ABSTRACT
Coleosoma octomaculatum (Bösenberg & Strand, 1906), previously known only from South East Asia, is recorded from New Zealand for the first time. The numerous specimen records between 1996 and 2007 suggest it is established. This species is probably an anthropogenic introduction and represents the first reported colonisation outside of its known range. We reverse the recent transfer to Chrysso O. Pickard-Cambridge, 1882 and suggest two possible species groups within the genus Coleosoma O. Pickard-Cambridge, 1882. A redescription including measurements and genitalic photographs is provided. We also report habitat preferences and egg clutch data for this species.
Introduction
Males of the genus Coleosoma are described as resembling ants (Paquin et al. Citation2008). Nine species are listed in this genus, of which one, Coleosoma blandum O. Pickard-Cambridge, 1882, is cosmopolitan (World Spider Catalog Citation2015). Another, Coleosoma floridanum (Banks, 1900), is cosmotropical (Central and South America (Levi Citation1959), Africa (Levi Citation1967), India (Srinivasulu et al. Citation2013), China (Zhu & Zhang Citation1992), the Philippines (Barrion & Litsinger Citation1995) and Pacific Islands (Marples Citation1955)). Coleosoma floridanum is also known from the southern United States (Levi Citation1959) and has become established in glasshouses in Britain (Spoczynska Citation1969), Europe (Pfliegler Citation2014), Canada (Paquin et al. Citation2008) and Japan (Yoshida Citation2009). The genus has not previously been recorded from the temperate regions of the southern hemisphere, although New Zealand's Ministry for Primary Industries (MPI) has intercepted C. floridanum in cargo entering the country (see Material examined). However, we have spiders from various New Zealand localities collected over the last two decades that we now recognise as Coleosoma octomaculatum (Bösenberg & Strand, Citation1906), transferred by Yoshida (Citation2009) to Chrysso. In this article, we redescribe the species (including measurements and genitalia photographs), and reinstate it in Coleosoma. We also note the possible existence of two species groups within Coleosoma and include observations on the biology and geographic range of the species.
Methods and conventions
Colour information is based on ethanol-preserved specimens. Unambiguous characters from the description of the genus are not repeated in the species redescription. All specimens are held at the Museum of New Zealand Te Papa Tongarewa unless otherwise noted and registration numbers are provided. Locality regions (noted in bold) for New Zealand follow Crosby et al. (Citation1998).
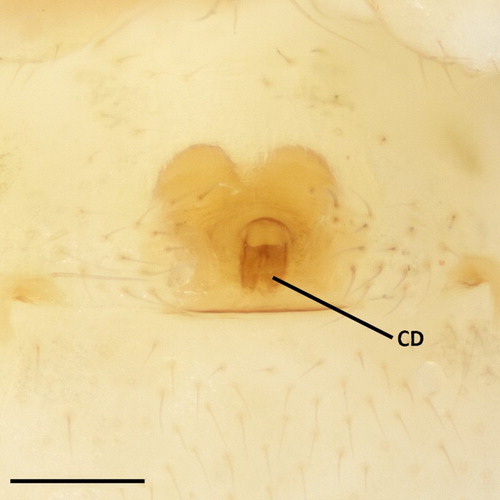
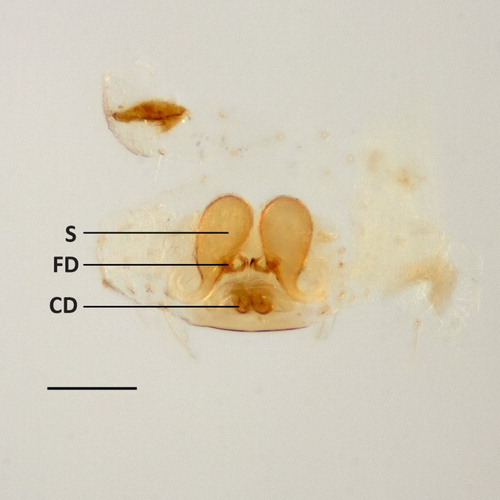
Abbreviations are as follows: Eyes: ALE = anterior lateral eyes; AME = anterior median eyes; PLE = posterior lateral eyes; PME = posterior median eyes. Male palp: C = conductor; ES = embolus spiral; EB = embolus base; MA = median apophysis; ST = subtegulum; T = tegulum; TTA = theridiid tegular apophysis. Female genitalia: CD = copulatory duct; FD = fertilisation duct; S = spermatheca.
Measurements (in mm) were made using an eyepiece micrometer on a Zeiss Stemmi 2000 binocular microscope and mean total lengths for males and females are also given. For the habitus figures (–), specimens were photographed using a Canon G2 digital camera on the same microscope, and a series of photographs was combined with focus-stacking software Combine ZP (© Alan Hadley) to produce images with an extended depth of field. Because the epigynum and palp were too small to photograph with this equipment, a series of photographs was taken on a Canon 450D fitted with a Nikon M Plan 10× microscope lens mounted on bellows. Images (–) were focus stacked with Zerene Stacker (Zerene Systems) before final cropping and editing in Photoshop CS6 (Adobe Systems). The map was prepared in SimpleMappr (Shorthouse Citation2010).
Figure 1. Habitus views of male Coleosoma octomaculatum. A, dorsal; B, ventral. Scale bars = 0.5 mm.

Figure 2. Habitus views of female Coleosoma octomaculatum. A, dorsal; B, ventral. Scale bars = 0.5 mm.

Figure 3. Coleosoma octomaculatum male palp, ventral view. C, conductor; ES, embolus spiral; EB, embolus base; MA, median apophysis; ST, subtegulum; T, tegulum; TTA, theridiid tegular apophysis. Scale bar = 0.1 mm.

Systematics
Family Theridiidae, subfamily Theridiinae
Genus Coleosoma O. Pickard-Cambridge, 1882
Coleosoma—O. Pickard-Cambridge, 1882: 426. Type species by monotypy Coleosoma blandum. Placed by Simon (Citation1894) as a junior synonym of Theridion Walckenaer 1805, repeated by Petrunkevitch, Roewer & Bonnet (as noted by Levi Citation1959). Removed from synonymy by Bryant (Citation1940: 303). We accept Agnarsson's (Citation2004: fig. 102) placement of Coleosoma within Theridiinae.
Description based on Pickard-Cambridge (Citation1882), Bryant (Citation1944), Levi (Citation1959) and Levi & Levi (Citation1962). Small spiders, less than 3 mm in length. Eyes very small, those of posterior row one to two diameters apart. Clypeus projecting. Chelicerae have no teeth, or 1–2 teeth on the promargin and none on the retromargin except in females of Coleosoma acutiventer (Keyserling 1884), which have one tooth. Leg formula typically 1-4-2-3, occasionally 1-2-4-3. Tarsal comb is present but indistinct. Colulus absent. Abdomen modified in males of all species and in females of some species. Abdomen of males is cylindrical and in some species constricted about midway. The abdomen always has a sclerotised ring encircling the pedicel and extending as a shield for more than half the length of the ventral surface of the abdomen. Males also have a prosoma-abdomen stridulatory mechanism comprising a pair of stridulatory pick rows on the abdomen that interact with a series of parallel ridges (pars stridens) on the posterior margin of the carapace (Agnarsson Citation2004). In females, the abdomen may be sub-oval or bear a tubercle above and posterior to the spinnerets. Paracymbium present, forming an incision on the margin of the cymbium, near the apex. Embolus spiral forms a half circle or almost a circle. Epigynum weakly sclerotised with indistinct opening in most species, but with distinct vulva in some. One pair of spermathacae present.
The genus currently contains nine species (World Spider Catalog Citation2015): Coleosoma acutiventer (Keyserling, 1884); Coleosoma africanum Schmidt & Krause, 1995; Coleosoma blandum O. Pickard-Cambridge, 1882 (type species); Coleosoma caliothripsum Barrion & Litsinger, Citation1995; Coleosoma floridanum Banks, 1900; Coleosoma matinikum Barrion & Litsinger, Citation1995; Coleosoma normale Bryant, Citation1944; Coleosoma pabilogum Barrion & Litsinger, Citation1995; Coleosoma pseudoblandum Barrion & Litsinger, Citation1995.
We consider Chrysso octomaculata (Bösenberg & Strand, Citation1906) is the tenth member of this genus.
Coleosoma octomaculatum (Bösenberg & Strand, Citation1906)
Theridion octomaculatum Bösenberg & Strand, Citation1906: 138, pl. 3, fig. 14, pl. 12, fig. 281.
Coleosoma octomaculatum (Bösenberg & Strand, Citation1906); Yoshida Citation1982: 38, figs –.
Chrysso octomaculata (Bösenberg & Strand, Citation1906); Yoshida Citation2009: 376, figs 198–200.
Remarks
Levi & Levi (Citation1962) differentiated Chrysso Pickard-Cambridge, 1882 and Coleosoma Pickard-Cambridge, 1882 on the basis of the presence of a sheathed anteroventral portion of the male abdomen in the latter genus. Males of Chrysso octomaculata have such a structure and for this reason, as well as a greater similarity of male palp morphology, we reverse the generic transfer of Yoshida (Citation2009) and return C. octomaculata to Coleosoma in agreement with Yoshida (Citation1982). We also note Agnarsson's (Citation2004) morphological analysis of theridiid spiders places Chrysso as sister to all other members of the Theridiinae, including C. floridanum, the species we regard as most closely related to C. octomaculatum, based on our discussion below of possible species groupings within the genus.
Diagnosis
Coleosoma octomaculatum and C. floridanum are separated from all other Coleosoma species by the well sclerotised vulva (genital opening unclear in most other described species) and the embolus spiral forming an approximate half circle (forming almost a full circle in other species).
Coleosoma octomaculatum and C. floridanum are separated from each other by the pair of stridulatory pick rows in males, barely projecting in C. octomaculatum but prominent in C. floridanum. In C. octomaculatum the copulatory ducts are narrower, looped posterolaterally (looped anteromedially in C. floridanum), and spermathecae larger. Males have a concave margin to the distal side of the embolus base (convex in C. floridanum).
Description
Intensity and area of the black parts of the colour pattern varies considerably. Carapace of typical New Zealand specimens straw-coloured, with blackish margin and a broad, blackish median band extending from behind the posterior row of eyes to the fovea, and more faintly to the posterior margin. Sternum yellow brown or grey with black margin. Abdomen has white blotching dorsomedially, sometimes extensive enough to form an irregular longitudinal stripe. A pair of broken black lines dorsolaterally, usually broken into four segments, with the anterior and posterior-most segments much longer than the middle segments. Lateral surfaces with white blotching above a band of black streaking. A blackish ring surrounds the spinnerets. Leg I and IV of some of the darker specimens has a distal black band on the tibia (, 2A). Legs 1-4-2-3. Cheliceral teeth minute or absent, spur present on distal end of prolateral margin of chelicera. Sternum longer than wide (, 2B). Female abdomen sub-oval. Male abdomen cylindrical and not constricted. Male abdominal ventral shield with slight lip on the posterior edge. Stridulatory pick rows comprised of four or five picks, each with a single spine, that match to grooved areas on the posterior cephalothorax (pars stridens). In the male left palp (), the embolus spiral originates retrolaterally about halfway up the bulb and circles clockwise around the bulb. Distal margin of embolus base concave. Theridiid tegular apophysis (sensu Agnarsson Citation2004) is conspicuous, black and tapers to a point. Female palp with slender, curved claw, without teeth. Epigynum () lightly sclerotised, atrium kidney-shaped, about twice as wide as long. Copulatory ducts open on the posterior side of the atrium, sclerotised and are visible through the anterior wall of the epigastric furrow. From the opening, the ducts run medially and parallel, then spread anteriolaterally in a loop to the large, ovoid spermathecae. Fertilisation ducts originate near the base of the spermathecae and converge at the midline ().
Measurements
Male
Mean total length: 1.412 (n = 15). Size range: 1.275–1.575.
Male, Hongiora, Aldermen Islands, CL, 22 Feb. 2002 (AS.004458). Total length: 1.500; carapace length/width: 0.634/0.634; sternum length/width: 0.341/0.329; labium length/width: 0.07/0.122; eye diameters and interdistances: AME 0.039, ALE 0.029, PME 0.029, PLE 0.039; AME–AME 0.039, AME–ALE 0.029, PME–PME 0.039, PME–PLE 0.039, ALE–PLE 0.002. See for leg measurements.
Table 1. Lengths of leg and palp segments (mm). Tarsus measurement for male palp is the length of the cymbium.
Female
Mean total length: 1.54 (n = 34). Size range: 1.225–1.925.
Female, Bream Head, ND, 18 Oct. 2001 (AS.004432). Total length: 1.550; carapace length/width: 0.578/0.486; sternum length/width: 0.395/0.365; labium length/width: 0.061/0.122; eye diameters and interdistances: AME 0.033, ALE 0.033, PME 0.029, PLE 0.029; AME–AME 0.039, AME–ALE 0.024, PME–PME 0.039, PME–PLE 0.039, ALE–PLE 0.002. See for leg measurements.
Remarks
The colour pattern is variable within this species. The illustrations of Bösenberg & Strand (Citation1906: pl. 3, fig. 14) and Barrion & Litsinger (Citation1995: fig. 253) show two longitudinal rows of four black spots, while Ono's depictions (Citation2011: figs 62, 65) show no black markings at all. This contrasts with the comparatively elongate shape of these markings in New Zealand specimens.
Material examined
Coleosoma octomaculatum
Northland: Taumataroa Flat, Te Paki, (34° 27.60° S, 172° 58.49′ E), O.J.-P. Ball, 14 Dec. 2006, 1♀, (AS.004442); Kerr Point Pines, Te Paki, (34° 27.73′ S, 172° 52.96′ E), O.J.-P. Ball, 13 Oct. 2006, 1♂, 1♀, (AS.004440); 12 Feb. 2007, 1♂, 1♀, (AS.004441); Spirit's Bay, Te Paki (pine site A), (34° 28.66′ S, 172° 52.72′ E), O.J.-P. Ball, 14 Dec. 2006, 1♂, 1♀, (AS.004437); 12 Feb. 2007, 1♀, (AS.004438); 13 Nov. 2006, 1♂, 1♀, (AS.004439); 12 Jan. 2007, 2♂, 1♀, (AS.004459–4461); Bream Head Scenic Reserve, (35° 51.00′ S, 174° 32.00′ E), B.M. Fitzgerald, 18 Oct. 2001, 1♀, (AS.004432); Hen I., (35° 54.60′ S, 174° 43.80′ E), B.M. Fitzgerald, 21 Oct. 2001, 1♂, (AS.004431); 24 Oct. 2001, 1♀, (AS.004434); 03 Dec. 2004, 7♀, 1 juv., (AS.004435); 06 Dec. 2004, 1♀ (plus egg sac), (AS.004436); Coromandel: Cuvier I., West Ridge track, (36° 26.05′ S, 175° 46.30′ E), B.M. Fitzgerald, 15 Dec. 1996, 2 juv., (AS.004444); Double I. (Western side), Mercury Is, (36° 37.20′ S, 175° 53.40′ E), B.M. Fitzgerald, 01 Mar. 2000, 4♀ (and 3 egg sacs) (AS.004433, AS.004447–4449); Korapuki I., Mercury Is, (36° 39.62′ S, 175° 50.92′ E), B.M. Fitzgerald, 29 Nov. 2000, 1♂, (AS.004443); Hongiora, Aldermen Is, (36° 57.00′ S, 176° 2.40′ E), B.M. Fitzgerald, 22 Feb. 2002, 1♂, 2♀ (plus egg sac and spiderlings) (AS.004456–4458); Middle Chain I., Aldermen Is, (36° 57.00′ S, 176° 3.60′ E), B.M. Fitzgerald, 05 Nov. 2002, 2♂, 1♀, (AS.004455); Ruamahuanui, Aldermen Is, (36° 57.00′ S, 176° 5.40′ E), B.M. Fitzgerald, 19 Nov. 2003, 2♀, (AS.004454); Ruamahuaiti, Aldermen Is, (36° 58.20′ S, 176° 3.60′ E), B.M. Fitzgerald, 08 Nov. 2002, 1♂, (AS.004453); Tuhua (= Mayor I.), (37° 16.80′ S, 176° 15.00′ E), B.M. Fitzgerald, 26 Mar. 2004, 3♀ 1 juv. (plus egg sac), (AS.004445–4456); Bay of Plenty: Moutohora (= Whale I.), (37° 51.00′ S, 176° 58.00′ E), B.M. Fitzgerald, 29 Mar. 2005, 1 juv., (AS.004450); 04 Feb. 1999, 6♂, 7♀, (AS.004451); 1♀ (plus egg sac), (AS.004452); Mackenzie: Tasman River [TA 013/8708, TA 021/3608], (43° 46.70′ S, 170° 8.26′ E), S. Anderson, 06 Dec. 2005, 3♂, (AS. 004462–004463).
Coleosoma floridanum
IDC ref. C13-0754 in packaging, country of origin: China, 8 Aug. 2013, MPI border quarantine 1♂.
Biology
In New Zealand, C. octomaculatum has been collected in pitfall traps, from twiggy litter and by beating low, scrubby vegetation and ferns. It has a preference for open, semi-modified habitat. At Te Paki, O.J.-P. Ball collected both sexes of this species in pitfall traps between October and February in young pine plantations and shrublands, but not in nearby native forest.
Several female specimens of C. octomaculatum collected by hand (between December and March) on the northern offshore islands carried an egg sac, attached to the spinnerets. This habit was also noted in Coleosoma floridanum (as Theridion aleipata) by Marples (Citation1955, p. 485) who commented that the egg sac, ‘is carried attached to the spinnerets and supported by one hind leg, an unusual habit in the Theridiidae’. Similar observations were made by Knoflach (Citation1999) for Coleosoma and the related genus Neottiura.
Egg sacs contained 7–19 eggs or hatchlings (n = 8, mean 11.4). For C. floridanum, Cutler (Citation1972) reported that eight egg sacs from females held in captivity had 3–13 eggs (mean 9.5 eggs per sac) and Knoflach (Citation1999) reported 10–12 eggs per egg sac (n = 5). Marples (Citation1955) reported three egg sacs with 26, 13 and nine eggs from females collected in vegetation and dead leaves.
Distribution and establishment in New Zealand
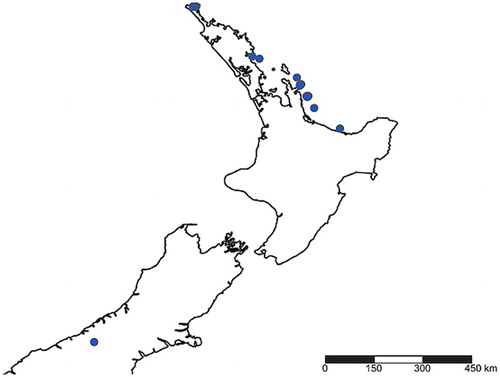
Coleosoma octomaculatum has previously been recorded from China, Japan, Korea and Taiwan (World Spider Catalog Citation2015) and, as far as we are aware, its presence in New Zealand since at least 1996 represents the first reported establishment of this species outside of its known range. In New Zealand records are disjunct (see ), with a single South Island locality and several records from the northern third of the North Island including uninhabited offshore islands that are infrequently visited (see ‘Material examined’). While the absence of records from countries between New Zealand and the South East Asian subregion does not prove the species is absent there, if we assume this is an accurate representation of the current distribution, it seems plausible that this species has arrived by anthropogenic dispersal. Subsequent dispersal via ballooning from the North Island could explain its presence on islands that are only rarely visited, but we cannot rule out anthropogenic dispersal as a possibility.
Potential species groupings within Coleosoma
Bryant (Citation1944, p. 55) observed that C. floridanum can be distinguished from other members of the genus ‘as the embolus completes about half a circle instead of almost a complete circle’, and Levi (Citation1959) noted that in C. floridanum the epigynum and internal genitalia are quite distinct from those of other members of the genus. We observe that most species of Coleosoma can be divided into two groups based on the form of these three characters as follows:
‘floridanum' group: Coleosoma floridanum, C. octomaculatum. In this group, the epigynum is more strongly sclerotised with a distinct vulva. The copulatory ducts are short while the spermathecae are large. The embolus spiral forms a half circle.
‘blandum' group: Coleosoma blandum, C. acutiventer, C. normale, C. caliothripsum, C. pseudoblandum. In these five species the epigynum is weakly sclerotised with indistinct openings and the copulatory ducts are long, slender and convoluted while the spermathecae are small. The embolus spiral almost completes a full circle in C. acutiventer, C. normale and C. blandum (males are unknown for C. caliothripsum and C. pseudoblandum).
Bryant (Citation1944) did not illustrate C. blandum or C. octomaculatum, but later figures (e.g. Yoshida Citation2009) show that the embolus spiral of C. blandum forms ‘almost a complete circle’ and of C. octomaculatum a ‘half circle’. The three remaining species of Coleosoma (C. africanum, C. matinikum, C. pabilogum) are known only from males and the illustrations of their palps are insufficiently clear to align them with either group.
We suggest that additional morphological and molecular study is needed to corroborate these prospective groupings.
Acknowledgements
We thank Olly Ball (NorthTec, Whangarei) for the series of specimens from Te Paki, Carol Muir (MPI, Christchurch) for lending specimens of C. floridanum, Sue Anderson for providing several specimens from the Department of Conservation (Twizel)—Project River Recovery, and Jean-Claude Stahl (Museum of New Zealand Te Papa Tongarewa, Wellington) for –. We also thank the two anonymous reviewers for their constructive comments that helped improve this manuscript.
Associate Editor: Dr Cor Vink.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Agnarsson I. 2004. Morphological phylogeny of cobweb spiders and their relatives (Araneae, Araneoidea, Theridiidae). Zool J Linn Soc. 141:447–626. doi: 10.1111/j.1096-3642.2004.00120.x
- Barrion AT, Litsinger JA. 1995. Riceland spiders of South and Southeast Asia. Wallingford, UK: CAB International.
- Bösenberg W, Strand E. 1906. Japanische Spinnen. Abh Senckenb Naturforsch Ges. 30:93–422.
- Bryant EB. 1940. Cuban spiders in the Museum of Comparative Zoology. Bull Mus Comp Zool. 86:249–532 + 22 pl.
- Bryant EB. 1944. Three species of Coleosoma from Florida (Araneae; Theridiidae). Psyche: A J Entomol. 51:51–58. doi: 10.1155/1944/42363
- Crosby TK, Dugdale JS, Watt JC. 1998. Area codes for recording specimen localities in the New Zealand subregion. New Zeal J Zool. 25:175–183. doi: 10.1080/03014223.1998.9518148
- Cutler B. 1972. Notes on the behavior of Coleosoma floridanum Banks. J Kansas Entomol Soc. 45:275–281.
- Knoflach B. 1999. The comb-footed genera Neottiura and Coleosoma in Europe (Araneae, Theridiidae). Bulletin de la Société entomologique suisse. 72:341–371.
- Levi HW. 1959. The spider genus Coleosoma (Araneae, Theridiidae). Breviora. 110:1–11.
- Levi HW. 1967. Cosmopolitan and pantropical species of theridiid spiders (Araneae: Theridiidae). Pacific Insects. 9:175–186.
- Levi HW, Levi LR. 1962. The genera of the spider family Theridiidae. Bull Mus Comp Zool. 127:3–71.
- Marples BJ. 1955. Spiders from Western Samoa. J Linn Soc-Lond, Zool. 42:453–504. doi: 10.1111/j.1096-3642.1955.tb02217.x
- Ono H. 2011. Spiders (Arachnida, Araneae) of the Ogasawara Islands, Japan. Mem Nat Museum Nat Sci Tokyo. 47:435–470.
- Paquin P, Dupérré N, Labelle S. 2008. Introduced spiders (Arachnida: Araneae) in an artificial ecosystem in eastern Canada. Entomol News. 119:217–226. doi: 10.3157/0013-872X(2008)119[217:ISAAIA]2.0.CO;2
- Pfliegler WP. 2014. Records of some rare and interesting spider (Araneae) species from anthropogenic habitats in Debrecen, Hungary. e-Acta Naturalia Pannonica. 7:143–156.
- Pickard-Cambridge O. 1882. New Araneidea. P Zool Soc Lond. 1882:428–442.
- Shorthouse, David P. 2010. SimpleMappr, an online tool to produce publication-quality point maps [Internet]. [cited 2015 Oct 5]. Available from: http://www.simplemappr.net
- Simon E. 1894. Histoire Naturelle des Araignées, seconde edition. Tome 1, Fascicule 3. Librairie encyclopédique de Roret, Paris; p. 489–760.
- Spoczynska JOI. 1969. A theridiid spider new to Britain established at Kew. Proc Trans Br Entomol Nat Hist Soc. 2:1–4.
- Srinivasulu C, Srinivasulu B, Javed SMM, Seetharamaraju M, Jyothi SA, Srinivasulu CA, Tampal F. 2013. Additions to the araneofauna of Andhra Pradesh, India—Part II. Records of interesting species of the comb-footed genera Latrodectus, Rhomphaea and Coleosoma (Araneae: Theridiidae). J Threat Taxa. 5:4483–4491. doi: 10.11609/JoTT.o2660.4483-91
- World Spider Catalog. 2015. World Spider Catalog: NMBE. Version 16 [Internet]. Bern, Switzerland: Natural History Museum of Bern; [cited 2015 June 24]. Available from: http://wsc.nmbe.ch
- Yoshida H. 1982. Spiders from Taiwan III. Three species of the genera Coleosome [sic] and Molione (Araneae: Theridiidae). Proc Jpn Soc Syst Zool. 24:37–40.
- Yoshida H. 2009. Uloboridae, Theridiidae, Ctenidae. In: Ono H, editor. The spiders of Japan with keys to the families and genera and illustrations of the species. Kanagawa: Tokai University Press; p. 142–147, 356–393, 467–468.
- Zhu MS, Zhang YQ. 1992. Notes of some species of Theridiidae in Guangxi (Arachnida: Araneae). J Guangxi Agr Coll. 11(1):20–29.