0.009), none of the repellents treatments was significantly different from the control (Fisher exact test 2 × 2 tables, P > 0.08).
Experiment 3.2 low concentration AQ and DP, addition of rat attractants (laboratory rats)
For the data averaged over the 4 days of the non-toxic exposure period, addition of any repellent combination reduced bait consumption relative to the control treatment (F8,72 = 13.67, P ≤ 0.001) (). Baits with AQ in combination with the higher concentration of DP (0.5 g kg−1) had lower consumption rates than baits with added bacon, peanut butter or a low concentration of DP (0.01 g kg−1). Female rats consumed significantly more non-toxic carrot bait than the males did (F1, 72 = 100.87, P ≤ 0.001), and there was no significant sex × treatment interaction. The repeated measures analysis showed significant differences in the pattern of intake over time (F8,72 = 4.87, P < 0.001) and the analysis of the change over the treatment period showed that this was due to an increase for the control rats but a decline for all the other treatments ().
Twelve of the 90 rats exposed to toxic baits died. Females ate more of the toxic baits in every treatment, but of the 12 rats that died, five were males and seven females. Seventy per cent of the control group rats died after consuming baits containing 0.08% 1080 (). This was significantly more than in the other 0.08% 1080 groups (G = 21.67, d.f. = 2, P ≤ 0.001 [cinnamon, bacon and peanut butter groups combined, DP groups combined]). No bait treatments containing 0.4 g kg−1 AQ achieved kill rates over 20%. The increased poison concentration (0.15% 1080) in baits containing 0.4 g kg−1 AQ and cinnamon or DP did not significantly improve the kill rate (G = 0.332, d.f. = 1, P > 0.5). No rats were killed when the baits contained a combination of AQ, cinnamon and DP.
Experiment 3.3 AQ concentration, addition of DP, cinnamon (ship rats)
There were no significant differences in bait consumption by ship rats between the treatment groups averaged over the 4 non-toxic days in Experiment 3.3 (F3,26 = 2.19, P = 0.114; ) or any single day. There was a decline in bait consumption over the 4 non-toxic presentation days (), but there was no significant treatment by time interaction (F9, 65 = 0.70, P = 0.706). Within 14 h of toxic bait presentation, four out of eight of the control group rats were dead, along with five in the 0.4 g kg−1 AQ + cinnamon group, four in the 0.4 g kg−1 AQ + DP group and six in the 0.8 g kg−1 AQ + cinnamon group (G = 1.47, d.f. = 3, P > 0.05). By the following day, only two rats were left alive in the 0.8 g kg−1 AQ + cinnamon group, and one in each of the other groups.
Experiment 3.4 high concentration AQ, addition of DP, cinnamon (possums)
None of the repellent treatments reduced bait consumption of carrot or cereal by possums (). There was a significant difference among the treatments in the percentage of bait eaten on day 1 (F6, 63 = 4.36, P ≤ 0.001) because of lower consumption of all three cereal baits (including the control without bird repellent) than the carrot baits (). There were no significant differences in consumption among the carrot treatments averaged over the 4 non-toxic bait presentation days (F6, 63 = 2.15, P = 0.060) or on any single day. Consumption of carrot baits (as a percentage of total daily food consumption) was relatively high on the day when the baits were toxic, because of rapid onset of illness after ingestion of the toxin and, thus, a subsequent low intake of the standard mash diet. All 10 possums in the control (green + cinnamon) group died. Nine died in each of the AQ and AQ + cinnamon + DP treatments, and seven in the AQ + cinnamon treatment (G = 4.92, d.f. = 3, P > 0.5).
Discussion
The series of palatability, field acceptance and poisoning efficacy experiments reported here have established the range of concentration of AQ and DP acceptable to possums and rats. The results have allowed other researchers to advance the work to improve the protection of native birds during pest control operations in New Zealand. The results also suggest that a way of making bird-repellent-treated baits more acceptable to rats would be to include additional flavours attractive to rodents.
The initial palatability trial directed our focus on trying to reduce the concentration of AQ to maximise acceptance by rats. The field acceptance experiments gave us a quick way to assess various repellent combinations, but we had no measure of the number of different animals visiting the stations. The low acceptance of salt-treated baits suggests that if any of the repellent-treated baits were particularly unpalatable to possums our method would have detected this. Wild rats also took the repellent baits from bait stations as readily as they took standard baits. Rats did not take salt-treated baits as readily as they took the other bait types, giving us confidence that our method would have identified highly unpalatable baits for rats as well as possums.
A low concentration of AQ was palatable to both the laboratory rats and the captive ship rats. While the high concentrations of the AQ + DP combination that are highly repellent to North Island robins (Day et al. Citation2003) and sparrows (Clapperton et al. Citation2012) were not palatable to rats in Experiment 1, there may be a compromise combination of lower AQ and higher DP that could ensure both bird repellence and rodent acceptance. Sparrows rejected the 0.4 AQ concentration that was acceptable to possums and ship rats used in the current trial when it was paired with a higher rate of DP (Day et al. Citation2012), but other bird species may not be deterred by AQ concentrations this low. More research is needed to determine whether rats would accept baits with DP concentrations higher than 2 g kg−1 (Cowan et al. Citation2014).
When the repellents are surface-coated on the baits rather than incorporated into the bait matrix, small bird species such as robins, tomtits and sparrows would receive a relatively larger concentration of repellent by just pecking at the surface of the bait than would rats, which are more likely to consume the whole bait. This suggests that surface-coating of baits may be a way of delivering an adequate dose of repellent to small bird species without deterring rats from eating the whole bait. The same system may not work for larger birds such as kea, which could consume larger pieces of bait. Incorporating AQ into the bait also has the potential problem of making it less effective as a repellent. Tupper et al. (Citation2014) found that AQ formulated into pelleted baits was less repellent to starlings than when it was applied as a surface coating to bird feed.
The high consumption rates of all the repellents at high concentrations in the field acceptance experiments suggest that the palatability experiment was probably conservative in its findings on repellency concentrations. As free-ranging wild animals are likely to be hungrier than captive animals, and more accustomed to sampling novel foods, they may be more likely to consume baits. Gentle et al. (Citation2006) showed that diet complexity can affect the development of taste aversions in Norway rats. They suggested that the experience of varied foods with no adverse effects early in life may pre-dispose rats to continuing to sample novel foods. However, Kare (Citation1970) noted that wild animals might be more sensitive to the toxicological consequences of food choice. Anthraquinone and DP proved acceptable to possums at rates that deter birds. This is consistent with our previous findings that possum kill was not affected in an aerial poison operation using carrot baits coated with a nominal rate of 0.9 g AQ kg−1 bait (Clapperton et al. Citation2014). The lower bait take at the rat bait stations during the field experiment in the current study limited our ability to detect differences in acceptance rates of repellents to rats, but there was no indication that either AQ or DP would be rejected by wild rats.
These findings were also confirmed by the palatability results in the efficacy experiments for both possums and rats. Even the less palatable cereal baits were taken by the possums, regardless of the presence of the bird repellents. While the laboratory-bred Norway rats ate less of the baits containing repellents, the wild-caught ship rats did not. The ship rats consumed concentrations of repellents that have been shown to be effective as bird repellents (Clapperton et al. Citation2014). This result, and the higher acceptance rates of repellent-treated baits that also contained rat attractants in efficacy Experiment 3.1 (AQ concentration, addition of DP, pennyroyal, rat attractants) suggest that bird repellents might still be used in control operations that target rats. The poor kill rate in efficacy Experiment 3.2 (low concentration AQ and DP, addition of rat attractants) indicates that care must be taken to select a suitable bait formulation when targeting Norway rats, and that such formulations should be tested on wild-caught or free-ranging Norway rats rather than laboratory-bred rats.
The efficacy experiments were designed to simulate a control operation. Application of 1080 baits is normally preceded by pre-feeding with non-toxic baits because this reduces the formation of aversions to 1080 baits in possums (Moss et al. Citation1998; Ross et al. Citation2000). Four days of ‘pre-feed’ were thus provided before the toxic baits were presented in this study. This pre-feed system is essential when using a secondary bird repellent such as AQ that works via the formation of a conditioned food aversion. Bait consumption patterns in the efficacy experiments indicate that neither the ship rats nor the possums developed aversions to the repellent-treated baits. While they ate less bait on the last day(s) of the experiment, this was true also for the control baits that did not contain repellent.
Because vision provides more effective cues than taste for the development of food aversion in birds (Avery Citation1984), the use of a colour cue with AQ might be just as effective as the AQ–DP combination for repelling birds from baits. This is supported by the results of our experiments on repellent formulations with sparrows (Clapperton et al. Citation2012) and with North Island tomtits (Petroica macrocephala toitoi) where their abundance was not reduced in an aerial field poison operation (Clapperton et al. Citation2014).
Blue colour is a suitable repellent for North Island robins (Hartley et al. Citation1999) but it might not be the most repellent colour for other species. Captive kea consumed fewer green-dyed baits than blue-dyed baits in a multi-choice situation (Weser & Ross Citation2013). The relative repellency of green- and dark blue-coloured baits when on the forest floor, rather than on a light-brown test surface, has not been assessed for this species. Acceptance by the pests must also be considered. While bait colour did not affect cereal bait choice by captive possums (Day & Matthews Citation1999), ship rats preferred green over blue or black (Prakash et al. Citation2003). If the main role of colour in a bird-repellent strategy is to act as a salient cue for the establishment of taste aversion to AQ rather than as a primary repellent, and rats are to be targeted in a pest control operation, then green-coloured baits might be as effective as blue-coloured baits for the protection of North Island robin populations, and might be more effective for other bird species.
The bird repellents tested here are likely to have no deleterious effect on the efficacy of possum control operations. Cowan et al. (Citation2014) showed that although AQ incorporated into cereal baits at 2.5 g kg−1 concentration reduced palatability to possums, it did not affect the acceptance of poison baits. While we recommend that AQ can be used at 1 g kg–1 bait and DP at 2 g kg−1 bait without deterring possums, a lower concentration of AQ and higher DP might be worth testing if rodent control is to be assured, as the rats tested in our experiments were more sensitive than the possums to bird repellents. Cowan et al. (Citation2014) found that even 1 g AQ kg−1 bait incorporated into cereal bait made the baits unacceptable to captive ship rats. This means that we must use our findings of consumption of coated carrot baits with some caution and the results do not imply success if the repellents are incorporated into cereal bait. However, the majority of captive ship rats were killed in our trial using the lower concentration (0.4 g kg−1 bait) of AQ with or without DP (0.5 g kg−1), and these were similar to the rates that deterred North Island robins from feeding (Clapperton et al. Citation2014), when these repellents were applied as a surface coating to baits.
Acknowledgements
We thank Kylie Flight and Shelley Aukett for assistance with the field acceptance experiments, the staff at the AgResearch SAC for their assistance with the laboratory experiments and Tony Day for veterinarian services. Catherine Cameron conducted some of the statistical analyses. Phil Cowan commented on an earlier draft of this paper. The Avex™ was provided by Elliot Technologies Ltd, Auckland, New Zealand. None of the authors will gain financially from the direct application of this research. The research was funded by the New Zealand Foundation for Research, Science and Technology, and by the New Zealand Animal Health Board.
Associate Editor: Dr Jonathan Banks.
References
- Avery ML 1984. Relative importance of taste and vision in reducing bird damage to crops with methiocarb, a chemical repellent. Agriculture Ecosystem & Environment 11: 299–308.10.1016/0167-8809(84)90003-3
- Avery ML, Decker DG, Humphrey JS, Laukert CC 1996. Mint plant derivatives as blackbird feeding deterrents. Crop Protection 15: 461–464.10.1016/0261-2194(96)00010-5
- Avery ML, Humphrey JS, Decker DG 1997. Feeding deterrence of anthraquinone, anthracene, and anthrone to rice-eating birds. The Journal of Wildlife Management 61: 1359–1365.10.2307/3802138
- Avery ML, Humphrey JS, Primus TM, Decker DG, McGrane AP 1998. Anthraquinone protects rice seed from birds. Crop Protection 17: 225–230.10.1016/S0261-2194(98)00002-7
- Beauchamp GK, Mason JR 1991. Comparative hedonics of taste. In: Bolles R ed. The hedonics of taste. Hillsdale, Lawrence Erlbaum Associates. Pp. 159–186.
- Belant JL, Ickes SK, Tyson LA, Seamans TW 1997. Comparison of d-pulegone and mangone as cowbird feeding repellents. International Journal of Pest Management 43: 303–305.10.1080/096708797228618
- Brown KP 1997. Impact of brodifacoum poisoning operations on South Island robins Petroica australis australis in a New Zealand Nothofagus forest. Bird Conservation International 7: 399–407.10.1017/S0959270900001726
- Caithness TA, Williams GR 1971. Protecting birds from poison baits. New Zealand Journal of Agriculture 12: 38–43.
- Clapperton BK 2006. A review of the current knowledge of rodent behaviour in relation to control devices. Science for Conservation 263. Wellington, Department of Conservation.
- Clapperton BK, Morgan DKJ, Day TD, Oates KE, Beath AM, Matthews LR 2014. Efficacy of bird repellents at deterring North Island robins (Petroica australis longipes) and tomtits (P. macrocephala toitoi) from baits. New Zealand Journal of Ecology 38: 116–123.
- Clapperton BK, Porter RER, Day TD, Waas JR, Matthews LR 2012. Designer repellents: combining olfactory, visual or taste cues with a secondary repellent to deter free-ranging house sparrows from feeding. Pest Management Science 68: 870–877.10.1002/ps.3244
- Cowan P, Brown S, Forrester G, Booth L, Crowell M 2014. Bird-repellent effects on bait efficacy for control of invasive mammal pests. Pest Management Science.10.1002/ps.3887
- Crocker DR, Scanlon CB, Perry SM 1993. Repellency and choice: feeding responses of wild rats (Rattus norvegicus) to cinnamic acid derivatives. Applied Animal Behaviour Science 38: 61–66.10.1016/0168-1591(93)90042-N
- Cunningham DM, Moors PJ 1996. Guide to the identification and collection of New Zealand rodents. Wellington, Department of Conservation.
- Day TD, Clapperton BK, Porter RER, Waas JR, Matthews LR 2012. Responses of free-ranging house sparrows to feed containing primary and secondary repellents. New Zealand Journal of Crop & Horticultural Science 40: 127–138.10.1080/01140671.2011.630738
- Day TD, Matthews LR 1999. Do colours that deter birds affect cereal bait acceptance by possums (Trichosurus vulpecula)? New Zealand Journal of Ecology 23: 261–266.
- Day TD, Matthews LR, Waas JR 2003. Repellents to deter New Zealand's North Island robin Petroica australis longipes from pest control baits. Biological Conservation 114: 309–316.10.1016/S0006-3207(03)00047-8
- Esther A, Tilcher R, Jacob J 2013. Assessing the effects of three potential chemical repellents to prevent bird damage to corn seeds and seedlings. Pest Management Science 69: 425–430.10.1002/ps.3288
- Fitzgerald G, Wilkinson R, Saunders L 2000. Public perceptions and issues in possum control. In: Montague TL ed. The brushtail possum: biology, impact, and management of an introduced marsupial. Christchurch, Manaaki Whenua Press. Pp. 187–197.
- Gentle M, Massei G, Quy R 2006. Diversity of diet influences the persistence of conditioned taste aversion in rats. Applied Animal Behaviour Science 97: 303–311.10.1016/j.applanim.2005.08.005
- Gurney JE, Watkins RW, Gill EL, Cowan DP 1996. Non-lethal mouse repellents: evaluation of cinnamamide as a repellent against commensal and field rodents. Applied Animal Behaviour Science 49: 353–363.10.1016/0168-1591(96)01051-9
- Hartley L, O'Connor C, Waas J, Matthews L 1999. Colour preferences in North Island robins (Petroica australis): implications for deterring birds from poisonous baits. New Zealand Journal of Ecology 23: 255–259.
- Hickling GJ 1997. Effect of green dye and cinnamon oil on consumption of cereal pest baits by captive North Island kaka (Nestor meridionalis). New Zealand Journal of Zoology 24: 239–242.10.1080/03014223.1997.9518119
- Innes JG 2005a. Norway rat. In: King CM ed. The handbook of New Zealand mammals. 2nd edition. Melbourne, Oxford University Press. Pp. 174–187.
- Innes JG 2005b. Ship rat. In: King CM ed. The handbook of New Zealand mammals. 2nd edition. Melbourne, Oxford University Press. Pp. 187–203.
- Jacobs GH, Fenwick JA, Williams GA 2001. Cone-based vision of rats for ultraviolet and visible lights. The Journal of Experimental Biology 204: 2439–2446.
- Kare MR 1970. The chemical senses of birds. Bird Control Seminars Proceedings Paper 184. Lincoln, Internet Center for Wildlife Damage Management, University of Nebraska.
- Marsh R 1985. Techniques used in rodent control to safeguard nontarget wildlife. Cal-Neva Wildlife Transactions 1985: 47–55.
- Mason JR 1990. Evaluation of d-pulegone as an avian repellent. The Journal of Wildlife Management 54: 130–135.10.2307/3808912
- Mason JR, Bean NJ, Shah PS, Clark L 1991. Taxon-specific differences in responsiveness to capsaicin and several analogues: correlates between chemical structure and behavioral aversiveness. Journal of Chemical Ecology 17: 2539–2551.10.1007/BF00994601
- Meehan AP 1984. Rats and mice: their biology and control. East Grinstead, Sussex, Rentokil.
- Montague TL 2000. The brushtail possum: biology, impact, and management of an introduced marsupial. Christchurch, Manaaki Whenua Press.
- Morgan DR 1990. Behavioral-response of brushtail possums, Trichosurus-vulpecula, to baits used in pest-control. Wildlife Research 17: 601–613.10.1071/WR9900601
- Morgan DR, Innes J, Frampton CM, Woolhouse AD 1995. Responses of captive and wild possums to lures used in poison baiting. New Zealand Journal of Zoology 22: 123–129.
- Morgan D, Waas JR, Innes J 2006. The relative importance of Australian magpies (Gymnorhina tibicen) as nest predators of rural birds in New Zealand. New Zealand Journal of Zoology 33: 17–29.10.1080/03014223.2006.9518427
- Moss ZN, O'Connor CE, Hickling GJ 1998. Implications of prefeeding for the development of bait aversions in brushtail possums (Trichosurus vulpecula). Wildlife Research 25: 133–138.10.1071/WR97018
- Nelms CO, Avery ML 1997. Reducing bird repellent application rates by the addition of sensory stimuli. International Journal of Pest Management 43: 187–190.10.1080/096708797228654
- Nolte DL, Mason JR, Clark L. 1993. Avoidance of bird repellents by mice (Mus musculus). Journal of Chemical Ecology 19: 427–432.10.1007/BF00994315
- Orr-Walker T, Adams NJ, Roberts LG, Kemp JR, Spurr EB 2012. Effectiveness of the bird repellents anthraquinone and d-pulegone on an endemic New Zealand parrot, the kea (Nestor notabilis). Applied Animal Behaviour Science 137: 80–85.10.1016/j.applanim.2012.01.002
- Powlesland RG, Knegtmans JW, Marshall ISJ 1999. Costs and benefits of aerial 1080 possum control operations using carrot baits to North island robins (Petroica australis longipes), Pureora Forest Park. New Zealand Journal of Ecology 23: 149–159.
- Powlesland RG, Knegtmans JW, Styche A 2000. Mortality of North Island tomtits (Petroica macrocephala toitoi) caused by aerial 1080 possum control operations, 1997-98, Pureora Forest Park. New Zealand Journal of Ecology 24: 161–168.
- Prakash S, Kumar S, Veer V, Gopalan N, Purnanand, Pandey KS et al. 2003. Laboratory evaluation of four rodenticides admixed in a cereal-based bait against commensal rat, Rattus rattus (L.) (Rodentia: Muridae: Murinae). Journal of Stored Products Research 39: 141–147.10.1016/S0022-474X(01)00026-1
- Ross JG, Hickling GJ, Morgan DR, Eason CT 2000. The role of non-toxic prefeed and postfeed in the development and maintenance of 1080 bait shyness in captive brushtail possums. Wildlife Research 27: 69–74.10.1071/WR98029
- Schmidt V, Schaefer HM, Winkler H 2004. Conspicuousness, not colour as foraging cue in plant-animal signalling. Oikos 106: 551–557.10.1111/j.0030-1299.2004.12769.x
- Spurr EB 2000. Impacts of possum control on non-target species. In: Montague TL ed. The brushtail possum: biology, impact, and management of an introduced marsupial. Christchurch, Manaaki Whenua Press. Pp. 175–186.
- Spurr EB, Porter RER 1998. Cinnamamide as a bird repellent for baits used in mammalian pest control. Proceedings of the 11th Australian Vertebrate Pest Conference. Bunbury, WA. Pp. 295–299.
- Spurr EB, Porter RER, Thomson C 2001. Palatability of bird repellents to Rattus norvegicus. Pest Management Science 57: 615–619.10.1002/ps.335
- Taylor RH, Thomas BW 1989. Eradication of Norway rats (Rattus norvegicus) from Hawea Island, Fiordland, using brodifacoum. New Zealand Journal of Ecology 12: 23–32.
- Tupper SK, Werner SJ, Carlson JC, Pettit SE, Wise JC, Lindell CA et al. 2014. European starling feeding activity on repellent treated crops and pellets. Crop Protection 63: 76–82.10.1016/j.cropro.2014.05.001
- Udy PD, Pracy LT 1981. Baits, birds and field operations. Counterpoint 6: 13–15.
- Veitch CR, Clout MN eds. 2002. Turning the tide: the eradication of invasive species. IUCN SSC Invasive Specialist Group Gland, Switzerland, Cambridge, UK, IUCN.
- Veltman CJ, Westbrooke IM 2011. Forest bird mortality and baiting practices in New Zealand aerial 1080 operations from 1986 to 2009. New Zealand Journal of Ecology 35: 21–29.
- Watkins RW, Gurney JE, Cowan DP 1998. Taste-aversion conditioning of house mice (Mus domesticus) using the non-lethal repellent, cinnamamide. Applied Animal Behaviour Science 57: 171–177.10.1016/S0168-1591(96)01182-3
- Werner SJ, Linz GM, Carlson JC, Pettit SE, Tupper SK, Santer MM 2011a. Anthraquinone-based bird repellent for sunflower crops. Applied Animal Behaviour Science 129: 162–169.10.1016/j.applanim.2010.11.010
- Werner SJ, Tupper SK, Pettit SE, Carlson JC, Linz GM 2011b. Anthraquinone repellent to reduce take of non-target birds from zinc phosphide rodenticide applications. Applied Animal Behaviour Science 135: 146–153.10.1016/j.applanim.2011.09.009
- Werner SJ, Tupper SK, Pettit SE, Ellis JW, Carlson JC, Goldade DA et al. 2014. Application strategies for an anthraquinone-based repellent to protect oilseed sunflower crops from pest blackbirds. Crop Protection 59: 63–70.10.1016/j.cropro.2014.01.016
- Weser C, Ross JG 2013. The effect of colour on bait consumption of kea (Nestor notabilis): implications for deterring birds from toxic baits. New Zealand Journal of Zoology 40: 137–144.10.1080/03014223.2012.710639
