ABSTRACT
There is growing concern about mitigation-driven translocations that move animals from anthropogenic threats at donor sites because of their failure rate and lack of application of scientific principles and best practice. We reviewed all known lizard translocations in New Zealand between 1988 and 2013 and identified 85 translocations of 30 lizard taxa to 46 release sites. Most translocations (62%) were motivated by conservation goals for the species or the release site, and one-third were mitigation-driven translocations, typically motivated by habitat loss due to development. Mitigation-driven translocations began in 2003, and since that time have equalled the number of conservation-motivated translocations. Conservation-motivated translocations usually released lizards on islands without mammalian predators, whereas mitigation-driven translocations usually relocated lizards to mainland sites with introduced predators. Long-term monitoring has been sparse and often rudimentary. Eight lizard translocations have recorded population growth, including one mitigation-driven translocation that was into a fenced reserve. Research on commonly used management techniques to mitigate human-related impacts is recommended to establish whether these techniques benefit lizards in the long term.
Introduction
Translocation of wildlife, the deliberate movement of organisms from one site for release in another, is an important conservation tool (Seddon et al. Citation2012; IUCN Citation2013) with thousands of translocations undertaken worldwide (Seddon et al. Citation2005). Translocations of terrestrial fauna in New Zealand date back to the 1880s, but the development of translocation techniques have become more sophisticated since the 1960s (Saunders Citation1994). Birds have been the main focus of translocations in New Zealand, with approximately three times as many bird translocations compared with translocations of all other taxonomic groups combined (Sherley et al. Citation2010; Miskelly & Powlesland Citation2013). However, since the 1980s, translocations of native bats, frogs, tuatara, invertebrates and lizards have become more popular (Sherley et al. Citation2010).
New Zealand's extant lizard fauna consists of 42 gecko (Diplodactylidae) and 55 skink (Scincidae) taxa (Hitchmough et al. Citation2013). They are distributed across the main islands (North, South and Stewart Islands) as well as many offshore islands, and occupy a range of habitats from the coast to the alpine zone (Jewell Citation2011). They are generally long-lived, have low annual reproductive output and all species, except the egg-laying skink (Oligosoma suteri), give birth to live young (Cree Citation1994; Hoare et al. Citation2005; Lettink & Whitaker Citation2006). Habitat destruction and the introduction of mammals, especially rats, have caused the extinction of two species and the range contraction of many others (Towns & Daugherty Citation1994). Of the 97 extant taxa, 81 are classified as Threatened or At Risk by the New Zealand Threat Classification system (Hitchmough et al. Citation2013).
The first translocations of New Zealand lizards occurred in 1988 (Thomas & Whitaker Citation1994; Towns Citation1994). Two developments paved the way for lizard translocations: (1) introduced rats were identified as the main agent of lizard decline (Whitaker Citation1973, Citation1978); and (2) the technology to eradicate rats from islands was developed (Towns & Broome Citation2003). In 1988, Whitaker's skink (O. whitakeri) was translocated to Korapuki Island after Polynesian rats (Rattus exulans) were eradicated. The abundance of resident lizard species increased in all habitats and there was a gradual increase in the numbers of O. whitakeri (Towns Citation1994). Fiordland skink (O. acrinasum) was translocated to Hawea Island after Norway rats (R. norvegicus) were eradicated and they rapidly increased in number (Thomas & Whitaker Citation1994). These projects confirmed that lizard populations were vulnerable to rodent predation, but could recover when rodents were eliminated, and that conservation-motivated translocations of lizard species could be successful in the short term. The success of the Korapuki Island and Hawea Island translocations paved the way for more lizard translocations to offshore islands.
In 1994, the first mainland translocation was undertaken when jewelled geckos (Naultinus gemmeus) were translocated from a site on the Otago Peninsula to another site nearby (Shaw Citation1994). Lizard translocations to non-fenced mainland sites face more challenges than translocations to islands because it is not possible to eliminate all introduced mammals that are known to prey on lizards, including ship rats (R. rattus), Norway rats (R. norvegicus), Polynesian rats (R. exulans), mice (Mus musculus), ferrets (Mustela furo), stoats (Mustela erminea), weasels (Mustela nivalis), cats (Felis catus) and hedgehogs (Erinaceus europaeus) (Towns & Daugherty Citation1994). To date, there is only one example of a predator control regime at a non-fenced mainland site that has allowed resident lizard populations to recover: multi-species pest animal control in native tussock/schist rock outcrop habitat at Macraes Flat has resulted in the recovery of grand skink (O. grande) and Otago skink (O. otagense) populations within the core of an intensive mammal control area that was surrounded by a trapping buffer (Reardon et al. Citation2012).
Lizard translocations to mainland sites with predator-proof fences began in 2005. These sites offer greater protection for lizards than non-fenced mainland sites because introduced mammals are eradicated inside the fences. Between 1999 and 2009, 28 conservation areas in New Zealand covering 8396 ha had been enclosed by 113 km of pest-proof fence (Burns et al. Citation2012). However, in some instances, mice were not eradicated or reinvaded the site after initial elimination (Speedy et al. Citation2007; Burns et al. Citation2012) and in the absence of mammalian predators and competitors, mice populations inside pest-proof fences can increase rapidly (Goldwater et al. Citation2012). High densities of mice can be detrimental to lizard populations (Newman Citation1994; Lettink & Cree Citation2006; Hoare et al. Citation2007; Knox et al. Citation2012; Norbury et al. Citation2013; Norbury et al. Citation2014b). Nonetheless, a study at Zealandia Sanctuary, Wellington, has shown that ornate skinks (O. ornatum) can recover in the presence of mice when mice are controlled to low densities for several years (Romijn Citation2013).
The early lizard translocations were mainly to offshore islands and motivated by conservation considerations. Mitigation-driven translocations began in 2003 (Sherley et al. Citation2010), initiated in response to lizards that must be removed from harm's way (Germano et al. Citation2015). The threat of illegal collection of lizards for the black market motivated some mitigation-driven translocations (Knox & Monks Citation2014); but in most cases, the immediate threat has been associated with land-use change. Lizards are legally protected by the Wildlife Act 1953 and their habitat is protected by the Resource Management Act 1991 (RMA). Therefore, if adverse effects on lizards cannot be avoided when developments are undertaken (e.g. lizard habitat destroyed for road projects), mitigation may require lizards to be rescued and relocated (Anderson et al. Citation2012).
Reptiles are considered suitable candidates for conservation translocation programmes, but there is concern about high failure rates when used in mitigation-driven translocations (Germano & Bishop Citation2009; Sullivan et al. Citation2015). Germano et al. (Citation2015) called mitigation-driven translocations a ‘misguided conservation strategy’ due to their failure rate and lack of application of scientific principles and best practice. Here, available information is assembled to: (1) provide a summary of lizard translocations in New Zealand between 1988 and 2013; and (2) compare mitigation-driven and conservation-motivated translocations. We investigated the number of translocations, the number of translocated taxa, threat status of translocated taxa, whether lizards were wild or captive-bred, whether wild lizards were sourced from islands or the mainland, the number of founders, whether release sites were islands or mainland sites, the introduced mammalian predators present at the release site, and whether disease screening was undertaken. We also compared the monitoring effort and outcomes of translocations.
Definition of terms
We follow the International Union for Conservation of Nature (IUCN Citation2013) definitions, and use the term ‘translocation' to mean the movement of organisms from one area to another. Translocations can be classified as ‘conservation translocations' if the intention is to establish or reinforce a viable population, and due care has been taken to maximise the likelihood of this outcome (IUCN Citation2013). A conservation translocation is: (1) a ‘reintroduction' when the target species is released into an area that was once part of its range but from which it has been extirpated; (2) a ‘reinforcement' when the target species is released into an existing population of conspecifics, in order to increase population size and reduce the risks of genetic or demographic collapse due to stochastic events; or (3) a ‘conservation introduction' when the target species is released outside its range to achieve conservation goals (IUCN Citation2013). A translocation is considered an ‘irresponsible release' when a species is released into an area that is not a suitable habitat or where the agents of decline have not been suitably addressed (IUCN Citation2013) ().
Table 1. Classification of lizard translocations intended to establish viable populations of species within their known range. Based on IUCN classifications (IUCN Citation2013).
In addition, we identified four motivating factors for lizard translocations: (1) conservation; (2) mitigation; (3) research; and (4) advocacy. The primary focus of conservation-motivated translocations is to benefit the target species or the recipient site. In contrast, the stimulus for mitigation-driven translocations is removing lizards from anthropogenic threats at the donor site such as habitat destruction and illegal collection. Research-motivated translocations either move animals specifically for research purposes, or relocate animals that had been used in laboratory experiments to a site different from where they were collected. Advocacy-motivated translocations move animals to a site for the purpose of raising public awareness of lizards.
The main motivation (e.g. conservation or mitigation) for undertaking a translocation does not determine whether or not the translocation is classified as a conservation translocation. This is determined by whether the translocation conforms to the IUCN best-practice guidelines (IUCN Citation2013; see ) to maximise the chances of a successful outcome at the recipient site. In this paper, we refer to translocations that adhere to the IUCN guidelines as best-practice translocations.
Methods
We collated data on 85 translocations of New Zealand lizards between 1988 and 2013. Data were gathered from the Department of Conservation's (DOC) translocations database and associated unpublished translocation proposals, translocation reports and monitoring reports—usually required as part of the translocation process (Cromarty & Alderson Citation2013)—as well as unpublished theses and published articles. We sent subsets of data to key people associated with each translocation to identify the primary motivation for the translocation, verify the data, fill any information gaps and make other comments. In some cases they informed us of other translocations that were not recorded in the above sources, so these translocations were also included.
This review included translocations of native lizards to offshore islands, fenced and non-fenced mainland sites, but excluded moving animals into captivity. Only translocations that transferred 10 or more individuals were included in the data set because this removed translocations that were unlikely to be beneficial at a population level. If a transfer of animals was conducted over several years with a few animals being added each year, it was classified as one event. If, however, there were two transfers of one species to the same location with a gap of three or more years, they were classified as two events. At one site, translocations of a species were counted as separate reintroductions because there was significant distance between the two release sites and the project leader advised that the populations were unlikely to merge initially.
Due to the relatively small network of people undertaking lizard translocations and their willingness to share data, we are confident that we have data for all conservation-motivated translocations. However, we did not locate information about all mitigation-driven translocations, as data from the main practitioner were not always available; in particular, no information was available for any translocations undertaken from 2009 to 2013. Therefore, mitigation-driven translocations are under-reported in this paper.
A successful translocation, by definition, will eventually produce a viable self-sustaining population living in the wild (Griffith et al. Citation1989; Dodd & Siegel Citation1991; Wolf et al. Citation1996; Fischer & Lindenmayer Citation2000). However, New Zealand lizards are susceptible to predation by introduced mammalian predators, so management intervention in the form of predator control is typically required to allow populations to be self-sustaining. As many lizard species are long-lived and/or have low reproductive output it may be difficult to classify translocations as being successful until many decades after the initial release (Dodd & Siegel Citation1991; Towns Citation1999; Miller et al. Citation2014). To deal with this difficulty we have modified a system outlined in Miller et al. (Citation2014) to assign progressive success criteria for projects, and have outlined appropriate timeframes at which these criteria may be assessed. The time frames when stages are likely to be reached are probably conservative for many taxa, as they are based on expected outcomes for some of New Zealand's slowest reproducing lizard taxa (Towns Citation1999) (see ).
Table 2. Stages of success of lizard translocations in New Zealand.
Translocation projects were categorised into two groups (conservation-motivated and mitigation-driven) based on information contained in reports or gathered from key people associated with the translocation.
Data analysis
Fisher's exact tests were used to compare the following attributes of lizard translocations motivated by conservation and mitigation: the number of gecko and skink translocations; threat status of translocated taxa; use of wild and/or captive animals as founders; source of wild animals used as founders; release locations; presence of introduced mammalian predators at the release sites; the intended type of translocation (e.g. reintroduction or reinforcement); and whether disease screening was undertaken. Monitoring effort across different time periods (0–4, 5–9 and 10+ years post release) and overall monitoring results with respect to the two groups were also compared. All data were analysed in the statistical programme ‘R’ and significance was assumed at P < 0.05 (R Development Core Team Citation2014).
Results
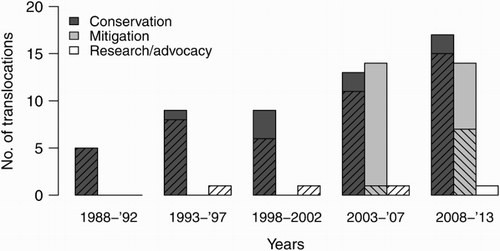
We reviewed 85 translocations that translocated 18 skink and 12 gecko taxa between1988 and 2013. A spreadsheet containing all lizard translocation data, and a reference list for translocations by taxa, are available in Table S1 and Dataset S1. Fifty-two projects translocated skinks and 33 projects translocated geckos. Most lizard translocations were motivated by conservation (62.4%) and mitigation (32.9%), while research (3.5%) and advocacy (1.2%) motivated only a small proportion of translocations. However, mitigation-driven translocations only began in 2003. Thereafter, the number of conservation-motivated and mitigation-driven translocations was similar (24 and 23, respectively) ().
Figure 1. Motivation for lizard translocations in New Zealand between 1988 and 2013 (n = 85). Hatching indicates the number of translocations that included some level of monitoring.
Of the eight lizard genera, Oligosoma skinks were translocated most often (61.2%) followed by the geckos Naultinus (14.1%), Hoplodactylus and Woodworthia (7.1% each), Dactylocnemis (5.9%) and Mokopirirakau (4.7%). There were no translocations of Toropuku and Tukutuku geckos (). summarises the taxa translocated.
Table 3. Translocations of New Zealand lizards between 1988 and 2013 summarised by genus.
Lizard taxa
The following results focus only on conservation-motivated and mitigation-driven translocations, therefore the following four research and advocacy translocations are excluded: N. gemmeus to Otago Peninsula, O. polychroma to Motueka, O. zelandicum to Awaiti Island and W. maculata to Whitireia Park.
There were 53 conservation-motivated translocations of nine gecko and 15 skink taxa: three Threatened (five translocations), 19 At Risk (37 translocations) and two Not Threatened (11 translocations) taxa. Six taxa (H. duvaucelii, O. alani, O. smithi, O. townsi, O. whitakeri and W. maculata) were translocated on three or more occasions, and together made up 52.3% of all conservation-motivated translocations. Most (92.5%) translocations used wild animals as founders (35 translocations were sourced from islands and 14 sourced from mainland sites) and seven of these translocations supplemented the wild animals with captive-bred animals. Only four translocations used entirely captive-bred animals. The most used captive-bred taxon was O. smithi (four translocations) followed by H. duvaucelii and N. punctatus (two translocations each) and M. ‘southern North Island’, O. otagense and O. suteri (one translocation each). The mean (± SD) number of founders used was 40.11 ± 19.84 (range 14–94, n = 53).
Of the 28 mitigation-driven translocations reviewed, six gecko and three skink taxa were translocated: eight At Risk (21 translocations) and one Not Threatened (seven translocations) taxa. While most translocations were due to impending habitat destruction, two translocations of N. gemmeus were undertaken to avoid them being illegally collected for export and trade (C. Knox, Ecogecko Consultants, pers. comm. 2014). Four species (N. gemmeus, O. aeneum, O. moco and O. ornatum) were translocated on three or more occasions and together made up 65.5% of all mitigation-motivated translocations. All mitigation-driven translocations used wild animals sourced from the mainland, and the mean (± SD) number of founders was 50.25 ± 52.26 (range 10–258, n = 28).
The lizards that were translocated in conservation-motivated and mitigation-driven translocations differed in two respects: (1) only conservation-motivated translocations used captive-bred animals; and (2) mitigation-driven translocations used only mainland lizards, whereas conservation-motivated translocations used a mix of mainland and island stock ().
Table 4. Attributes of lizard translocations motivated by conservation or mitigation.
Release sites
Conservation-motivated translocations utilised 23 release sites: 21 islands (51 translocations), one fenced mainland site (one translocation) and one non-fenced mainland site (one translocation). summarises the conservation-motivated release sites and the lizards translocated to them. Most sites (91%) were free of exotic predators when lizards were released. The exceptions were Matakohe/Limestone Island where mice were present at low density, and Macraes Flat where mammalian predators were controlled to levels that were known to allow the translocated species to recover (Reardon et al. Citation2012). Forty-six translocations were intended to be reintroductions and seven were intended to be reinforcements. However, one reintroduction was later found to be a reinforcement when a resident population of the translocated species was found (D. pacificus on Lady Alice Island). Another reintroduction was unintentionally a conservation introduction as the species was translocated outside its historic range. Oligosoma infrapunctatum were translocated to Mana Island, but genetic evidence later split this species complex so that the appropriate species to translocate would have been O. aff. infrapunctatum ‘southern North Island’ (L. Adams, DOC, pers. comm. 2014). Seven islands (Korapuki, Lady Alice, Mana, Matakohe/Limestone, Matiu/Somes, Motuora and Tiritiri Matangi) have received three or more translocations, and together have received 60.4% of all conservation-motivated translocations. Since 2005, 85.2% (n = 27) of translocations have undertaken disease screening for Cryptosporidia and Salmonella. All translocations to Crusoe Island, Matakohe/Limestone Island, Matiu/Somes Island, Motuihe Island, Motuora Island, Tiritiri Matangi Island and Mokomoko Dryland Sanctuary screened lizards before release.
Twenty-eight of the mitigation-driven translocations utilised 19 release sites: 15 non-fenced mainland sites (21 translocations), three fenced mainland sites (six translocations) and one island (one translocation). summarises the mitigation-driven release sites and the lizards translocated to them. Information on introduced predators was received for only 12 sites (63.2%): four sites had mice present (Orokonui Ecosanctuary, Tawharanui Regional Park, Zealandia Sanctuary and Quail Island) and eight sites had introduced mammalian predators present. The seven sites for which data were not received were mainland sites that were likely to have mammalian predators present. Although Shakespear Regional Park had predators present when two lizard translocations were undertaken, there were plans to erect a predator-proof fence, and this was completed several years after the translocations. Seven translocations were intended to be reintroductions, 11 translocations were reinforcements and no data were available for the other translocations. Two intended reintroductions were later found to be reinforcements when resident populations of the translocated species were found (M. granulatus and N. elegans at Tawharanui Regional Park). Disease screening for Salmonella was carried out for the translocation to Quail Island.
There were four significant differences between the release sites for conservation- and mitigation-motivated translocations: (1) conservation-motivated translocations were mainly to islands, whereas mitigation-driven translocations were mainly to mainland sites; (2) most conservation-motivated translocations were to sites with no introduced mammalian predators, whereas all mitigation-driven translocations were to sites that had some mammalian predators present; (3) most conservation-motivated translocations were introductions, whereas most mitigation-driven translocations were reinforcements; and (4) nearly half of all conservation-motivated translocations undertook disease screening of founders before they were released, whereas this was the case for only one mitigation-driven translocation ().
Monitoring and outcomes
Lizard monitoring data were collected for 84.9% (n = 45) of the conservation-motivated translocations (). The majority (95.2%, n = 21) of translocations monitored between 0–4 years after release located some founders, thus indicating initial establishment. Most translocations (88.9%, n = 9) monitored between 5–9 years after release confirmed that breeding had taken place. Two translocations resulted in an increase in the size of the translocated lizard population within 4 years of release (O. acrinasum on Hawea Island [Thomas & Whitaker Citation1994] and O. grande at Macraes Flat [Whitmore et al. Citation2011]). Long-term success (i.e. more animals located than released ≥10 years after release) was recorded in five of 15 (33%) monitored translocations, at only two release sites (O. alani, O. suteri and O. whitakeri on Korapuki Island [Towns & Ferreira Citation2001; Miller Citation2009] and H. duvaucelii and O. lineoocellatum on Mana Island [Bell & Herbert forthcoming]). Five other translocations confirmed that reproduction was occurring 10 years after release (O. oliveri on Korapuki Island; D. pacificus and O. macgregori on Lady Alice Island; O. alani on Motuopau Island; O. whitakeri on Stanley Island), but more time may be needed to determine whether populations are self-sustaining. The remaining five translocations appear to have failed with either no lizards located (D. ‘Matapia’ Motuopau Island; N. punctatus on Mana Island; O. alani on Red Mercury Island; O. whitakeri on Red Mercury Island) or only a few founders located (e.g. N. manukanus on Motuara Island) more than 10 years after release.
Table 5. Monitoring of lizard translocations (n = 54).
Monitoring data were collected for 32.1% (n = 9) of mitigation-driven translocations (). All eight translocations monitored between 0–4 years after release located some founders. One translocation in this period recorded an increase in the translocated population (N. gemmeus at Orokonui Ecosanctuary [Knox & Monks Citation2014]). Only one translocation was monitored between 5–9 years after release and it showed evidence of reproduction.
Only conservation-motivated translocations were monitored 10+ years after release (Fisher's exact test, P = 0.042, ). Usually fewer animals were located than were released for both conservation- and mitigation-motivated translocations (Fisher's exact test, P = 0.831, ).
Discussion
Monitoring and outcomes
As Miller et al. (Citation2014) found in their review of New Zealand herpetofaunal translocations, there was no overall difference in translocation outcomes between conservation-motivated and mitigation-driven translocations of New Zealand lizards. Significantly, no mitigation-driven translocations to non-fenced mainland sites have been monitored for 10 or more years after release, so it has yet to be determined whether they can produce viable self-sustaining populations. A third of conservation-motivated translocations to predator-free islands, where monitoring was carried out for 10 or more years post-release, were classified as self-sustaining. Although this may appear to be a low success rate, it is within the success range recorded for translocations of reptiles and amphibians (19%, Dodd & Siegel Citation1991; 41%, Germano & Bishop Citation2009) and other fauna (Griffith et al. Citation1989; Wolf et al. Citation1996; Fischer & Lindenmayer Citation2000; Miskelly & Powlesland Citation2013).
The reintroduction of O. whitakeri highlights the potential conservation benefits and uncertainties of translocations. This species was once widespread throughout the North Island, but by 1988 it survived only on Middle Island (13 ha) in the Mercury Islands, Castle Island (3 ha) off the Coromandel Peninsula, and at Pukerua Bay (1 ha) near Wellington. Between 1988 and 1995, O. whitakeri was reintroduced to Korapuki Island (18 ha), Red Mercury Island (225 ha) and Stanley Island (100 ha), all in the Mercury Islands, dramatically increasing the potential habitat and survival of the species (Towns Citation1992, Citation1999). All these translocations have been monitored 10 years after release with mixed results: success on Korapuki Island (self-sustaining population), failure on Red Mercury Island (no founder located) and success still to be determined on Stanley Island (evidence of reproduction, but the number of lizards located was less than the number released).
It is possible to create self-sustaining populations of some of New Zealand's most vulnerable lizard species when introduced predators are eliminated or adequately controlled. However, translocated populations are still at risk of demographic and stochastic problems due to the relatively small number of founders used (Towns & Ferreira Citation2001). It can take many years to create a self-sustaining population; therefore, translocations require a long-term commitment, especially at mainland sites where the risks are higher and more difficult to manage.
Concerns about mitigation-driven translocations
Studies overseas have shown that mitigation-driven translocations of reptiles have high failure rates (Germano & Bishop Citation2009; Germano et al. Citation2015; Sullivan et al. Citation2015). This review has identified some areas of concern about mitigation-driven translocations of lizards in New Zealand. It is widely accepted that before any translocations are undertaken the agent of decline must be identified, removed or reduced, to allow population recovery (Dodd & Siegel Citation1991; IUCN Citation2013). Seven mitigation-motivated translocations can be classified as best-practice translocations because they adequately dealt with the agent of decline by releasing lizards in sanctuaries with pest-proof fences or on islands where only mice were present (e.g. N. gemmeus translocated to Orokonui Ecosanctuary [Knox & Monks Citation2014]). Most projects, however, released animals at sites where a suite of introduced mammalian predators were still present. Translocations undertaken under the RMA must comply with the requirements of their resource consent, which involves the development of a lizard management plan that outlines management activities to be undertaken at the release site (e.g. legal protection of the site, habitat enhancement and predator control [Anderson et al. Citation2012]). However, it is questionable whether these requirements are sufficient to generate self-sustaining populations.
The risks associated with translocating lizards to mainland sites without pest-proof fences are high as very little is known about the pest mammal densities required to allow particular lizard taxa to recover in different habitat types (exception, Reardon et al. Citation2012). Control of introduced predators to a level that allows establishment, growth and persistence of lizard populations is costly and needed in perpetuity (Norbury et al. Citation2014a). There is no information to suggest that predator control operations that benefit birds or general ecosystem health are sufficient to allow translocated lizard populations to establish or persist. In some instances, control operations may be detrimental, as they may cause meso-predator release (Crooks & Soule Citation1999) or competitor release (Caut et al. Citation2007). For example, controlling predators (e.g. cats, ferrets and stoats) in a grassland/shrubland ecosystem in the South Island caused meso-predator release of mice, which had negative effects on lizards (Norbury et al. Citation2013).
Most mitigation-driven translocations released lizards into sites where there were resident conspecifics. If the habitat is at carrying capacity, then it is likely that released animals will either die or displace resident lizards (Sullivan et al. Citation2015). If the habitat is below carrying capacity, but the refuges that offer protection from predation are fully occupied, the translocated individuals will be vulnerable to predation by introduced mammals (Norbury et al. Citation2014b). In both scenarios there may be no overall change in the population and no benefit from translocating animals. Mitigation-driven translocations to mainland sites do not undertake disease screening so there is also the risk of introducing disease into resident populations when lizards are moved between sites (Sullivan et al. Citation2015).
Some projects create or enhance habitat at release sites through planting programmes and/or by providing additional cover (Anderson et al. Citation2012). However, it is yet to be proven whether enhanced habitats benefit lizard populations in the long term in the presence of introduced predators, as some lizard populations decline in areas of excellent habitat where predators are present (e.g. O. aeneum and O. whitakeri at Pukerua Bay [Hoare et al. Citation2007]).
In contrast, all conservation-motivated translocations in this review can be classified as best-practice translocations because they released lizards in suitable habitat where the agent of decline had been adequately managed (IUCN Citation2013). Most translocations were undertaken at sites that were free of introduced mammalian predators. The two exceptions involved releasing lizards on an island where mice were present at low density, and at a mainland site where a proven multi-species control programme was in place to protect resident lizards (Whitmore et al. Citation2011; Reardon et al. Citation2012).
Long-term monitoring is required to assess the effectiveness of habitat enhancement and predator control (as opposed to predator eradication): two management techniques that are commonly used for mitigation-motivated translocations under the RMA. Until these management techniques are proven, a precautionary approach should be taken by only allowing best-practice translocations to mitigate human-related impacts. In other words, RMA and DOC consents should only be granted to translocate lizards to predator-free offshore islands or fenced sanctuaries, and if no suitable release site is available, the consent should not be granted. If predator control and habitat enhancement are not successful, it would be unethical to continue to translocate animals into areas where they are unlikely to survive (i.e. an irresponsible release [IUCN Citation2013]), and new solutions to mitigate the destruction of lizard habitat would be needed. If the management techniques are successful, it will be necessary to ensure that practitioners comply with consent conditions as there is generally a low level of regulatory compliance, with 35.2% of requirements not being achieved (Brown et al. Citation2013).
Conclusions and recommendations
These findings are important and concerning. Few lizard translocations, even those intended to derive a conservation benefit, resulted in significant growth of the re-established population. There were no mitigation-driven translocations before 2003, and since then nearly half were initiated by habitat loss due to human land use. The effectiveness of management techniques applied to non-fenced mainland sites (as typically used by mitigation-driven translocations) are unproven, and research is required to determine whether or not they are a ‘misguided conservation strategy’ (see Germano et al. Citation2015).
The following recommendations will improve outcomes of lizard translocations:
All lizard translocations should be approved and assessed to ensure they meet the requirements of a best-practice conservation translocation, as outlined in the IUCN guidelines (IUCN Citation2013).
Research is required on the long-term effects of predator control and habitat enhancement on lizards in mitigation-motivated translocations. The oldest translocation sites should be revisited, monitored and the results published in peer reviewed journals, whether the translocations were successful or not.
Research is required to ascertain the level to which pest mammal densities must be reduced to allow particular lizard species to recover in different habitat types on the mainland (see Norbury et al. Citation2015 for a review of pest density-impact functions). Mainland species and habitats that are most at risk from development should be the focus of this research.
Long-term monitoring of all lizard translocations is needed to inform future translocations. There must be a commitment by organisations that undertake translocations to long-term monitoring (Germano & Bishop Citation2009). Towns’ (Citation1999) recommendation of monitoring long-lived species every 5 years (up to 15 years after release) is appropriate for all lizard species.
Lizard translocation projects that are more than 10 years old, where data are lacking, should be revisited to monitor progress (e.g. Hawea Island).
Information on lizard translocations should be more accessible to researchers. National databases, such as those managed by DOC, should be reviewed and improved to make it easier to obtain summary data about key aspects of all translocations. Practitioners must ensure that monitoring reports are provided to the national regulatory body in a timely manner.
Supplementary data
Table S1. Spreadsheet containing data on all lizard translocations referred to in the article text.
Dataset S1. Summary information about all translocations by taxa.
Dataset S1. Summary information about all translocations by taxa.
Download MS Word (34.2 KB)Table S1. Spreadsheet containing data on all lizard translocations referred to in the article text.
Download MS Excel (58.5 KB)Acknowledgements
We thank the Department of Conservation (DOC) for allowing access to the translocation database and associated reports. We are extremely grateful to the people who helped us find data, or checked portions of the data set and provided additional information about their particular translocation(s), including: Lynn Adams (DOC), Mike Aviss (DOC), Chris Birmingham (DOC), Marleen Baling (Massey University), Ben Barr (North Tec), Manuela Barry (Massey University), Raewyn Empson (Zealandia Sanctuary), Brett Gartrell (Massey University), Peter Gaze, John Heaphy (DOC), Rod Hitchmough (DOC), Hal Hovell (DOC), Benno Kappers (DOC), Carey Knox (Ecogecko Consultants), Marieke Lettink (Fauna Finders), John Lucas (DOC), Matt Maitland (Auckland Council), Chris McClure (DOC), Trudi Ngawhare (DOC), Grant Norbury (Landcare Research), Richard Parrish, James Reardon (DOC), Melinda Rixon, Ivan Roger (DOC), Su Sinclair (Auckland Council), Brent Tandy (DOC), Bruce Thomas, Dave Towns (Auckland University of Technology), Gavin Udy (DOC), Graham Ussher (Tonkin and Taylor) and Chris Wedding (Bioresearches Group). Thanks to Lynn Adams, Doug Armstrong (Massey University), Simon Chapman (Andrew Stewart), Nikki MacArthur (Greater Wellington Regional Council) and Phil Seddon (University of Otago) for discussions on various aspects of translocations, and Lynn Adams, Jen Germano, Grant Norbury and an anonymous reviewer for helpful comments on the manuscript.
Associate Editor: Dr Christina Painting.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Anderson P, Bell B, Chapman S, Corbett K. 2012. New Zealand lizards conservation toolkit—a resource for conservation management of lizards of New Zealand. Wellington: Society for Research on Amphibians and Reptiles of New Zealand (SRARNZ); [cited 2016 Feb 9]. Available from: http://www.srarnz.org.nz/Toolkit.aspx
- Bell TP, Herbert SM. Forthcoming. Establishment of a self-sustaining population of a long-lived, slow-breeding gecko (Diplodactylidae: Hoplodactylus duvaucelii) evident 15 years after translocation. J Herpetol.
- Brown MA, Clarkson BD, Barton BJ, Joshi C. 2013. Ecological compensation: an evaluation of regulatory compliance in New Zealand. Impact Assess Proj Apprais. 31:34–44. doi: 10.1080/14615517.2012.762168
- Burns B, Innes J, Day T. 2012. The use and potential of pest-proof fencing for ecosystem restoration and fauna conservation in New Zealand. In: Somers MJ, Hayward MS, editors. Fencing for conservation: restriction of evolutionary potential or a riposte to threatening processes? New York, NY: Springer; p. 65–90.
- Caut S, Casanovas JG, Virgos E, Lozano J, Witmer GW, Courchamp F. 2007. Rats dying for mice: modelling the competitor release effect. Austral Ecol. 32:858–868. doi: 10.1111/j.1442-9993.2007.01770.x
- Cree A. 1994. Low annual reproductive output in female reptiles from New Zealand. New Zeal J Zool. 21:351–372. doi: 10.1080/03014223.1994.9518005
- Cromarty PL, Alderson SL. 2013. Translocation statistics (2002–2010), and revised Department Of Conservation translocation process. Notornis. 60:55–62.
- Crooks KR, Soule ME. 1999. Mesopredator release and avifaunal extinctions in a fragmented system. Nature. 400:563–566. doi: 10.1038/23028
- Dodd CK, Siegel RA. 1991. Relocation, repatriation, and translocation of amphibians and reptiles: are they conservation strategies that work? Herpetologica. 47:336–350.
- Fischer J, Lindenmayer DB. 2000. An assessment of the published results of animal relocations. Biol Conserv. 96:1–11. doi: 10.1016/S0006-3207(00)00048-3
- Germano JM, Field KJ, Griffiths RA, Clulow S, Foster J, Harding G, Swaisgood RR. 2015. Mitigation-driven translocations: are we moving wildlife in the right direction? Front Ecol Environ. 13:100–105. doi: 10.1890/140137
- Germano LM, Bishop PJ. 2009. Suitability of amphibians and reptiles for translocation. Conserv Biol. 23:7–15. doi: 10.1111/j.1523-1739.2008.01123.x
- Goldwater N, Perry GLW, Clout MN. 2012. Responses of house mice to the removal of mammalian predators and competitors. Austral Ecol. 37:971–979. doi: 10.1111/j.1442-9993.2011.02356.x
- Griffith B, Scott JM, Carpenter JW, Reed C. 1989. Translocation as a species conservation tool: status and strategy. Science. 245:477–480. doi: 10.1126/science.245.4917.477
- Hitchmough R, Anderson P, Barr B, Monks J, Lettink M, Reardon J, Tocher M, Whitaker T. 2013. Conservation status of New Zealand reptiles, 2012. New Zealand Threat Classification Series 2. Wellington: Department of Conservation.
- Hoare JM, Adams LK, Bull LS, Towns DR. 2007. Attempting to manage complex predator-prey interactions fails to avert imminent extinction of a threatened New Zealand skink population. J Wildlife Manage. 71:1576–1584. doi: 10.2193/2006-488
- Hoare JM, Stephens CL, Daugherty CH, Phillpot PMS. 2005. Oligosoma lineoocellatum (spotted skink). Longevity, site fidelity. Herpetol Rev. 36:181.
- IUCN/SSC. 2013. Guidelines for reintroductions and other conservation translocations. Version 1.0. Gland, Switzerland: IUCN; [cited 2016 Feb 9]. Available from: http://www.issg.org/pdf/publications/RSG_ISSG-Reintroduction-Guidelines-2013.pdf
- Jewell T. 2011. A photographic guide to reptiles and amphibians of New Zealand. Revised ed. Auckland: New Holland Publishers.
- Knox CD, Cree A, Seddon PJ. 2012. Direct and indirect effects of grazing by introduced mammals on a native, arboreal gecko (Naultinus gemmeus). J Herpetol. 46:145–152. doi: 10.1670/10-315
- Knox CD, Monks JM. 2014. Penning prior to release decreases post-translocation dispersal of jewelled geckos. Anim Conserv. 17:18–26. doi: 10.1111/acv.12149
- Lettink M, Cree A. 2006. Predation, by the feral house mouse (Mus musculus), of McCann's skinks (Oligosoma maccanni) constrained in pitfall traps. Herpetofauna. 36:61–62.
- Lettink M, Whitaker AH. 2006. Hoplodactylus maculatus (common gecko) longevity. Herpetol Rev. 37:223–224.
- Miller KA. 2009. Founding events and the maintenance of genetic diversity in reintroduced populations [Unpublished PhD thesis]. Wellington: Victoria University of Wellington.
- Miller KA, Bell TP, Germano JM. 2014. Understanding publication bias in reintroduction biology: an assessment of translocations in New Zealand's herpetofauna. Conserv Biol. 28:1045–1056. doi: 10.1111/cobi.12254
- Miskelly CM, Powlesland RG. 2013. Conservation translocations of New Zealand birds, 1863–2012. Notornis. 60:3–28.
- Newman DG. 1994. Effect of a mouse Mus musculus eradication programme and habitat change on lizard populations on Mana Island, New Zealand, with special reference to McGregor's skink, Cyclodina macgregori. New Zeal J Zool. 21:443–456. doi: 10.1080/03014223.1994.9518015
- Norbury G, Byrom A, Smith J, Clarke D, Anderson D, Forrester G. 2013. Invasive mammals and habitat modification interact to generate unforeseen outcomes for indigenous fauna. Ecol Appl. 23:1707–1721. doi: 10.1890/12-1958.1
- Norbury G, Hutcheon A, Reardon J, Daigneault A. 2014a. Pest fencing or pest trapping: a bio-economic analysis of cost-effectiveness. Austral Ecol. 39:795–807. doi: 10.1111/aec.12147
- Norbury G, van den Munchof M, Neitzel S, Hutcheon A, Reardon J, Ludwig K. 2014b. Impacts of invasive house mice on post-release survival of translocated lizards. New Zeal J Ecol. 38:1–6.
- Norbury GL, Pech RP, Byrom AE, Innes J. 2015. Density-impact functions for terrestrial vertebrate pests and indigenous biota: guidelines for conservation managers. Biol Conserv. 191:409–420. doi: 10.1016/j.biocon.2015.07.031
- R Development Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; [cited 2016 Feb 9]. Available from: http://www.R-project.org/
- Reardon JT, Whitmore N, Holmes KM, Judd LM, Hutcheon AD, Norbury G, MacKenzie DI. 2012. Predator control allows critically endangered lizards to recover on mainland New Zealand. New Zeal J Ecol. 36:141–150.
- Romijn RL. 2013. Can skinks recover in the presence of mice? [Unpublished BSc (Hons) thesis]. Wellington: Victoria University of Wellington.
- Saunders A. 1994. Translocations in New Zealand: an overview. In: Serena M, editor. Reintroduction biology of Australian and New Zealand fauna. Chipping Norton, NSW: Surrey Beatty & Sons; p. 43–46.
- Seddon PJ, Soorae PS, Launay F. 2005. Taxonomic bias in reintroduction projects. Anim Conserv. 8:51–58. doi: 10.1017/S1367943004001799
- Seddon PJ, Strauss WM, Innes J. 2012. Animal translocations: what are they and why do we do them? In: Ewen JG, Armstrong DP, Parker KA, Seddon PJ, editors. Reintroduction biology: integrating science and management. Oxford, UK: Wiley-Blackwell; p. 1–32.
- Shaw T. 1994. Population size, distribution, home range and translocation of the jewelled gecko. Wildlife Management Report 56. Dunedin: University of Otago.
- Sherley GH, Stringer IAN, Parrish GR. 2010. Summary of native bat, reptile, amphibian and terrestrial invertebrate translocations in New Zealand. Science for Conservation 303. Wellington: Department of Conservation.
- Speedy C, Day T, Innes J. 2007. Pest eradication technology – the critical partner to pest exclusion technology: the Maungatautari experience. In: Witmer GW, Pitt WC, Fagerstone KA, editors. Managing vertebrate invasive species: proceedings of an international symposium. USDA/APHIS National Wildlife Research Centre Symposia, 7–9 August 2007, Fort Collins, CO, USA. Lincoln, NE: University of Nebraska, USDA/APHIS; [cited 2016 Feb 9]. Available from: https://www.aphis.usda.gov/wildlife_damage/nwrc/symposia/invasive_symposium/content/Speedy115_126_MVIS.pdf
- Sullivan BK, Nowak EM, Kwiatkowski MA. 2015. Problems with mitigation translocation of herpetofauna. Conserv Biol. 29:12–18. doi: 10.1111/cobi.12336
- Thomas BW, Whitaker AH. 1994. Translocation of the Fiordland skink, Leiolopisma acrinasum to Hawea Island, Breaksea Sound, Fiordland, New Zealand. In: Serena M, editor. Reintroduction biology of Australian and New Zealand fauna. Chipping Norton, NSW: Surrey Beatty & Sons; pp. 91–95.
- Towns D. 1992. Recovery plan for Whitaker's skink and robust skink. Threatened Species Unit Recovery Plan Series No. 3. Wellington: Department of Conservation.
- Towns DR. 1994. The role of ecological restoration in the conservation of Whitaker's skink (Cyclodina whitakeri), a rare New Zealand lizard (Lacertilia: Scincidae). New Zeal J Zool. 21:457–471. doi: 10.1080/03014223.1994.9518016
- Towns DR. 1999. Cyclodina spp. skink recover plan 1999–2004. Threatened Species Recovery Plan No. 27. Wellington: Department of Conservation.
- Towns DR, Broome KG. 2003. From small Maria to massive Campbell: forty years of rat eradications from New Zealand Islands. New Zeal J Zool. 30:377–398. doi: 10.1080/03014223.2003.9518348
- Towns DR, Daugherty CH. 1994. Patterns of range contractions and extinctions in the New Zealand herpetofauna following human colonisation. New Zeal J Zool. 21:325–339. doi: 10.1080/03014223.1994.9518003
- Towns DR, Ferreira SM. 2001. Conservation of New Zealand lizards (Lacertilia: Scincidae) by translocation of small populations. Biol Conserv. 98:211–222. doi: 10.1016/S0006-3207(00)00156-7
- Whitaker AH. 1973. Lizard populations on islands with and without Polynesian rats. Rattus exulans (Peale). Proc New Zeal Ecol Soc. 20:121–130.
- Whitaker AH. 1978. The effects of rodents on amphibians and reptiles. In Atkinson IAE, Dingwell PR, Hay C, editors. The ecology and control of rodents in New Zealand nature reserves. New Zealand Department of Lands and Survey Information Series 4. Wellington: Department of Lands and Survey; p. 75–88.
- Whitmore N, Judd LM, Mules RD, Webster TA, Madill SC, Hutcheon AD. 2011. A trial wild-wild translocation of the critically endangered grand skink Oligosoma grande in Otago, New Zealand. Conserv Evid. 9:28–35.
- Wolf CM, Griffith B, Reed C, Temple SA. 1996. Avian and mammalian translocations: update and reanalysis of 1987 survey data. Conserv Biol. 10:1142–1154. doi: 10.1046/j.1523-1739.1996.10041142.x
