Abstract
The fiords of southwestern New Zealand may promote the existence of genetically divergent populations as a result of geological and ecological isolation. The ophiuroid Astrobrachion constrictum lives on black coral in these fiords, and is thought to have a relatively short pelagic larval duration that may limit dispersal among populations. Samples of A. constrictum were collected from seven sites (four fiords) in Fiordland. Analysis of the mitochondrial COI gene using single-stranded conformational polymorphism revealed eight haplotypes, representatives of which were sequenced. These included nine variable sites, none of which were phylogenetically informative. G-test and AMOVA revealed little structuring of samples within or among fiords, but there was evidence for reduced gene flow among the most geographically separated fiords. We conclude that fiords do not house relict populations from earlier glaciations, and that little differential sorting of haplotypes has occurred in the last 10,000 years.
Introduction
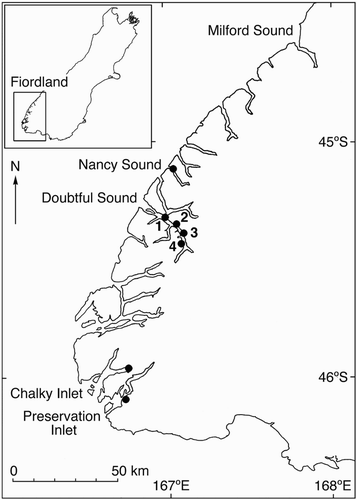
The southwest coast of New Zealand comprises 14 glacial fiords () extending from Milford Sound in the north to Preservation Inlet c. 200 km to the south (Augustinus Citation1992). Although information on early glacial activity in this area is lacking due to intense erosion during subsequent stages (McKellar & Soons Citation1982), speleothem deposits in the Te Ana-au cave system suggest seven glacial events in the last 230 ka (Williams Citation1996). The mountainous nature of Fiordland and prevailing westerly weather systems result in high regional rainfall (Stanton & Pickard Citation1982). The resulting freshwater run-off forms a low-salinity surface layer in fiords (Stanton & Pickard Citation1981), creating an estuarine-like circulation system in the upper layers. The freshwater surface layer flows seaward entraining water of higher salinity beneath, driving this lower layer towards the head of the fiord (Garner Citation1964; Stanton Citation1978; Stanton & Pickard Citation1981; Stanton Citation1986). Seawater below sill depth tends to stay in fiords, with limited displacement from above when incoming coastal water is denser than the deep water of the fiords (Stanton & Pickard Citation1981; Stanton Citation1986). High concentrations of chromophoric dissolved organic matter in the low salinity surface layer (Peake & Mosley Citation2004) reduce light penetration to deeper water in the fiord (Grange et al. Citation1981). Other deep-water conditions such as low current and little wave action are also promoted within the fiords due to their sheltered nature (Grange et al. Citation1981). These factors combine to allow species that normally occupy deep offshore habitats, such as the snake star Astrobrachion constrictum, to exist within the fiords (Grange et al. Citation1981). These populations have the potential to be genetically distinct from each other as a result of the geological age and hydrological disconnectedness of fiords (Turan et al. Citation1998; Wallis & Trewick Citation2009).
Figure 1 Fiordland region of South Island, New Zealand, showing collection locations for the snake star Astrobrachion constrictum. Doubtful Sound sites are: 1, Espinosa Point; 2, Tricky Cove; 3, Oz; 4, Crowded House.
Astrobrachion constrictum lives exclusively on the antipatharian black coral Antipathes fiordensis (Stewart & Mladenov Citation1997) in a mutualistic relationship (Vasques Citation1997). The apparent lecithotrophic larval phase of A. constrictum (Strathmann & Rumrill Citation1987; Stewart & Mladenov Citation1993; Stewart Citation1995) suggests that its dispersal potential is relatively low, further increasing the opportunity for genetic differentiation among fiords. Alternatively, gene flow through offshore populations could maintain genetic connectedness of fiord populations.
This study was undertaken to assess the level of genetic differentiation of populations of A. constrictum among fiords with three possible scenarios in mind (Graham Citation2008; Wallis & Trewick Citation2009): 1. genetic differentiation consistent with initial glacial activity and fiord formation (high; 2.7 Ma); 2. differentiation indicative of the end of the last glacial period (low; 0.1 Ma); and 3. differentiation consistent with recent or ongoing gene flow within the last interglacial (none; 0 Ma). We include multiple population samples within one fiord to contrast levels of differentiation within and among fiords.
Materials and methods
Specimens of A. constrictum were collected by SCUBA from four sites (Espinosa Point, Tricky Cove, Oz, Crowded House) in Doubtful Sound, and one site in Nancy Sound, Chalky Inlet and Preservation Inlet (). Several collecting trips were made between October 1996 and September 1998. Due to the patchy distribution of this species (Stewart & Mladenov Citation1997), sample numbers vary considerably among sites. At all sites except Crowded House and Oz, animals were collected from several different host coral colonies within the dive site. All animals (n = 117) were kept alive in seawater for transport to the laboratory, where they were immediately stored at –80 °C. No samples were collected from the continental shelf outside the fiords as A. constrictum lives beyond SCUBA depth and the only collecting method is dredging. Dredging was not a viable option as the black coral on which A. constrictum lives is a protected species.
Approximately 50 mg of gonad tissue was dissected from the upper arm of each specimen and macerated with 50 μl of ultrapure water. Samples were placed in a boiling water bath for c. 10 min then centrifuged at 15,000 g for 2 min. The supernatant was transferred to a clean tube and DNA precipitated with 1/20 volume of 5 M NaCl and 2×volume of cold 100% ethanol. Tubes were centrifuged at 15,000 g for 10 min, ethanol removed and the dried DNA dissolved in 30 μl of ultrapure water. This DNA solution was used directly for polymerase chain reaction (PCR).
Two mitochondrial gene regions were amplified (Simon et al. Citation1994): a 473-bp fragment of the cytochrome c oxidase subunit I (COI) gene using primers C1-J-1718 and C1-N-2191, and a 511-bp fragment of the large ribosomal subunit (16S) using primers LR-J-12887 and LR-N-13398. Polymerase chain reactions were carried out using 3.5 mM MgCl2, 200 μM each dNTP, 0.25 μM each primer, 0.025 units of Qiagen© Taq polymerase, 10× Qiagen© buffer in a total volume of 10 μl (1 μl of template) for single-stranded conformational polymorphism (SSCP) and 25 μl (2 μl of template) for sequencing and RFLP analysis. The following thermocycling protocol was used for amplification: initial denaturation at 94 °C for 60 s; 40 cycles of denaturation at 94 °C for 15 s, annealing at 50 °C for 30 s and extension at 72 °C for 60 s; final extension at 72 °C for 3 min.
Sequence variation in COI and 16S amplicons was surveyed across individuals using SSCP (Trewick et al. Citation2000). We sequenced at least one representative of each COI haplotype to ascertain underlying sequence differences. Twenty-five microlitre PCR products and a 1-kb Plus DNA ladder (Gibco BRL, Grand Island, NY) were electrophoresed in 2% agarose gels stained with EtBr. Products of expected length were excised and purified using Prep-A-Gene® (BioRad, Hercules, CA), and quantified by electrophoresis with 1-kb Plus DNA ladder. Cycle sequencing was carried out with BigDye™ chemistry according to the manufacturer's recommendations (Applied Biosystems, Carlsbad, CA). The product was precipitated using EtOH/NaAc and the sequence read on an ABI377.
Sequences were aligned by eye and nucleotide differences noted using SeqEd v1.03 (ABI, Perkin Elmer, Foster City, CA). A minimum spanning network showing relationships between haplotypes and their frequencies across sites was drawn using PopArt (Population Analysis with Reticulate Trees; http://popart.otago.ac.nz/index.shtml). Haplotype frequencies were analysed using G-tests (Sokal & Rohlf Citation1981) and sequence data by AMOVA (Excoffier et al. Citation1992).
Results
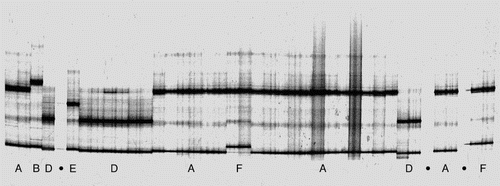
Using SSCP, 114 individuals were scored for COI and 19 individuals (representing at least one example of every COI haplotype present in each fiord) were scored for 16S. Eight COI haplotypes (A–H; ; ) and a single 16S haplotype were identified from the SSCP gels. Four individuals of COI haplotype A, two of haplotypes D and E, and one each of haplotypes B, C, F, G and H were sequenced (haplotypes A–H; Genbank Accession #KR140027-34). One individual was heteroplasmic for two haplotypes (A/D, Steel et al. Citation2000). Because only one 16S haplotype was identified from the SSCP gels, no individuals were sequenced for 16S. All SSCP-resolved haplotypes could be distinguished from each other at the sequence level, and no additional ‘hidden’ variation was found. That is, there was a one-to-one matching of SSCP band to haplotype.
Figure 2 Minimum spanning network for eight snake star COI haplotypes (A–H; ) constructed from sequence data. Black dots on the C and D lineages indicate hypothetical intermediates. Sample size (as assessed by SSCP) is reflected by area of each circle.
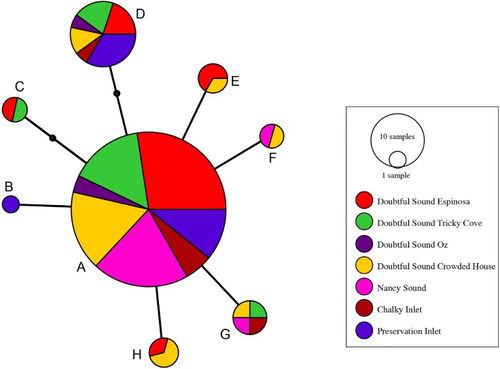
Table 1 Summary of COI SSCP haplotype data for snake stars from Nancy Sound, Chalky Inlet and Preservation Inlet, and four sites within Doubtful Sound (Espinosa Point, Tricky Cove, Oz, Crowded House).
Of the 473-bp of COI sequenced, there were nine variable nucleotide sites, all representing synonymous polymorphisms (one in the first position and eight in the third position of the codon). The transition:transversion ratio was 7:2. All haplotypes appear to derive from the most common haplotype A by one (B, E–H) or two (C–D) substitutions ().
G-test analysis (Sokal & Rohlf Citation1981) showed no significant difference in haplotype frequencies among all seven sampling sites (G[36] = 37.9, P > 0.2), among the four sites in Doubtful Sound alone (G[21] = 13.1, P > 0.9), or among the four fiords after pooling Doubtful Sound sites (G[21] = 24.8, P > 0.2). Due to the large number of haplotypes with low or zero frequency at some locations, G-test analysis was repeated having pooled low-frequency haplotypes (i.e. A, D, others). The results of these three tests were similarly not significant (G[12] = 13.7, P > 0.3; G[6] = 3.49, P > 0.7; G[6] = 10.21, P > 0.1). However, the two geographically most distant fiords (Preservation Inlet and Nancy Sound) do show a significant difference (G[4] = 13.1, P < 0.02), further enhanced by pooling of rare haplotypes (G[2] = 9.3, P < 0.01). This result reflects a higher frequency of D haplotypes to the south. AMOVA analysis (Excoffier et al. Citation1992) showed no significant difference among fiords (FST = 0.013, P > 0.05) or among sites within Doubtful Sound (FSC = –0.037, P > 0.05). These results suggest that nucleotide variation among sites or fiords is not significantly greater than variation within a sample.
Discussion
Analysis of the genetic structure of seven population samples of Astrobrachion constrictum showed no significant overall differentiation among samples as a whole within, or among, fiords. Within Doubtful Sound, there is no evidence for heterogeneity among samples whatsoever (P > 0.9). The lack of deep genetic subdivision among fiords is consistent with recolonisation since the most recent glaciation, as has been described for Chilean pycnogonids (Weis & Melzer Citation2012) and limpets (Gonzalez-Wevar et al. Citation2012). The relatively recent development of New Zealand fiords has probably not allowed sufficient time for differentiation to accrue. Associated with the end of the last glacial period 10,000–18,000 years ago (Pillans et al. Citation1992) was a rise in sea level of 110–120 m, stabilising at today's level around 6500 years ago (Pickrill et al. Citation1992). The timing of marine incursions into each fiord would be dependent on the height of the entrance sill; fiords such as Preservation Inlet with high sills would have been flooded later than fiords with low sills. Therefore, Fiordland as it is today has only been in existence for a maximum of 10,000 years (Pickrill et al. Citation1992; Graham Citation2008). Although this time is long enough to detect a correlation between age of fiord and species richness (Smith Citation2001), it is too short for detectable DNA substitutions to have occurred in situ (Hickerson et al. Citation2006).
Although these results are inconsistent with the first of our hypotheses (high differentiation), some weak genetic subdivision among fiords might, however, still be expected over this time frame. If effective population sizes were restricted, differential sorting of haplotypes from an original heterogeneous population would be likely. There is some evidence for subdivision at the level of haplotype frequency differences among samples of A. constrictum for Nancy Sound and Preservation Inlet, the most geographically separated fiords. Thus, although our sample sizes are small, there is evidence for restricted gene flow among fiords. The nature of the differentiation is best exemplified as a clinal increase in frequency of haplotype D from north to south: Nancy 0/19 (0%); Doubtful 9/73 (12%); Chalky 1/7 (14%); and Preservation 5/15 (33%). This finding is consistent with strong currents flowing southwest (Stanton Citation1986; Graham Citation2008): once a haplotype is lost to the north, it is hard for it to be reintroduced. Our findings are therefore probably best characterised by our second hypothesis of low differentiation.
Other research reports similar findings. The Fiordland asteroid Coscinasterias muricata shows two main haplotypes differing by a single substitution, also with a north–south cline in frequency (Perrin et al. Citation2004). Genetic differentiation through random genetic drift is also reported among fiord populations of black coral A. fiordensis (Miller Citation1997) and C. muricata (Skold et al. Citation2003) using allozymes. Despite prevailing ocean current towards the southwest, Mladenov et al. (Citation1997) showed some differentiation between coastal and Doubtful Sound populations of sea urchin (Evechinus chloroticus), suggesting some limitation to gene flow. Evechinus chloroticus has a long pelagic larval duration and thus high dispersal potential (Dix Citation1969; Walker Citation1984), and also inhabits shallow coastal waters between fiords (Dix Citation1970). These two factors should make gene flow among fiords more likely for this echinoid than for deepwater A. constrictum.
It is worth mentioning that COI is often highly variable within populations (Muths et al. Citation2006; Richards et al. Citation2007) and has been useful in assessing connectedness between populations of marine invertebrates (Burton & Lee Citation1994; Duran et al. Citation2004; Richards et al. Citation2007; Christensen et al. Citation2008; Hunter & Halanych Citation2008). Indeed, the overall lack of divergence in our samples () is striking, and (on the basis of this single marker) argues for recent ancestry of populations. Although we cannot rule out some gene flow occurring among fiords (our third hypothesis), our results are more consistent with recent origins of fiord populations without invoking ongoing gene flow. Further finescale assessment of gene flow among populations of fiord echinoderms using microsatellite analysis would be useful, though even these markers have shown little differentiation in some other studies (Jorde et al. Citation2007).
Associate Editor: Dr Jonathan Banks.
Acknowledgements
Paul Meredith helped to organise trips to Fiordland, and Peter Stratford, Nicki Alcock and Jo Duncan assisted in collection of samples. Anonymous reviewers provided very helpful comments on earlier versions of this article. This work was funded by a University of Otago Divisional grant to GPW, and by the Department of Marine Science, University of Otago.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Augustinus PC 1992. Outlet glacier trough size-drainage area relationships, Fiordland, New Zealand. Geomorphology 4: 347–361. doi: 10.1016/0169-555X(92)90028-M
- Burton RS, Lee B-N 1994. Nuclear and mitochondrial gene genealogies and allozyme polymorphism across a major phylogeographic break in the copepod Tigriopus californicus. Proceedings of the National Academy of Sciences, USA 91: 5197–5201. doi: 10.1073/pnas.91.11.5197
- Christensen AB, Christensen EF, Weisrock DW 2008. Population genetic structure of North American Ophiactis spp. brittle stars possessing hemoglobin. Marine Biology 154: 755–763. doi: 10.1007/s00227-008-0968-1
- Dix TG 1969. Larval life span of the echinoid Evechinus chloroticus (Val.). New Zealand Journal of Marine and Freshwater Research 3: 13–16. doi: 10.1080/00288330.1969.9515273
- Dix TG 1970. Biology of Evechinus chloroticus (Echinoidea: Echinometridae) from different localities: 3. Reproduction. New Zealand Journal of Marine and Freshwater Research 4: 385–405. doi: 10.1080/00288330.1970.9515355
- Duran S, Palacín C, Becerro MA, Turon X, Giribet G 2004. Genetic diversity and population structure of the commercially harvested sea urchin Paracentro tus lividus (Echinodermata, Echinodea). Molecular Ecology 13: 3317–3328. doi: 10.1111/j.1365-294X.2004.02338.x
- Excoffier L, Smouse PE, Quattro JM 1992. Analysis of molecular variance inferred from metric distance among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131: 479–491.
- Garner DM 1964. The hydrology of Milford Sound. In: Skerman TM ed. Studies of a New Zealand fiord. New Zealand Oceanographic Institute Memoir. Wellington, DSIR. Pp. 25–33.
- Gonzalez-Wevar CA, Huene M, Canete JI, Mansilla A, Nakano T, Poulin E 2012. Towards a model of postglacial biogeography in shallow marine species along the Patagonian Province: lessons from the limpet Nacella magellanica (Gmelin, 1791). BMC Evolutionary Biology 12: 1–16. doi: 10.1186/1471-2148-12-139
- Graham IJ ed. 2008 A continent on the move: New Zealand geoscience into the 21st century. Wellington, The Geological Society of New Zealand in association with GNS Science. 388 p.
- Grange KR, Singleton RI, Richardson JR, Hill PJ, Main WdeL 1981. Shallow rock-wall biological associations of some southern fiords of New Zealand. New Zealand Journal of Zoology 8: 209–227. doi: 10.1080/03014223.1981.10427963
- Hickerson MJ, Stahl EA, Lessios, HA 2006. Test for simultaneous divergence using approximate Bayesian computation. Evolution 60: 2435–2453. doi: 10.1111/j.0014-3820.2006.tb01880.x
- Hunter RL, Halanych KM 2008. Evaluating connectivity in the brooding brittle star Astrotoma agassizii across the Drake Passage in the Southern Ocean. Journal of Heredity 99: 137–148. doi: 10.1093/jhered/esm119
- Jorde PE, Knutsen H, Espeland SH, Stenseth NC 2007. Spatial scale of genetic structuring in coastal cod Gadus morhua and geographic extent of local populations. Marine Ecology Progress Series 343: 229–237. doi: 10.3354/meps06922
- McKellar IC, Soons JM 1982. Fiordland. In: Soons JM, Selby MJ eds. Landforms of New Zealand. 2nd edition. Auckland, Longman Paul. Pp. 505–515.
- Miller KJ 1997. Genetic structure of black coral populations in New Zealand's fiords. Marine Ecology Progress Series 161: 123–132. doi: 10.3354/meps161123
- Mladenov PV, Allibone RM, Wallis GP 1997. Genetic differentiation in the New Zealand sea urchin Evechinus chloroticus (Echinodermata: Echinoidea). New Zealand Journal of Marine and Freshwater Research 31: 261–269. doi: 10.1080/00288330.1997.9516763
- Muths D, Davoult D, Gentil F, Jollivet D 2006. Incomplete cryptic speciation between intertidal and subtidal morphs of Acrocnida brachiata (Echinodermata: Ophiuroidea) in the Northeast Atlantic. Molecular Ecology 15: 3303–3318. doi: 10.1111/j.1365-294X.2006.03000.x
- Peake BM, Mosley LM 2004. Hydrogen peroxide concentrations in relation to optical properties in a fiord (Doubtful Sound, New Zealand). New Zealand Journal of Marine and Freshwater Research 38: 729–741. doi: 10.1080/00288330.2004.9517272
- Perrin C, Wing SR, Roy MS 2004. Effects of hydrographic barriers on population genetic structure of the sea star Coscinasterias muricata (Echinodermata: Asteroidea) in the New Zealand fiords. Molecular Ecology 13: 2183–2195. doi: 10.1111/j.1365-294X.2004.02241.x
- Pickrill RA, Fenner JM, McGlone MS 1992. Late quaternary evolution of a fjord environment in Preservation Inlet, New Zealand. Quaternary Research 38: 331–346. doi: 10.1016/0033-5894(92)90042-H
- Pillans B, Pullar WA, Selby MJ, Soons JM 1992. The age and development of the New Zealand landscape. In: Soons JM, Selby MJ eds. Landforms of New Zealand. 2nd edition. Auckland, Longman Paul. Pp. 31–62.
- Richards VP, Thomas JD, Stanhope MJ, Shivji MS 2007. Genetic connectivity in the Florida reef system: comparative phylogeography of commensal invertebrates with contrasting reproductive strategies. Molecular Ecology 16: 139–157. doi: 10.1111/j.1365-294X.2006.03145.x
- Simon C, Frati F, Beckenbach A, Crespi B, Liu H, Flook P 1994. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Annals of the Entomological Society of America 87: 651–701. doi: 10.1093/aesa/87.6.651
- Skold M, Wing SR, Mladenov PV 2003. Genetic subdivision of a sea star with high dispersal capability in relation to physical barriers in a fjordic seascape. Marine Ecology Progress Series 250: 163–174. doi: 10.3354/meps250163
- Smith F 2001. Historical regulation of local species richness across a geographic region. Ecology 82: 792–801. doi: 10.1890/0012-9658(2001)082[0792:HROLSR]2.0.CO;2
- Sokal RR, Rohlf FJ 1981. Biometry. 2nd edition. San Francisco, WH Freeman & Co. 859 p.
- Stanton BR 1978. Hydrology of Caswell and Nancy Sounds. In: Glasby GP ed. Fiord studies: Caswell and Nancy Sounds, New Zealand. New Zealand Oceanographic Institute Memoir. Wellington, DSIR. Pp. 73–82.
- Stanton BR 1986. Winter oceanographic observations in some New Zealand fiords. New Zealand Journal of Marine and Freshwater Research 20: 299–314. doi: 10.1080/00288330.1986.9516151
- Stanton BR, Pickard GL 1981. Physical oceanography of the New Zealand fjords. New Zealand Oceanographic Institute Memoir. Wellington, DSIR: 88. 37 p.
- Stanton BR, Pickard GL 1982. Physical oceanography of the New Zealand fjords. In: Freeland HJ, Farmer DM, Levings CD eds. Fjord oceanography. New York, Plenum Press. Pp. 329–332.
- Steel DJ, Trewick SA, Wallis GP 2000. Heteroplasmy of mitochondrial DNA in the ophiuroid Astrobrachion constrictum. Journal of Heredity 91: 146–149. doi: 10.1093/jhered/91.2.146
- Stewart B 1995. The biology of the euryalid snake star Astrobrachion constrictum (Echinodermata: Ophi uroidea). Unpublished PhD thesis. Dunedin, New Zealand, University of Otago.
- Stewart B, Mladenov P 1993. Aspects of reproduction and anatomy of the euryalinid snake star Astrobrachion constrictum. In: David B, Guille A, Feral JP, Roux M eds. Echinoderms through time: Proceedings of the 8th International Echinoderm Conference, Dijon, France. Rotterdam, Balkema AA. Pp. 491–497.
- Stewart BG, Mladenov PV 1997. Population structure, growth and recruitment of the euryalinid brittle-star Astrobrachion constrictum (Echinodermata: Ophiuroidea) in Doubtful Sound, Fiordland, New Zealand. Marine Biology 127: 687–697. doi: 10.1007/s002270050059
- Strathmann MF, Rumrill SS 1987. Phylum Echinodermata, class Ophiuroidea. In: Strathmann MF ed. Reproduction and development of marine invertebrates of the northern Pacific coast. Data and methods for the study of eggs, embryos, and larvae. Seattle, University of Washington Press. Pp. 556–573.
- Trewick SA, Wallis GP, Morgan-Richards M 2000. Phylogeographical pattern correlates with Pliocene mountain building in the alpine scree weta (Orthoptera, Anostostomatidae). Molecular Ecology 9: 657–666. doi: 10.1046/j.1365-294x.2000.00905.x
- Turan C, Carvalho GR, Mork J 1998. Molecular genetic analysis of Atlanto-Scandian herring (Clupea harengus) populations using allozymes and mitochondrial DNA markers. Journal of the Marine Biological Association UK 78: 269–283. doi: 10.1017/S002531540004008X
- Vasques J 1997. Sedimentation and housecleaning: the interactions of Astrobrachion constrictum and its host Antipathes fiordensis. Unpublished MARI 480 project. Dunedin, New Zealand, University of Otago. 39 p.
- Walker MM 1984. Larval life span, larval settlement, and early growth of Evechinus chloroticus (Valenciennes). New Zealand Journal of Marine and Freshwater Research 18: 393–397. doi: 10.1080/00288330.1984.9516060
- Wallis GP, Trewick SA 2009. New Zealand phylogeography: evolution on a small continent. Molecular Ecology 18: 3548–3580. doi: 10.1111/j.1365-294X.2009.04294.x
- Weis A, Melzer RR 2012. How did sea spiders recolonize the Chilean fjords after glaciation? DNA barcoding of Pycnogonida, with remarks on phylogeography of Achelia assimilis (Haswell, 1885). Systematics and Biodiversity 10: 361–374. doi: 10.1080/14772000.2012.716462
- Williams PW 1996. A 230 ka record of glacial and interglacial events from Aurora Cave, Fiordland, New Zealand. New Zealand Journal of Geology and Geophysics 39: 225–241. doi: 10.1080/00288306.1996.9514707