Abstract
Two curculionid weevils, Orthochaetes setiger (Beck, 1817) and Exomias pellucidus (Boheman, 1834) are recorded in New Zealand for the first time. The former has a wide distribution through the eastern South Island, while the latter has so far only been located in a single suburban garden in Dunedin. Both species are polyphagous and flightless. Although neither is expected to cause notable economic damage, their potential to invade native ecosystems makes them worthy of further investigation.
Introduction
Invasive species threaten biodiversity and economic values in New Zealand and globally (Simberloff Citation2005; Brockerhoff et al. Citation2010). Increasing global trade and commerce have made the establishment of alien organisms a regular occurrence despite diligent biosecurity in New Zealand (Kriticos et al. Citation2005). Some invasions have significant economic impacts (e.g. Didymo, Pseudomonas syringae pv. actinidiae (Psa), tomato potato psyllid, clover root weevil); however, invasive species also have the potential to threaten New Zealand's unique biodiversity (Snyder & Evans Citation2006; Brockerhoff et al. Citation2010). While economic consequences are well recognised, non-economic environmental impacts of invasive species attract less attention (Simberloff Citation2005; Brockerhoff et al. Citation2010).
Within the invertebrates, the weevils (Coleoptera: Curculionoidea) are a group that is particularly invasive. In New Zealand, over 60 species have become established (Kuschel Citation1972; Macfarlane et al. Citation2011), with some species (e.g. clover root weevil [Sitona obsoletus (Gmelin)], lucerne weevil [Sitona discoideus Gyllenhal], Argentine stem weevil [Listronotus bonariensis (Kuschel)], eucalyptus weevil [Gonipterus platensis Marelli], and vine weevils [Otiorhynchus Germar spp.]) harming New Zealand's primary industries (Bain Citation1977; Prestidge et al. Citation1991; Eerens et al. Citation2005). Identifying recently arrived species is a critical first step towards evaluating and responding to them (Phillips & Barratt Citation2004). Here, we provide the first reports of two European weevils in New Zealand.
Exomias pellucidus (Boheman, 1837) (until recently placed in the genus Barypeithes Jacquelin du Val, see Löbl & Smetana Citation2013) is commonly known as the juniper root weevil, hairy broad-nosed weevil or hairy spider weevil and is classified in the tribe Sciapithini, subfamily Entiminae (Alonso-Zarazaga & Lyal Citation1999). Originally from Europe, it is a pest in North America in forestry nurseries and horticultural crops, where it is distributed across the continent between latitudes 34°N and 60°N (Coyle et al. Citation2008). The genus Exomias Bedel, 1883 has 35 described species in Europe and Asia (Löbl & Smetana Citation2013), and was most recently revised (as Barypeithes) by Fremuth (Citation1971).
Orthochaetes setiger (Beck 1817) is in the tribe Styphlini, subfamily Curculioninae (Alonso-Zarazaga & Lyal Citation1999). Orthochaetes Germar 1824 contains 15 described species distributed throughout Europe and North Africa. Most species of Orthochaetes are locally distributed in southwestern Europe and North Africa, but O. setiger and Orthochaetes insignis Aubé 1863 have wider distributions through Europe and into the British Isles. The most recent revision of the genus was published by Gonzalez (Citation1967), who provided a key to the known species of Orthochaetes.
Exomias pellucidus
Discovery
Specimens of E. pellucidus were found by pitfall trapping during a survey of biodiversity in 55 Dunedin domestic gardens in mid to late November 2009. The invertebrate survey was part of a larger study carried out by researchers in the Departments of Geography, Zoology and Botany, University of Otago, Dunedin (Barratt et al. Citation2015). Pitfall traps were set for a 10-day period with three to five traps set in each garden, depending upon garden area, and were positioned in areas of lawn, shrubs and open flower/vegetable beds. Only three E. pellucidus specimens were found, all from the same property. After the Ministry of Primary Industries was alerted to this discovery, one larva was found from around ivy roots by Ministry of Primary Industries response officers and was confirmed as E. pellucidus through DNA sequencing. Since the initial discovery, little effort has gone into locating further specimens, so it remains uncertain if this species is established.
New Zealand specimens were determined by BIPB using keys in Morris (Citation1997) and Fremuth (Citation1971), and the identity was confirmed by RS Anderson (Canadian Museum of Nature, Ottawa, Canada; pers. comm.). The length and curvature of the aedeagus match the illustrations provided by Fremuth (Citation1971) and Dieckmann (Citation1980). DNA sequencing for confirming the identity of the larva was done through EcoGene (Auckland, New Zealand), and involved sequencing 386 base pairs of the cytochrome c oxidase subunit 2 (COII) gene using the primers C2-J-3400 and TK-N-3785 (Simon et al. Citation1994) (D Jones pers. comm.). Sequences from an adult and a larva matched each other, and had a 91% identity to E. pellucidus sequences from GenBank (GU182497–GU182499; Hirsch et al. Citation2010). Sequences for nine other species of Exomias (as Barypeithes) are also available (DQ836068–DQ836076, Lackowska et al. unpub. data), with identities to these sequences ranging between 83% and 87%. This identity is low for absolute confidence in the identity of these specimens, as other studies of COII in weevils show intraspecific distances in the region of 0.7%–7.7% (Emerson et al. Citation2000; Machado Citation2008); however E. pellucidus has a wide range through Europe and is known to have infraspecific morphological variation. The sequence from the adult specimen has been deposited in GenBank (KM359706).
Specimens of E. pellucidus were deposited in the Ministry of Primary Industries synoptic collection (Christchurch; two males), and the Invermay Agricultural Research Collection (Mosgiel, Dunedin; one male).
Identification
Exomias pellucidus () can be distinguished from all other entimine weevil species present in New Zealand by the combination of its smaller size (2.9–4.0 mm); the small, dorsally situated eyes; shiny castaneous elytra clothed with sparse, long hairs; connate tarsal claws; and a tooth on each trochanter (RG Oberprieler pers. comm.). Similar species in New Zealand include Otiorhynchus ovatus Linnaeus, from which E. pellucidus can be distinguished by the long hairs on the elytra, the lack of teeth on the femora, and its castaneous elytra with lighter legs. The aedeagus was illustrated by Dieckmann (Citation1980) and the pupa was described and illustrated by Gosik & Sprick (Citation2013).

In Kuschel's (Citation1972) key to introduced Curculionoidea, E. pellucidus keys out to O. ovatus, from which it differs in the characters given above. Confusion may be encountered at couplet 64, where E. pellucidus shares with Maleuterpes spinipes Blackburn connate claws, but differs by having the pronotum not humped, and by having long, silky hairs on the dorsum.
Biology
Exomias pellucidus is polyphagous, with records coming from a wide range of plant species and families (). It is not of major economic importance, but has been recorded as a pest in horticultural nurseries (Morris Citation1997) and strawberries (Borg Citation1981; Bomford & Vernon Citation2005). In Canada, E. pellucidus occurs in forestry nurseries and vineyards (Brandt et al. Citation1996; Bouchard et al. Citation2005), and in the Great Lakes region it is one of a complex of nine European weevils that is threatening indigenous hardwood forests where it can reach densities of over 100 adults/m/day at peak emergence (Coyle et al. Citation2012). Outbreaks and aggregations have been recorded (Petherbridge Citation1915); however, the cause of these aggregations is not known.
Table 1 Host plants recorded for Exomias pellucidus.
Larvae of E. pellucidus are root-feeders, and are usually found in the top 10–30 cm of the soil (Brandt et al. Citation1996; Coyle et al. Citation2011). The species overwinters as larvae, and the nocturnal adults emerge in early summer (Brandt et al. Citation1996; Coyle et al. Citation2011, Citation2012) when they can be found on the ground or low vegetation (Morris Citation1997). It reproduces sexually, with males and females present in roughly equal proportions (Morris Citation1997). In western Ukraine, E. pellucidus is associated with moist and warm environments, including deciduous and mixed forest as well as other managed and modified environments (Mazur Citation2002). It is often found in ruderal plant communities, associated with herbaceous plants (Mazur Citation2002). Exomias pellucidus is capable of sustained movement, and is a strong climber (Bomford & Vernon Citation2005), but its dispersal is limited by it being flightless (Morris Citation1997).
Orthochaetes setiger
Discovery
The first specimens to be recognised were collected by SDJB at Lake Evelyn in the Lake Coleridge area on 11 December 2010. Specimens were shown in January 2011 to G Kuschel, who was also unfamiliar with the species. When dissections of a number of specimens showed them all to be female, the possibility that the specimens represented a previously unrecognised exotic parthenogenetic species became a consideration. After consulting Morris (Citation2002), it became likely that these specimens were Orthochaetes setiger, an identity confirmed by SDJB by referring to the available literature and comparing specimens with those in the Natural History Museum; London, UK).
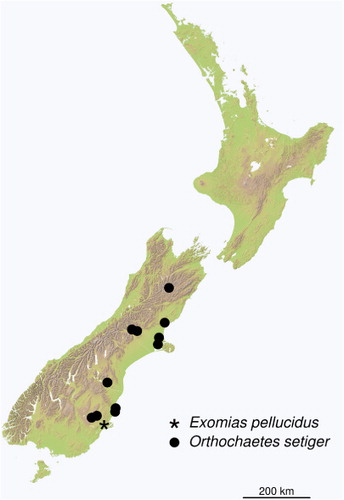
After this initial discovery, O. setiger has been widely collected east of the main divide of the South Island (). However, it is generally found in low numbers, and had therefore escaped notice by researchers sampling weevil pests in pasture (CB Phillips, pers. comm.). Historical specimens have been found in unsorted weevil collections from Canterbury and Central Otago tussock grassland, with the earliest found so far being two specimens collected from Broadfield, Canterbury on 5 January 1996.
New Zealand specimens were determined as Orthochaetes setiger by the combination of costate odd-numbered interstriae, apically inserted antennae, unarmed fore femora, simple tarsal claws, six funicle segments, oval elytra, and erect elytral setae (Gonzalez Citation1967; Morris Citation2002; Caldara et al. Citation2014). The length of the arms of the eighth sternite and stout spermatheca are also consistent with O. setiger (Davidian & Savitsky Citation2000).
Sequences were obtained from a single specimen collected in Sutton (CO, Crosby et al. Citation1998) for the 28S ribosomal DNA and cytochrome c oxidase I (COI) regions and deposited in GenBank (28S: KM359704, COI: KM359705). 28S was amplified using the primers S3660/28SFF (Sequeira et al. Citation2000; Gómez-Zurita et al. Citation2005), and COI using primers TY-J-1460/TL2-N-3014 (Simon et al. Citation1994). COI showed no close matches (i.e. identity over 90%) when searched for in GenBank or BOLD.
Specimens of O. setiger were deposited in the New Zealand Arthropod Collection (Manaaki Whenua Landcare Research, Tamaki, Auckland), Ministry of Primary Industries synoptic collection (Tamaki, Auckland and Christchurch), Museum of New Zealand Te Papa Tongarewa (Wellington), the Lincoln University Entomology Research Museum (Lincoln, Christchurch), and the Natural History Museum (London, UK).
Identification
Orthochaetes setiger () can be distinguished from all other weevil species in New Zealand by a combination of its small size (2.3–2.9 mm), grey coloration, oval outline, having six funicle segments, a heavily pitted metasternum, and costate elytra with numerous erect scales. Similar species in New Zealand include Listronotus bonariensis (Kuschel), and species within Baeosomus Broun, Microcryptorhynchus Lea, and Notacalles Kuschel. It differs from Listronotus bonariensis and Baeosomus by having costate elytra with erect scales. It differs from Microcryptorhynchus and Notacalles by not having a prosternal canal. In Kuschel (Citation1972), O. setiger keys out to Sitophilus Schoenherr (couplet 21), from which it differs by being grey, having clubbed upright scales on the elytra, in the form of the antennal club, and by not being found in stored products.
Biology
Orthochaetes setiger is polyphagous, but has an apparent preference for members of the Asteraceae (). It has negligible economic impact, but has been noted as causing aesthetic damage to ornamental plants (Halstead Citation1977).
Table 2 Host plants recorded for Orthochaetes setiger.
All eight specimens of O. setiger that were dissected were female. Hence the population in New Zealand is probably parthenogenetic, as is the case throughout most of northern Europe and Britain (Gonzalez Citation1967). Orthochaetes setiger and O. insignis are the only species in the subfamily Curculioninae that are known to be parthenogenetic (Caldara et al. Citation2014); though parthenogenesis is also known in the subfamilies Brachycerinae, Cyclominae and Entiminae (Marvaldi et al. Citation2014; Oberprieler Citation2014).
Larvae of O. setiger are leaf miners, with mines appearing as pale-brown, irregularly shaped blotches (Halstead Citation1977). Larvae develop over about 2 months in spring (Halstead Citation1977). Pupation occurs in the soil, and takes about 3 weeks. The species apparently overwinters as adults. It is assumed to be univoltine, though this has not been investigated in detail. In Europe, O. setiger has a wide ecological tolerance, being found in xerophytic habitats as well as cooler humid environments (Dieckmann Citation1986). It has a broad altitudinal distribution and is found up to 2100 m in Switzerland (Dieckmann Citation1986). A preference for drier habitats is consistent with its known distribution through the eastern South Island of New Zealand (). The inherent dispersal capacity of O. setiger is very low, due to this species being flightless (Morris Citation2002) and particularly slow moving.
Discussion
These weevils are two of a number of adventive weevils that have been accidentally introduced to New Zealand over the past 50 years. While some of these weevils pose little threat to the economy or environment of New Zealand, several have caused substantial economic losses, particularly within the agricultural sector. It is also notable that species such as Sitona obsoletus and Listronotus bonariensis have reached a greater level of severity as pests in New Zealand compared with other regions where they have invaded and established. This might be a reflection of the relatively low level of species diversity, and hence natural enemy complex, in New Zealand agricultural systems compared with larger continental land masses (Tylianakis & Romo Citation2010; Goldson et al. Citation2014), combined with New Zealand's heavy reliance on ryegrass and white clover as forage crops. Hence predicting impacts of invasive species in New Zealand can be challenging.
Discoveries such as those reported here are reminders that regular collecting post-border, with follow-up identification and reporting of specimens that are suspected of being new to New Zealand, is important in the rapid detection of invasive species.
The discovery of O. setiger in remote localities such as Lake Coleridge and Sedgemere suggests that it is readily dispersed, despite its apparently poor capacity for dispersal. This was probably achieved through movement of soil or plant matter, especially stock feed. Its behaviour makes it unlikely to have been dispersed through hitch-hiking on vehicles, as has been implicated in the spread of Sitona obsoletus (CB Phillips pers. comm.). The presence of O. setiger in areas with high native plant diversity, including native species of genera that are known to be attacked as hosts (such as Myosotis and Ranunculus), makes it a potential conservation concern.
Further research into the genetic diversity of O. setiger may offer insight into the origin of the New Zealand population, and potential pathways for its invasion.
The ability of E. pellucidus to invade indigenous hardwood forest in North America (Coyle et al. Citation2012) makes this species also worthy of monitoring. In the first instance, a survey to verify its establishment and determine its distribution in New Zealand needs to be carried out. The composition of New Zealand forests is quite different from those in North America, so they may not be at risk from E. pellucidus; however, trees in amenity areas might be damaged.
Acknowledgements
Thanks to Rolf Oberprieler (Australian National Insect Collection, CSIRO, Canberra, Australia) for useful discussion of key characters. Birgit Rhode (Landcare Research, Tamaki, Auckland) provided photographs of E. pellucidus. Basemap of Figure 2 courtesy of Geographx. Joana Cristovao (NHM) translated Gonzalez's key to Orthochaetes. Diane Jones (Ministry of Primary Industries, Tamaki, Auckland) provided details of the molecular identification of the E. pellucidus larva. Craig Phillips (AgResearch, Lincoln) made helpful comments on the manuscript. This research was funded by Lincoln University, the Miss EL Hellaby Indigenous Grasslands Research Trust and AgResearch (MSI contract LINX0304, Ecosystems Bioprotection), as part of SDJB's doctoral programme. SDJB's trip to the NHM was funded by the Spragg Agricultural Research and Development Award. BIPB was supported by AgResearch as part of the Better Border Biosecurity (B3, www.b3nz.org) collaborative research programme, and a University of Otago Research Grant.
Associate Editor: Dr Rob Cruickshank.
References
- Alonso-Zarazaga MA, Lyal CHC 1999. A world catalogue of families and genera of Curculionoidea (Insecta: Coleoptera) (excepting Scolytidae and Platypodidae). Barcelona, Entomopraxis.
- Bain J 1977. Gum-tree weevil Gonipterus scutellatus Gyllenhal (Coleoptera: Curculionidae). Forest and Timber Insects in New Zealand 8: 1–3.
- Barratt BIP, Dickinson KJM, Freeman C, Porter S, Johnstone PD, Wing J et al. 2015. Biodiversity of Coleoptera and other invertebrates in urban gardens: a case study in a New Zealand city. Insect Conservation and Diversity. doi:10.1111/icad.12120
- Bomford MK, Vernon RS 2005. Root weevil (Coleoptera: Curculionidae) and ground beetle (Coleoptera: Carabidae) immigration into strawberry plots protected by fence or portable trench barriers. Environmental Entomology 34: 844–849.10.1603/0046-225X-34.4.844
- Borg Å 1981. Viveln Barypeithes pellucidus Boh., en liten skadegörare på jordgubbar. Växtskyddsnotiser 45: 99–101.
- Bouchard P, Lesage L, Goulet H, Bostanian NJ, Vincent C, Zmudzinska A et al. 2005. Weevil (Coleoptera: Curculionoidea) diversity and abundance in two Quebec vineyards. Annals of the Entomological Society of America 98: 565–574.10.1603/0013-8746(2005)098[0565:WCCDAA]2.0.CO;2
- Brandt JP, Smith SM, Hubbes M 1996. Distribution and sampling of root weevil larvae in young ornamental conifer plantations. The Canadian Entomologist 128: 1125–1133.10.4039/Ent1281125-6
- Brockerhoff EG, Barratt BIP, Beggs JR, Fagan LL, Kay MK, Phillips CB et al. 2010. Impacts of exotic invertebrates on New Zealand's indigenous species and ecosystems. New Zealand Journal of Ecology 34: 158–174.
- Caldara R, Franz NM, Oberprieler RG 2014. 3.7.10 Curculioninae Latreille, 1802. In: Leschen RAB, Beutel RG eds. Handbook of zoology, Arthropoda: Insecta. Coleoptera, Beetles volume 3: morphology and systematics (Phytophaga). Berlin, Walter De Gruyter. Pp. 589–628.
- Coyle DR, Duman JG, Raffa KF 2011. Temporal and species variation in cold hardiness among invasive rhizophagous weevils (Coleoptera: Curculionidae) in a Northern hardwood forest. Annals of the Entomological Society of America 104: 59–67.10.1603/AN10112
- Coyle DR, Mattson WJ, Jordan MS, Raffa KF 2012. Variable host phenology does not pose a barrier to invasive weevils in a northern hardwood forest. Agricultural and Forest Entomology 14: 276–285.10.1111/j.1461-9563.2012.00567.x
- Coyle DR, Mattson WJ, Raffa KF 2008. Invasive root-feeding insects in natural forest ecosystems of North America. In: Johnson SN, Murray PJ eds. Root feeders: an ecosystem perspective. Wallingford, CAB International. Pp. 134–149.
- Crosby TK, Dugdale JS, Watt JC 1998. Area codes for recording specimen localities in the New Zealand subregion. New Zealand Journal of Zoology 25: 175–183.10.1080/03014223.1998.9518148
- Davidian GE, Savitsky VY 2000. To the knowledge of weevils of the tribe Orthochaetini Winkler (Coleoptera, Curculionidae) in the fauna of Russia and neighbouring countries. Entomological Review 80: 746–768.
- Dieckmann L 1980. Beiträge zur Insektenfauna der DDR: Coleoptera—Curculionidae (Brachycerinae, Otiorhynchinae, Brachyderinae). Beiträge zur Entomologie 30: 145–310.
- Dieckmann L 1986. Beiträge zur Insektenfauna der DDR: Coleoptera—Curculionidae (Erirhinae). Beiträge zur Entomologie 36: 119–181.
- Eerens JPJ, Hardwick S, Gerard PJ, Willoughby BE 2005. Clover root weevil (Sitona lepidus) in New Zealand: the story so far. Proceedings of the New Zealand Grassland Association 67: 19–22.
- Emerson BC, Oromí P, Hewitt GM 2000. Colonization and diversification of the species Brachyderes rugatus (Coleoptera) on the Canary Islands: evidence from mitochondrial DNA COII gene sequences. Evolution 54: 911–923.10.1111/j.0014-3820.2000.tb00091.x
- Fremuth J 1971. Die mitteleuropaischen Arten der gattung Barypeithes. Annotationes Zoologicae et Botanicae 65: 1–30.
- Galford JR 1987. Feeding habits of the weevil Barypeithes pellucidus (Coleoptera: Curculionidae). Entomological News 98: 163–164.
- Goldson SL, Wratten SD, Ferguson CM, Gerard PJ, Barratt BIP, Hardwick S et al. 2014. If and when successful classical biological control fails. Biological Control 72: 76–79.10.1016/j.biocontrol.2014.02.012
- Gómez-Zurita J, Jolivet P, Vogler AP 2005. Molecular systematics of Eumolpinae and the relationships with Spilopyrinae (Coleoptera, Chrysomelidae). Molecular Phylogenetics and Evolution 34: 584–600.
- Gonzalez M 1967. El género Orthochaetes Germar (Col. Curculionidae). Publicaciones del Instituto de Biologia aplicada 42: 49–85.
- Gosik R, Sprick P 2013. Morphology and identification of the pupae of several species of soil-dwelling broad-nosed weevils from Central Europe. Zootaxa 3731: 445–472.10.11646/zootaxa.3731.4.2
- Halstead AJ 1977. Leaf mining of hardy cyclamen by Orthochaetes setiger (Beck) (Coleoptera: Curculionidae). Plant Pathology 26: 102.
- Hirsch J, Sprick P, Reineke A 2010. Molecular identification of larval stages of Otiorhynchus (Coleoptera: Curculionidae) species based on polymerase chain reaction-restriction fragment length polymorphism analysis. Journal of Economic Entomology 103: 898–907.10.1603/EC09381
- Kriticos D, Phillips CB, Suckling DM 2005. Improving border biosecurity: potential economic benefits to New Zealand. New Zealand Plant Protection 58: 1–6.
- Kuschel G 1972. The foreign Curculionoidea established in New Zealand (Insecta: Coleoptera). New Zealand Journal of Science 15: 273–289.
- Löbl I, Smetana A eds. 2013. Catalogue of Palaearctic Coleoptera. Volume 8. Curculionoidea II. Brill, Leiden.
- Macfarlane RP, Maddison PA, Andrew IG, Berry JA, Johns PM, Hoare RJB et al. 2011. Chapter nine. Phylum Arthropoda; subphylum Hexapoda; Protura, springtails, Diplura and insects. In: Gordon DP ed. New Zealand inventory of biodiversity, volume 2. Kingdom Animalia, Chaetognatha, Ecdysozoa, Ichnofossils. Canterbury, Canterbury University Press. Pp. 233–467.
- Machado A 2008. New taxa of Laparocerus Schönherr, 1834 from Madeira and Porto Santo, with a key to the species of the Madeiran Archipelago (Coleoptera, Curculionidae, Entiminae). Graellsia 64: 307–328.10.3989/graellsia.2008.v64.i2.40
- Marvaldi AE, Lanteri AA, Guadelupe del Rio M, Oberprieler RG 2014. 3.7.5 Entiminae Schoenherr, 1823. In: Leschen RAB, Beutel RG eds. Handbook of zoology, Arthropoda: Insecta. Coleoptera, Beetles volume 3: morphology and systematics (Phytophaga). Berlin, Walter De Gruyter. Pp. 503–522.
- Mazur M 2002. The distribution and ecology of weevils (Coleoptera: Nemonychidae, Attelabidae, Apionidae, Curculionidae) in western Ukraine. Acta Zoologica Cracoviensia 45: 213–244.
- Metcalfe DJ 2005. Biological Flora of the British Isles No. 240 Hedera helix L. Journal of Ecology 93: 632–648.10.1111/j.1365-2745.2005.01021.x
- Morris MG 1997. Broad-nosed weevils. Coleoptera: Curculionidae (Entiminae). London, Royal Entomological Society.
- Morris MG 2002. True weevils (Part I): Coleoptera: Curculionidae (Subfamilies Raymondionyminae to Smicronychinae). London, Royal Entomological Society.
- Oberprieler RG 2014. 3.7.4 Cyclominae Schoenherr, 1826. In: Leschen RAB, Beutel RG eds. Handbook of zoology, Arthropoda: Insecta. Coleoptera, Beetles volume 3: morphology and systematics (Phytophaga). Berlin, Walter De Gruyter. Pp. 483–502.
- Osella G, Zuppa AM 1993. Gli Orthochaetini Italiani (Ceoloptera Curculionidae Notarinae). Memorie della Societa Entomologica Italiana, Genova 72: 277–309.
- Petherbridge FR 1915. Exomias pellucidus as a plant pest. Annals of Applied Biology 1: 390–392.10.1111/j.1744-7348.1915.tb08006.x
- Phillips CB, Barratt BIP 2004. A guide to assist detection of newly arrived Sitona species (Coleoptera: Curculionidae) in New Zealand and Australia. Proceedings of the 8th Australasian Grassland Invertebrate Ecology Conference, AgResearch Ltd, Lincoln, New Zealand. Pp. 22–33.
- Prestidge RA, Barker GM, Pottinger RP 1991. The economic cost of Argentine stem weevil in pastures in New Zealand. Proceedings of the 44th New Zealand Weed and Pest Control Conference, The New Zealand Weed and Pest Control Society Inc., Tauranga, New Zealand. Volume 1. Pp. 165–170.
- Sequeira AS, Normark BB, Farrell BD 2000. Evolutionary assembly of the conifer fauna: distinguishing ancient from recent associations in bark beetles. Proceeding of the Royal Society of London B 267: 2359–2366.
- Simberloff DS 2005. Non-native species do threaten the natural environment! Journal of Agricultural and Environmental Ethics 18: 595–607.
- Simon C, Frati F, Beckenbach AT, Crespi B, Liu H, Flook P 1994. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Annals of Applied Biology 87: 651–701.
- Snyder WE, Evans EW 2006. Ecological effects of invasive arthropod generalist predators. Annual Review of Ecology, Evolution and Systematics 37: 95–122.10.1146/annurev.ecolsys.37.091305.110107
- Tylianakis JM, Romo CM 2010. Natural enemy diversity and biological control: making sense of the context-dependency. Basic and Applied Ecology 11: 657–668.10.1016/j.baae.2010.08.005
