ABSTRACT
Natural habitat is not always available for translocations due to habitat modification or pressure from introduced predators. We followed the release of 30 captive-bred Otago skinks (Oligosoma otagense) into a 109 m2 outdoor enclosure of artificial habitat at Orokonui Ecosanctuary in southeastern New Zealand. We evaluated the short-term success of the translocation by assessing visibility and survival of the skinks over the 3 summer months following release in spring 2013, plus survival the following spring. Skinks were visible most days, especially in warm, dry conditions. Survival was high over the first summer; 80% of skinks were seen at least once during February 2014. The following spring 63% of skinks were resighted during three surveys and breeding has since been reported. Thus, it is feasible to maintain captive-bred adults of this Nationally Endangered skink in artificially constructed, outdoor habitat in a coastal location. However, to avoid predation of juveniles by adult skinks, future translocations should avoid releasing juveniles close to adults.
Introduction
The Otago skink (Oligosoma otagense) is New Zealand's largest species of skink and is classified as Nationally Endangered under national threat classification criteria (Hitchmough et al. Citation2013). The remaining wild population is restricted to the Otago region of southern South Island and is estimated to be just over 2000 individuals (Chapple Citation2010). Most individuals occur at Macraes Flat in eastern Otago, with a small population near Lake Hāwea (Norbury et al. Citation2007), and a recently established population of captive-bred skinks inside a mammal-proof enclosure containing natural habitat near Alexandra (Hare et al. Citation2012; Norbury et al. Citation2014).
Otago skinks are diurnal and their emergence is strongly weather-dependent (Coddington & Cree Citation1997). They inhabit schist rock outcrops (Coddington & Cree Citation1997) and can travel reasonably long distances across grassland and tussock between outcrops (Germano Citation2007). Their diet consists mainly of fleshy fruit, small-bodied Coleoptera and large-bodied Diptera (Calliphoridae) (Tocher Citation2003). The current low population density, combined with late maturation (4–6 years) and low annual reproductive output (1.41–2.34 offspring per female per year), limits population growth and makes them vulnerable to decline (Cree Citation1994; Tocher Citation2009). The decline in Otago skink abundance has been attributed mainly to mammalian predators and conversion of tussock to pasture (Whitaker Citation1996; Tocher Citation2003; Norbury et al. Citation2007). The remains of Otago skinks have been found in the stomachs of feral cats (Middlemiss Citation1995) and mice have been witnessed attacking adult Otago skinks, with a detrimental effect on survival (Norbury et al. Citation2014). Other New Zealand skink species are also preyed upon by ferrets, stoats and weasels (Miskelly Citation1997; Norbury Citation2001), magpies (McIlroy Citation1968) and hedgehogs (Jones et al. Citation2005). When a suite of mammalian predators are removed or suppressed in an area, populations of Otago skinks recover, with the greatest population growth in the centre of the predator-suppressed area, or inside a mammal-proof fence (Reardon et al. Citation2012). However, predator control in situ is expensive and may not be feasible throughout the range of the species; as such, translocations into predator-proof fences are being considered to ensure species security. Translocations of captive skinks into a predator-controlled area of natural habitat have shown promise (Norbury et al. Citation2014), but until this study no other translocation of captive-reared Otago skinks had occurred.
Otago skinks have been bred in captivity over several generations. Some differences from wild skinks (e.g. relatively high body mass and low sprint speed) have been noted (Connolly & Cree Citation2008). Captive populations that are accessible to the public provide an opportunity to raise community awareness and support for skink conservation (Norbury et al. Citation2007). Here, we assessed the short-term survival in the establishment phase (sensu IUCN/SSC Citation2013) of Otago skinks translocated into artificial habitat within the Orokonui Ecosanctuary, a more coastal location than the current geographic range of wild Otago skinks (Norbury et al. Citation2007). We investigated survival and visibility over the first 3 months after release, and then returned after the winter to get an index of the minimum number alive the following spring, 10–11 months after release. We hypothesised that survival over the summer months would be high, and that skinks would be visible most days, with more skinks visible when weather conditions are warm and dry as this is when Oligosoma skinks are most likely to emerge (Coddington & Cree Citation1997; Hoare et al. Citation2009).
Materials and methods
Orokonui Ecosanctuary in the southern South Island (45°46′S, 170°36′ E) contains 307 ha of remnant and regenerating forest, shrubland and exotic grassland. The ecosanctuary is surrounded by an 8.7 km mammal-resistant fence erected in 2007 and mammalian predators have since been almost entirely eradicated. Thirty Otago skinks from private collections or zoos in the North Island were released into an enclosure at the ecosanctuary on 20 November 2013 (late austral spring). This location is outside (at least 35 km east of) the known past or present range (Norbury et al. Citation2007). The enclosure is roughly oval, measuring 16.2 m × 9.8 m in maximum dimensions, with an area of approximately 109 m2. Habitat was created by ecosanctuary staff and volunteers using schist and other rock slabs to mimic rock tors used by Otago skinks in the wild elsewhere in Otago. Tussocks and native shrubs (e.g. Chionochloa rigida, Melicytus alpinus, at least three species of Coprosma, including C. propinqua and C. rotundifolia, and a species of Corokia) were planted amongst the rocks and rank grass to simulate the natural habitat (). Density of skinks in the enclosure (30 skinks in 109 m2 including eight rock tors) was chosen to reflect densities seen at areas with intensive predator control in the wild (K Ludwig, formerly Department of Conservation [DOC], pers. comm. 8 April 2015). Monitoring during the previous winter showed that temperatures of potential retreat sites at 200–650 mm depth within the rock piles remained above 1.5 °C, enabling skinks to avoid freezing (Sophie Penniket and Alison Cree, pers. obs.). The enclosure is surrounded by a low (85 cm) solid fence, thought to be escape-proof for the size of skinks released. The fence allows the skinks to be viewed (including by members of the public) without entering the enclosure.
Figure 1. Otago skink enclosure, showing the fence, schist rock piles, and some vegetation. Photograph taken on 12 October 2014 by Alison Cree.

Prior to release, all Otago skinks were photographed from both sides for future identification of individuals, focusing on the snout to foreleg region as this area has distinct markings that are stable over time (Reardon et al. Citation2012). Observations such as scars or tail loss were also recorded. Snout-vent length (SVL), vent-tail length (VTL) and tail regeneration length (R) were measured to the nearest 1 mm with a ruler. Adult males were sexed based on the swelling produced by hemipenes in the tail base and their generally wider heads. The reproductive condition of adult females was assessed as non-reproductive, vitellogenic (with detectable ovarian follicles undergoing yolking) or pregnant (with uterine conceptuses) by palpation of the abdomen (Cree Citation1994; Holmes & Cree Citation2006).
Skinks available for release were those deemed surplus to requirements in the captive breeding programme and do not necessarily reflect the optimal demographic for a translocation (K Ludwig, formerly DOC, pers. comm. 8 April 2015). The released skinks consisted of 12 adult males (102–142 mm SVL), 11 adult females (103–126 mm SVL), five subadult or small adult skinks whose sex was uncertain (92–108 mm SVL) and two juveniles (66–70 mm SVL). Of the adult females, four had detectable vitellogenic follicles and one was possibly pregnant; the remaining six were non-reproductive. Skinks were released by hand, individually over a 2 h period during a warm, sunny afternoon and were spaced evenly around the enclosure with approximately one skink every 3–4 m in order to minimise intraspecific interactions upon release and to assist each skink to find a suitable refuge. Many skinks disappeared under rocks immediately after release; however, within 30 min of the last release some skinks were out basking and foraging.
An iButton DS1921G data logger was placed 20 cm into a rock retreat to measure retreat temperature. Data loggers were also placed inside two copper models placed on rock surfaces in the sun to infer temperatures that might be achieved by basking. Only one that was in full sun all day was used in the analysis as the other was shaded by a small tree between 1000 and 1100 h for 2 months of the study. The copper models were of a size (110 mm long × 24 mm wide × 11 mm high) and colour (brown) that simulated smaller lizards including Oligosoma maccanni (McCann's skink) and Woodworthia ‘Otago/Southland' (Otago/Southland gecko). Such models have been validated to replicate body temperatures of these smaller species when placed alongside the models (Hare et al. Citation2009; Penniket & Cree Citation2015). The copper models may not exactly simulate the body temperatures of basking Otago skinks (which are larger in size and black-and-gold in colour, differences that could influence heating rates, though in opposite directions; e.g. Stevenson Citation1985; Clusella Trullas et al. Citation2007). However, effects of size and colour on temperatures of copper models that simulate basking lizards are typically small, and unlikely to be more than a few °C at times when skinks choose to bask (Shine & Kearney Citation2001).
Twenty observations were made over 17 days between 20 November 2013 and 28 February 2014. Human observations were made for 60 min. The observer (MB) would slowly approach the fence and attempt to photograph the area including the head and front legs of every skink that was visible. The location and any interactions with other skinks were also recorded. If no skinks were sighted within the first 20 min, the observer would record no skinks for that day. The presence of the observer did not appear to cause the skinks to alter their behaviour, and due to the public position of the skink enclosure there were often people talking and pointing beside the fence. The skinks received supplementary food of 100 crickets a week from ecosanctuary staff, which they were seen eating, and could also feed on invertebrates and small lizards (presumed to be common skinks, Oligosoma polychroma, and Otago/Southland geckos) that were present within the enclosure.
Time-lapse photography was used to gather more observational data on skink activity and interactions. A UOVision UV565 time-lapse camera was set up on the northwestern perimeter at a height of 155 cm looking SSE and set to photograph every 10 min between 0600 h and 2100 h as Otago skinks are diurnal (Norbury et al. Citation2007).
Generalised additive models (GAMs) were used to test for a relationship between environmental factors and skink detection by an observer. The statistical program R (v2.15.3; The R Foundation for Statistical Computing 2013) was used to construct the GAMs using the packages ‘gam’ and ‘bbmle’. For copper model and retreat temperature, where the relationship between model parameters and skink counts was non-linear, a smoothing function was used.
The global starting models specified that skink detection was an additive function of {skinks ∼ hour + moisture + TCM + Tretreat}, where hour was the starting time of the observation, moisture was scored as 0 = no moisture, 1 = airborne moisture or low cloud, or 2 = precipitation, TCM was the temperature inside the copper model skink with a smoothing function, and Tretreat was the temperature in the retreat at the start of the observation. This model was compared with eight less complicated models that included different additive combinations of the parameters from the global model, and a null model of {skinks∼1}. Akaike's Information Criterion adjusted for small sample sizes (AICc) was used to assess relative model fit. The difference between the score of the top model and the scores of the other models (ΔAIC) was used to select the model(s) that best estimated the number of skinks detected. All models with ΔAICc < 2 were considered to have substantial support, models with ΔAIC < 4 were considered to have moderate support, and models with ΔAIC > 10 were essentially not supported (Burnham & Anderson Citation2002).
Three additional days of observations were made the following spring (between 20 September and 16 October 2014) to get an index of minimum survival over winter. All observations were made on days when the rocks had been warmed by the sun.
Results
Survival over the first 3 months was high, with 24 of the 30 skinks originally released sighted at least once after 1 February 2014. Of the two juvenile skinks, one was seen and photographed being consumed by an adult skink within 2 h of release (with only its tail visible); the other has not been seen since the release date. The other recorded death was of an adult male found on an exposed rock 1.5 months after the release (Elton Smith, Orokonui Ecosanctuary, pers. comm. 6 January 2014); the cause of death was not determined. Another adult male skink and a skink of uncertain sex were not seen after 13 December and 23 December 2013, respectively. Six skinks lost parts of their tails; one adult female that lost a large portion of its tail within the first month of release was not seen again after 31 January 2014. The cause(s) of tail loss were not determined. Two confrontations with physical contact between skinks (both between a male and another unidentified skink) were recorded during surveys on 29 January 2014 and 6 February 2014 in the late morning and at midday, respectively. The confrontations were very fast, lasting only a few seconds. A male skink was also seen chasing another skink off a rock without any physical contact mid-morning on 13 December 2013. The observer witnessed four peaceful male–female interactions, and two pairs of male skinks in close proximity, but not interacting.
The time-lapse camera detected 43 occasions where skinks were interacting or in close proximity. Most of the interactions appeared peaceful, with skinks basking together and, on some occasions, touching. Where photo identification was possible, all interactions were between one individual female and one of three known males.
Between 20 September and 16 October 2014, 19 skinks were sighted during three surveys and are therefore known to have survived the winter (eight females, six males and five of uncertain sex).
Skinks were visible for all hours that the ecosanctuary was open. Although the number of skinks detected appeared to decrease after 1300 h (A), this relationship was not supported statistically (AIC weight of the highest ranking model containing ‘hour’ < 0.001; ). The time-lapse footage showed that, during the summer months, skinks emerged between 0730 and 2030 h. Skinks were only visible when there was no precipitation (B). We found a weak positive relationship between the number of skinks detected and the temperature inside the copper model of a skink, although on the two warmest observation days (model temperature 37.5–41.0 °C) few skinks were visible (C). No relationship was detected between retreat temperature and skink visibility (D; ).
Figure 2. Number of skinks detected over an hour of visual observations. A, At different times of the day during the hours that Orokonui Ecosanctuary was open; B, in relation to precipitation (Dry = no precipitation, 1 = light precipitation from low cloud, 2 = rain); C, in relation to temperature measured using a copper model of a skink placed in full sunlight (n = 20 observations; fitted line from the generalised additive model is shown); D, in response to the temperature measured in shade under a rock 20 cm into a retreat (n = 20 observations).
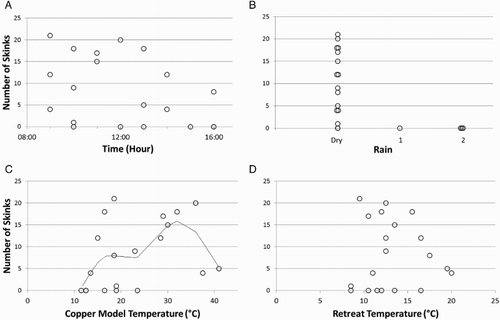
Table 1. Candidate generalised additive models of Otago skink (Oligosoma otagense) counts at Orokonui Ecosanctuary, December 2013–February 2014.
The highest ranking model was {skinks∼rain} which had 45% support as indicated by the model weight. The second- and third-highest ranking models were {skinks∼TCM + rain} and {skinks∼TCM}, with 28% and 17% support, respectively (). Thus, the main factors that favoured skink visibility were the absence of rain and the temperature of the copper model (up to a probable maximum of about 36 °C). The null model also received 8% support.
Discussion
After 3 months, 80% of all skinks released (and 86% excluding juveniles) were resighted over three observations in February 2014; however, after winter only 63% (68% excluding juveniles) were resighted over 3 days in September–October 2014 (10–11 months after release). Due to the limited number of observation days this can only be used as an index of the minimum number surviving; skinks may have been present, but not basking, at the times observations were made. Also, areas in the centre of the enclosure were not visible from the outside of the enclosure.
The minimum survival rate found in our study (68% of subadults and adults after 11 months) was low compared to those for established populations inside mammal-proof fences and with intensive predator control. Reardon et al. (Citation2012) compared survival rates in wild populations under three treatments and found that annual survival was between 0.83 and 0.94. Norbury et al. (Citation2014) found that survival of a captive-reared translated cohort over 3 years was 0.83 per annum. First year survival in our study was slightly lower than that in a translocation of Otago skinks at Alexandra (Hare et al. Citation2012; note that these results pertain to the first year of the same study as Norbury et al. Citation2014), in which 75% were still alive after 12 months. However, in the study by Hare et al. (Citation2012) more observations were made, and only after 15 consecutive non-sightings was it assumed that skinks were unlikely to have survived. Due to the very low number of monitoring days 10–11 months after translocation our results give only a minimum estimate of survival. Factors that could have influenced survival are that the naïve captive-bred Otago skinks may not have been familiar with the locations of more optimal refugia and may have fallen victim to the cold during winter, to predation by magpies or to injuries from other skinks competing for the best refugia. When naïve skinks were introduced to an enclosure with mice present their survival was much lower than that of established skinks (Norbury et al. Citation2014). Further studies are needed to assess ongoing survival, reproduction and density in this and other enclosures.
At Orokonui, one juvenile skink was witnessed being eaten by a larger skink on the day of the release, and the other juvenile skink was not seen after the release. Cannibalism is not uncommon among species of lizards (Mitchell Citation1986), so the incident observed here is not entirely surprising. The captive-reared juveniles may also have been relatively unwary and/or slow to run compared with wild juveniles (Connolly & Cree Citation2008). To avoid cannibalism of juvenile skinks in future translocations, juveniles should not be released in the immediate vicinity of adult skinks. Five skinks also lost some of their tails; however, it is not clear whether tail loss was due to interspecific predation attempts or intraspecific interactions. Tail loss can occur even in the absence of any predators. Fighting and tail loss were common in 13 Otago skinks released into a large, fully-enclosed outdoor cage (c. 8 × 3 m; G Norbury, Landcare Research, pers. comm. 10 July 2015). Magpies, which are known predators of lizards (Whitaker Citation1972), were also seen around the skink enclosure in our study; however, no predation attempts were witnessed.
Rain and temperature inside a copper model skink were the main predictors of skink detectability during surveys. No skinks were detected on days with precipitation, and skink sightings increased with copper model temperature up to a maximum of 36 °C, beyond which there was presumably no need to emerge for thermoregulation. The weather variables identified in the model are similar to those shown by Coddington & Cree (Citation1997) to influence the number of Oligosoma skinks visible in natural habitat at Macraes Flat and by Hoare et al. (Citation2009) to influence detections of Oligosoma polychroma beneath artificial retreats in the Eglinton Valley, Fiordland.
The temperature measured in the copper models simulated (at least in relative terms) the potential body temperature achievable by basking. In our study at Orokonui Ecosanctuary, detections of Otago skinks increased as copper model temperature increased up until 36 °C, but appeared to decrease when temperatures reached or exceeded 37.5 °C (C). At Macraes Flat, wild Otago skinks have been observed basking, but with body temperatures not exceeding about 30 °C, even when pregnant (Wilson Citation1998). We suspect that the copper models used here overestimated the body temperatures chosen by Otago skinks on the warmest days; however, the relationship between TCM and skink sightings, with more skinks observed at intermediate temperatures, is likely to still be valid. Otago skinks are likely to seek shade or retreat sites to avoid body temperatures above about 31–32 °C (as do the congeneric McCann's skinks in the wild and on a thermal gradient; Hare et al. Citation2009). At high temperatures the skinks may take less time to reach their optimum temperature by basking and they can avoid over-heating by using their retreats more frequently.
One limitation to this study was the small selection of weather variables; wind has also been found to influence skink emergence, with fewer Otago skinks seen on windy days (Coddington & Cree Citation1997; see also Hare et al. Citation2009 for McCann's skinks). Observations were also limited to the ecosanctuary opening hours, and to the summer months. During the winter months skinks may be less visible, and less effective for advocacy purposes, but this was not assessed in the present study.
The skinks used in this study were captive-bred and reared and did not appear to respond to the presence of people, allowing the observer the ability to get close and manoeuvre to get a clear photograph. Wild skinks are more sensitive to approach by humans with an average distance of 2 m before an observer elicits a startle response (Roughton Citation2005). There are also phenotypic differences between captive and wild skinks, with captive skinks having a heavier body weight and slower sprint speed (Connolly & Cree Citation2008). These traits may affect visibility, though it is not certain whether they affect survival immediately after release (Hare et al. Citation2012).
No evidence of mating or births was observed during our study, which is not unexpected given that mating is thought to occur in autumn (Coddington & Cree Citation1997; Germano Citation2007) and, with one possible exception, none of the adult females were pregnant at release. However, 12 neonates were observed in the enclosure during February–March 2015, following the completion of our study (Elton Smith, Orokonui Ecosanctuary, pers. comm. 4 July 2015), indicating that the founders bred successfully within 15 months of translocation. The high visibility of Otago skinks over the summer months after their release at Orokonui Ecosanctuary should be beneficial to raising community awareness (Towns & Williams Citation1993; Hoban & Vernesi Citation2012). Future studies should look into the long-term survival and reproductive output of captive-raised Otago skinks after release in order to ensure that the translocated populations are self-sustaining. Future releases of skinks should take care to release skinks of similar size to avoid adults cannibalising juvenile skinks after the release.
Acknowledgements
We thank the Otago Natural History Trust for allowing us to conduct this study, which was conducted with the assistance of Karin Ludwig of the Department of Conservation and the approval of the University of Otago Animal Ethics Committee. Our thanks to Elton Smith and other staff of Orokonui Ecosanctuary for their help and advice with setting up and positioning the camera, and for additional observations. We also thank Sophie Penniket for assistance with temperature recording and camera equipment. We thank Grant Norbury and two anonymous reviewers for feedback that improved this paper.
Associate Editor: Dr Grant Norbury.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information-theoretic approach. New York: Springer.
- Chapple DG. 2010. Oligosoma otagense. In: IUCN 2013. IUCN Red List of Threatened Species. Version 2013.2. [cited 2014 Mar 30]. Available from: http://www.iucnredlist.org/details/15260/0
- Clusella Trullas S, van Wyk JH, Spotila JR. 2007. Thermal melanism in ectotherms. J Therm Biol. 32(5):235–245. doi: 10.1016/j.jtherbio.2007.01.013
- Coddington EJ, Cree A. 1997. Population numbers, response to weather, movements and management of the threatened New Zealand skinks Oligosoma grande and O. otagense in tussock grassland. Pac Conserv Biol. 3(4):379–391. doi: 10.1071/PC980379
- Connolly JD, Cree A. 2008. Risks of a late start to captive management for conservation: phenotypic differences between wild and captive individuals of a viviparous endangered skink (Oligosoma otagense). Biol Conserv. 141(5):1283–1292. doi: 10.1016/j.biocon.2008.02.026
- Cree A. 1994. Low annual reproductive output in female reptiles from New Zealand. New Zeal J Zool. 21:351–372. doi: 10.1080/03014223.1994.9518005
- Germano JM. 2007. Movements, home ranges, and capture effect of the endangered Otago skink (Oligosoma otagense). J Herpetol. 41(2):179–186. doi: 10.1670/0022-1511(2007)41[179:MHRACE]2.0.CO;2
- Hare JR, Holmes KM, Wilson JL, Cree A. 2009. Modelling exposure to selected temperature during pregnancy: the limitations of squamate viviparity in a cool-climate environment. Biol J Linn Soc. 96(3):541–552. doi: 10.1111/j.1095-8312.2008.01151.x
- Hare KM, Norbury G, Judd LM, Cree A. 2012. Survival of captive-bred skinks following reintroduction to the wild is not explained by variation in speed or body condition index. New Zeal J Zool. 39(4):319–328. doi: 10.1080/03014223.2012.662160
- Hitchmough R, Anderson P, Barr B, Monks J, Lettink M, Reardon J, Tocher M, Whitaker T. 2013. Conservation status of New Zealand reptiles, 2012. New Zealand Threat Classification Series, 2. Wellington: Department of Conservation.
- Hoare JM, O'Donnell CF, Westbrooke I, Hodapp D, Lettink M. 2009. Optimising the sampling of skinks using artificial retreats based on weather conditions and time of day. Appl Herpetol. 6(4):379–390. doi: 10.1163/157075309X12531848432985
- Hoban S, Vernesi C. 2012. Challenges in global biodiversity conservation and solutions that cross sociology, politics, economics and ecology. Biol Letters. doi:10.1098/rsbl.2012.0596.
- Holmes KM, Cree A. 2006. Annual reproduction in females of a viviparous skink (Oligosoma maccanni) in a subalpine environment. J Herpetol. 40:141–151. doi: 10.1670/235-04.1
- IUCN/SSC. 2013. Guidelines for reintroductions and other conservation translocations. Version 1.0. Gland, Switzerland: IUCN Species Survival Commission.
- Jones C, Moss K, Sanders M. 2005. Diet of hedgehogs (Erinaceus europaeus) in the upper Waitaki Basin: implications for conservation. New Zeal J Ecol. 29(1):29–35.
- McIlroy JC. 1968. The biology of magpies (Gymnorhina spp.) in New Zealand [PhD thesis]. Christchurch: Lincoln College, University of Canterbury.
- Middlemiss A. 1995. Predation of lizards by feral house cats (Felis catus) and ferrets (Mustela furo) in the tussock grassland of Otago [MSc thesis]. Dunedin: University of Otago.
- Miskelly CM. 1997. Whitaker's skink Cyclodina whitakeri eaten by a weasel Mustela nivalis. Conservation Advisory Science Notes 146. Wellington: Department of Conservation.
- Mitchell JC. 1986. Cannibalism in reptiles: a worldwide review. Herpetological circular No. 15. Oxford: Society for the Study of Amphibians and Reptiles.
- Norbury G. 2001. Conserving dryland lizards by reducing predator-mediated apparent competition and direct competition with introduced rabbits. J Appl Ecol. 38(6):1350–1361. doi: 10.1046/j.0021-8901.2001.00685.x
- Norbury G, van den Munckhof M, Neitzel S, Hutcheon A, Reardon J, Ludwig K. 2014. Impacts of invasive house mice on post-release survival of translocated lizards. New Zeal J Ecol. 38(2):322–327.
- Norbury G, Reardon J, McKinlay B. 2007. Grand and Otago skink recovery plan 2006–2016. Working draft. Dunedin: Department of Conservation.
- Penniket S, Cree, A. 2015. Adherence to Bergmann's rule by lizards may depend on thermoregulatory mode: support from a nocturnal gecko. Oecologia. doi:10.1007/s00442-015-3239-0.
- Reardon JT, Whitmore N, Holmes KM, Judd LM, Hutcheon AD, Norbury G, Mackenzie DI. 2012. Predator control allows critically endangered lizards to recover on mainland New Zealand. New Zeal J Ecol. 36(2):141–150.
- Roughton CM. 2005. Assessment of methods to monitor Otago skink and grand skink populations. DOC Research & Development Series 211. Wellington: Department of Conservation.
- Shine R, Kearney M. 2001. Field studies of reptile thermoregulation: How well do physical models predict operative temperatures? Functional Ecol. 15(2):282–288. doi: 10.1046/j.1365-2435.2001.00510.x
- Stevenson RD. 1985. Body size and limits to the daily range of body temperature in terrestrial ectotherms. Am Naturalist. 125(1):102–117. doi: 10.1086/284330
- Tocher MD. 2003. The diet of grand skinks (Oligosoma grande) and Otago skinks (O. otagense) in Otago seral tussock grasslands. New Zeal J Zool. 30(3):243–257. doi: 10.1080/03014223.2003.9518342
- Tocher MD. 2009. Life history traits contribute to decline of critically endangered lizards at Macraes Flat, Otago. New Zeal J Ecol. 33(2):125–137.
- Towns DR, Williams M. 1993. Single species conservation in New Zealand: towards a redefined conceptual approach. J Roy Soc New Zeal. 23(2):61–78. doi: 10.1080/03036758.1993.10721218
- Whitaker AH. 1972. Lizard predators. Pepeke. 19:4–5.
- Whitaker AH. 1996. Impact of agricultural development on grand skink (Oligosoma grande)(Reptilia: Scincidae) populations at Macraes Flat, Otago, New Zealand. Science for Conservation 33. Wellington: Department of Conservation.
- Wilson J. 1998. The relationship between body temperature and gestation length in Otago lizard species [BSc Hons dissertation]. Dunedin: University of Otago.
