ABSTRACT
Brushtail possums are controlled extensively in New Zealand because they are a livestock disease vector and have an impact on native biodiversity. Reinvasion of controlled areas and subsequent population recovery is a significant management problem but little attention has been paid to what influences the settlement of possums in depopulated areas. To address this gap we trapped possums out of an area of about 24 ha in native podocarp–hardwood forest and studied reinvasion and settlement in the central c. 14 ha over 22 months. Most new possums were young males, but adults were also trapped. Many of the new possums caught on the study site post-depopulation did not settle there, most likely because they continued to disperse, but some may have returned to their ranges nearby or were residents with a very low probability of capture. This finding highlights the need for better information about the origins and settlement of possums in depopulated areas to improve management of population recovery and long-term sustained control of possums.
Introduction
Introduced common brushtail possums (Trichosurus vulpecula) are a major pest in New Zealand where they act as the main wildlife host responsible for reinfecting livestock with bovine tuberculosis (Mycobacterium bovis), their browsing damages native forests, and they compete with and prey on native animals (Montague Citation2000; Cowan Citation2005). To mitigate those impacts they are controlled extensively. Recent improvements in aerial and ground control mean that possum numbers are now often reduced to extremely low levels, at which changes in behaviour and trapability may occur (Sweetapple & Nugent Citation2009). In New Zealand, areas depopulated of possums have been used extensively as buffers to minimise the spread of bovine tuberculosis, so the likelihood of movement of possums from adjacent areas into buffers has been much studied (e.g. Pech et al. Citation2010; Byrom et al. Citation2015).
Population recovery after control is an ongoing management problem. Because control usually leaves few survivors, the initial stages of population recovery generally involve invasion and settlement of dispersing possums. Movement into controlled areas by immediate neighbours occurs but is not a significant factor unless the area controlled is small (Efford et al. Citation2000; Pech et al. Citation2010). Possum dispersal from surrounding source populations into controlled areas is reasonably well characterised (Clout & Efford Citation1984; Cowan Citation2000a; Cowan & Clout Citation2000; Ji et al. Citation2004), but the factors that influence dispersing possums to settle and establish a home range are largely unknown. This is of wide interest for the management of pest species and for more general questions about dispersal ecology (Cowan Citation2000a; Sutherland et al. Citation2002). The three stages of dispersal—leaving, travelling and settling—each involve different costs and trade-offs (Bonte et al. Citation2012), and settlement is the least studied stage (Travis et al. Citation2012).
Previous studies on recovery of possum populations, with the exceptions of Clinchy et al. (Citation2001) and Ji et al. (Citation2004), have mainly focused on large-scale influences or invasion from surrounding areas (e.g. Pech et al. Citation2010). Much remains to be clarified about the fine detail of resettlement after local depopulation, particularly the probability of ongoing residence (i.e. settlement) at a depopulated site. Arthur et al. (Citation2002) suggested such areas might preferentially attract possums, although evidence to date suggests not (Pech et al. Citation2010; Byrom et al. Citation2015). While possum dispersal from natal areas appears to be independent of density (Cowan et al. Citation1997), factors that might influence settlement at a site include, for example, local possum density, habitat quality, opportunities for breeding, inbreeding risk, and the presence and location of survivors of control (Cowan Citation2000a; Sweetapple & Nugent Citation2009; Banks & Lindenmayer Citation2014; Shaw & Kokko Citation2014). Depopulated areas offer effectively unlimited choices of food and shelter, so it was hypothesised that such areas would be attractive for settlement of dispersing possums. Therefore, this study characterised the arrival and settlement behaviour and population characteristics of previously unmarked possums trapped in an area depopulated of its original resident possums.
Methods
Study site
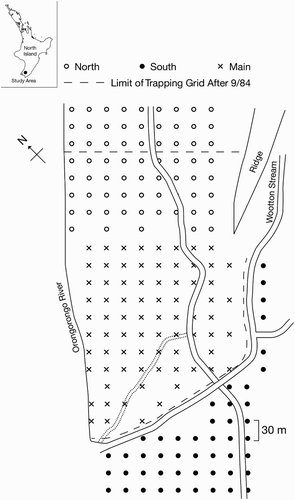
The study was conducted in three contiguous areas of predominantly lowland podocarp, mixed hardwood forest in the vicinity of Wootton Stream at its junction with the Orongorongo River () in the Orongorongo Valley, southern Rimutaka Range (41°22′S, 174°57′E). The vegetation at the 8 ha main site and the 10 ha north site was broadly similar (Campbell Citation1984). The south site (6 ha) on the southern side of Wootton Stream comprised forest regenerating after fires and logging prior to 1950, with no trees taller than 10 m, and with ground cover predominantly of introduced grasses rather than the litter, seedlings, ferns and mosses of the main and north sites. Possum density in unmanaged podocarp–broadleaved forest in the Orongorongo Valley is about 6–14 possums/ha (Efford & Cowan Citation2004).
Figure 1. Location of study site, Orongorongo Valley, near Wellington, New Zealand, and layout of trapping grid showing main, north and south grids. Resettlement of possums after depopulation of the study site was studied in the grey shaded area (referred to in the text as the resettlement study area).
Possum capture–mark–recapture
The entire site was covered by a trapping grid, with 194 live-catch cage traps for brushtail possums set at the intersections of a 30 m grid that covered all three areas (). Mark–recapture studies of possums on the main and north trapping grids began 5.5 and 2.5 years, respectively, before the present study. Trapping was conducted for 1–3 nights monthly, using apple coated with aniseed-lured flour as an attractant. The south site had not been trapped before the start of depopulation.
On their initial capture, all live-trapped possums were uniquely identified with an ear tattoo and ear tag. Head length was measured with callipers to the nearest millimetre, total body length and tail length to the nearest 5 mm with a flexible tape, and weight was recorded to the nearest 50 g to assist with assigning animals to age classes (adult/juvenile). Sexual maturity was judged from pouch and nipple development in females and testis size in males (Gilmore Citation1969). Possums were released at their point of capture. The project was approved and conducted under legislation prevailing prior to the 1999 New Zealand Animal Welfare Act.
Depopulation and settlement
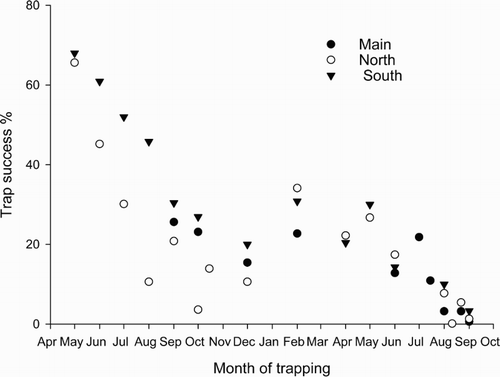
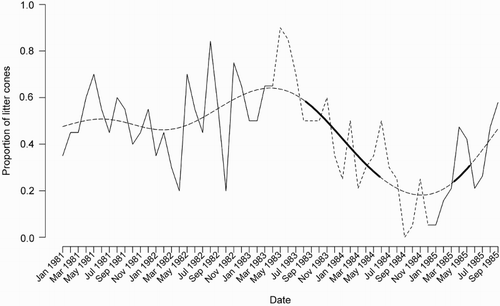
From May 1983 until September 1984, all three sites were cleared gradually of possums (Table S1). Possums were captured in cage traps set at the grid sites and killed humanely. Teeth were extracted and aged by counting annual lines in the molar cementum (Pekelharing Citation1970). The effectiveness of the depopulation was assessed from the progressive decline in possum catch rates (), supported by an index based on the presence/absence of possum faeces in litter-collection cones sited under 20 hīnau (Elaeocarpus dentatus) trees on the main site and checked monthly () (methods fully described in Cowan & Waddington [Citation1990]). Possums often use hīnau trees for denning and feeding (Cowan Citation1989).
Figure 3. Proportion of 20 litter-collection cones found to contain possum faeces on monthly checks from January 1981 to September 1985. Dotted line indicates the period during which possums were removed from the Orongorongo Valley study site. Bold parts of the fitted curve indicate statistically significant trends.
From October 1984 to August 1985, possums were allowed to resettle over the whole area naturally. During that time, live-traps were set for three consecutive nights each month on a subsection of the original trapping area, namely the main site and the southern half of the north site (together referred to as the resettlement study area; see shaded area on ). The omission of part of the previously trapped area to the north and south aimed to minimise any effect of home range expansion by resident possums in adjacent areas, so that new possums trapped were more likely to be immigrants (Efford et al. Citation2000). Trapped possums were tagged and tattooed, weighed, measured and aged as before, and released at point of capture. The resettlement study area was then largely cleared of possums again by intensive cage trapping, with traps set for 10 nights between 3 and 25 September 1985 for a total of 1160 trap-nights. Captured possums were killed and measured as before. The whole area was then left undisturbed for a further 12 months and possums that had moved into the resettlement area removed again during 6 nights of intensive cage trapping (657 trap-nights) between 23 September and 17 October 1986.
Data analysis comprised contingency table comparisons of frequencies using chi-square tests in Genstat 14th edition (www.vsni.co.uk). Analysis of the faecal pellet index was done by fitting a generalised additive model with a smoothed term for collection date to the data (using the ‘mgcv’ package for R [Woods Citation2011]) giving a smoothed trend line for the proportion of litter cones containing possum droppings with time. To identify periods of change in the trend line, first derivatives of the smoothed function (i.e. slopes) and their standard errors were evaluated using the trapezoidal method at 200 evenly spaced points over the time series. Confidence intervals (95% CI) around each evaluation point were computed from the derivatives and standard errors. If these intervals did not include zero then the derivative was considered to be significant at that evaluation point, with a significant positive derivative indicating an increasing trend, and a negative derivative a decreasing trend.
Results
Initial depopulation of the site
During the initial depopulation of the study site, totals of 139, 80 and 50 possums were killed on the north, main and south grids, respectively. Judged from near-to-zero trap catches at the end of that time () and the lack of previously tagged possums (Table S1), possums were effectively eliminated from the study site. Data on possum faecal pellets also indicated a reduction in possum numbers to near zero on the main site ().
Initial resettlement–mark–recapture
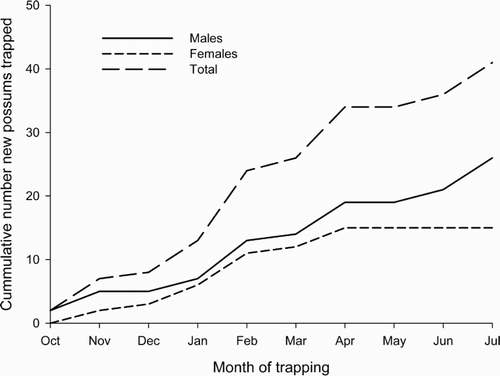
During the initial resettlement period (October 1984 and July 1985), 41 possums were marked and released on the resettlement study area (i.e. main grid and the southern half of the north grid), with males outnumbering females by 1.73:1 (, P < 0.1). However, excluding four males that were caught for the first time in the last live-trapping session, 57% of the possums live-trapped were only caught in one trapping session. Overall, trapability (number of captures excluding first capture as a percentage of total trap nights post initial capture) during the resettlement period was very low, ranging from 0%–66% (median 0%) and 0%–89% (median 0%) for individual males and females, respectively. Three of the captured possums (one male, two females) had been tagged previously, and so had survived the depopulation. Of the other 13 females caught, eight were juveniles with immature pouches; by contrast, 18 out of the other 25 males caught were adult (as judged by testis size) (
, P < 0.1). The cumulative total of possums caught showed a steady increase rather than a seasonally biased influx ().
Depopulations after resettlement
Twenty five possums were trapped and killed (13 males, 12 females) in the depopulation of the resettlement study area in September 1985, of which 16 were ear-tagged (nine males, seven females). The nine new possums caught ranged from 1–12 years old, but most (67%) were 1–2 years old. Previously tagged possums accounted for only 39% of the 41 possums tagged and released on the resettlement study area during the initial resettlement period. The proportion of previously tagged males trapped (0.35) during the depopulation did not differ from that of females (0.47; , P > 0.5).
Tagged possums not recaptured included both juveniles and adults. Six of the 11 males tagged during live-trapping of the resettlement study area and not recaptured in the subsequent removal trapping in September 1985 were mature adults. One of the other five was the immature offspring of a female live-trapped in the resettlement study area, and the remaining four were immature males 1–2 years old of unknown origin. Two of the eight tagged females not recaptured were adults tagged before the depopulation; two of the remaining six were young of the year, three were 1–2 years old with immature pouches and one was a fully mature adult.
In the resettlement study area a further 12 months later in September–October 1986, 24 possums were caught (15 males, nine females), including five previously tagged males trapped 30–210 m from their last known location 12 months earlier. One other previously tagged male survived, as it was trapped about 4 km to the north 4 months later.
Comparison of immigrants with the prior resident possum population
As expected from previous studies, the age structure of the immigrants was significantly different from that of the original population (). Young animals dominated the immigrant population, comprising 57.1% of animals ≤2 years old, compared with 38.6% of the original population. Animals older than 6 years comprised only 6.1% of the immigrant population, compared with 14.3% in the previous year (, P = 0.03). However, ages of immigrants ranged up to 12 years.
Table 1. Frequency of tooth ring ages of possums trapped at Orongorongo Valley during depopulations in September 1985 and September–October 1986.
Discussion
The possum population in the 1200 ha area of the Orongorongo Valley surrounding the study site has not been subjected to widespread possum control since the late 1950s. For that reason, while the data from this study were collected c. 30 years ago, they remain relevant to uncontrolled populations in similar habitat elsewhere in New Zealand. Although the findings of this study generally agree with characteristics of possum dispersers and dispersal identified in other studies (e.g. Cowan & Clout Citation2000; Ji et al. Citation2004), some findings require further discussion. First, there was no strong seasonal influx of immigrants. The possum population in Orongorongo Valley breeds once a year in April to June; dispersal of young of the year occurs between January and May (Ward Citation1985; Efford & Cowan Citation2004) and between September and February for animals in their second year (Efford Citation1998). These two extended periods of dispersal may explain the lack of a seasonal peak, although their relative importance to population recovery requires further clarification, as does the generality of the extended period of dispersal to other populations. Repopulation of the resettlement study area began in October and, particularly for males, increased linearly. This suggests that new possums arriving on the resettlement study area were a mixture of dispersing possums <1 and 1–2 years old and, occasionally, adult dispersers and nearby adults expanding their ranges or making occasional forays beyond their usual ranges (Cowan & Clout Citation2000; Efford et al. Citation2000; Byrom et al. Citation2015). Annual trap-revealed minimum convex polygon home range size on a nearby area with similar vegetation averaged 0.2–1.4 ha with extensive overlap between individuals (Crawley Citation1973; Ward Citation1984), although estimates from radio-tracking were two to 20 times larger (Ward Citation1984). The capture of adults and these estimates of home range size suggest that our attempt to minimise the influence of home range expansion of surrounding resident possums on the results of the study by restricting the resettlement study area to the central part of the original possum removal area may not have been entirely successful.
Figure 4. Cumulative number of new possums trapped at Orongorongo Valley resettlement study site during the initial population recovery from October 1983 to July/August 1984.
If that were the case it would explain the mixture of juveniles and adults among the new possums, both those that remained and took up residence on the resettlement area and those that were not recaptured because they had either continued their dispersal or returned to their usual home ranges. Clinchy et al. (Citation2001) noted that most of the experimentally removed possums in his study were replaced by range expansion of nearby residents. However, the number of unmarked possums immediately adjacent to (at least) the north and main grids should have been minimised as a result of the 2.5 years of 3 nights per month capture–mark–release trapping done there before depopulation began.
Second, the recapture rate of possums during the capture–mark–release trapping was low (<50%) and less than half of the new possums tagged on the study site after depopulation were recaptured. Ji et al. (Citation2004) observed a similar phenomenon in a different habitat, i.e. forest remnants on farmland. Transient possums have often been noted in mark–recapture studies of possum populations at carrying capacity (e.g. Crawley Citation1973; Efford Citation2000), but in that situation the opportunity to settle is thought to be limited in some way by existing residents. It is not however, completely prevented, as some possums translocated into populations with no recent history of control (and hence potentially at carrying capacity) are able to settle and establish home ranges in the vicinity of their release sites (Cowan Citation2000b; G. Nugent, pers. comm.). Some of the juveniles tagged on the resettlement grid and not recaptured may have been the offspring of adult residents with low capture probabilities that subsequently underwent natal dispersal, since such dispersal is known to continue even after 90% population reduction (Cowan et al. Citation1997). They are unlikely, however, to have been the unmarked offspring of adult females caught on the resettlement study area, as young of the year in this population are seldom capable of an independent existence by September (Bell Citation1981), when the depopulations ended.
Mortality cannot be ruled out as an explanation for the disappearance of immigrants in the present study, but it is not usually that high in the studied possum population (Efford & Cowan Citation2004). Changes in movement behaviour and reduced trapability at low density and short-term exploratory movements by established residents in the surrounding forest may account for some of the apparent disappearance of immigrants (Sweetapple & Nugent Citation2009; Byrom et al. Citation2015). However, the simplest explanation is that most of these possums did not remain on the study site, despite the initial almost complete absence of possums and, even with reinvasion, significantly fewer possums than originally. The pre-control possum density at the study site was moderate to high compared with other areas of surrounding forest (K. Richardson, unpubl. data), so habitat quality alone was unlikely to have contributed significantly to rejection of the site for settlement.
This finding highlights the current lack of understanding of factors influencing possum settlement and suggests that residual possum density may not be an important influence. Broad-scale surveys of post-control low-density possum populations have shown individuals are highly aggregated and that aggregation increases with time (Sweetapple & Nugent Citation2009). Such aggregation could be driven by possums seeking proximity to other possums, mating opportunities and/or more favourable habitat (e.g. topographic position and vegetation), suggesting reduced density per se is not a vital factor in settlement decisions. Some dispersing possums make multiple movements, settling for a few days in an area and then moving on (Cowan & Rhodes Citation1993). Such behaviour suggests individuals are sampling sites and habitats as part of the settlement process. Individual factors, such as phenotype (Cote et al. Citation2008) and body condition (Stamps Citation2006) may also influence settlement decisions. Clearly, better information about factors influencing settlement is needed to improve management of population recovery and long-term sustained control. Intensive tracking of dispersing possums fitted with global positioning system collars would be one suitable approach to address this information gap, especially when combined with replicated experimental settlement sites where density and/or sex ratio of resident possums were manipulated.
Supplementary data
Table S1. Details of initial depopulation trapping sessions on Main, North and South grids during May 1983 to September 1984. Numbers of traps set and possums caught and killed (T, previously tagged; N, new). More than one trapping session occurred in some months.
Table S1.
Download MS Word (21.3 KB)Acknowledgements
Don Waddington and Mark Hearfield assisted with possum trapping, Jim White assisted with the possum autopsies and completed the tooth ageing, and Don and Nyree Fea helped with data entry and checking. Mandy Barron analysed the faecal pellet index data. Bruce Warburton and Al Glen commented on the draft manuscript, which was edited by Christine Bezar and significantly improved by comments from the three referees.
Associate Editor: Dr Grant Norbury.
Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Funding
References
- Arthur A, Ramsey DS, Efford M. 2002. Changes in possum behaviour at reduced density—a review. Landcare Research Contract Report LC0102/102 for Animal Health Board. 28pp.
- Banks SC, Lindenmayer DB. 2014. Inbreeding avoidance, path isolation, and matrix permeability influence dispersal and settlement choices by male agile antechinus in a fragmented landscape. J Anim Ecol. 83:515–524. doi: 10.1111/1365-2656.12128
- Bell BD. 1981. Breeding and condition of possums Trichosurus vulpecula in the Orongorongo Valley, near Wellington, New Zealand, 1966–1975. Zoology Publications Victoria University of Wellington. 74:87–138.
- Bonte D, Van Dyke H, Bullock JM, Coulon A, Delgado MDM, Gibbs M, Lehouck V, Matthysen E, Mustin K, Saastamoinen M, et al. 2012. Costs of dispersal. Biological Reviews of the Cambridge Philosophical Society. 87:290–312. doi: 10.1111/j.1469-185X.2011.00201.x
- Byrom AE, Anderson DP, Coleman M, Thomson C, Cross ML, Pech RP. 2015. Assessing movements of brushtail possums (Trichosurus vulpecula) in relation to buffer zones for the management of wildlife tuberculosis in New Zealand. PLoS ONE. 10(12):e0145636. doi:10.1371/journal.pone.0145636.
- Campbell DJ. 1984. The vascular flora of the DSIR study area, lower Orongorongo Valley, Wellington, New Zealand. New Zeal J Bot. 22:223–270. doi: 10.1080/0028825X.1984.10425254
- Clinchy M, Krebs CJ, Jarman PJ. 2001. Dispersal sinks and handling effects: interpreting the role of immigration in common brushtail possum populations. J Anim Ecol. 70:515–526. doi: 10.1046/j.1365-2656.2001.00510.x
- Clout MN, Efford MG. 1984. Sex differences in the dispersal and settlement of brushtail possums (Trichosurus vulpecula). J Anim Ecol. 53:737–749. doi: 10.2307/4656
- Cote J, Boudsocq S, Clobert J. 2008. Density, social information, and space use in the common lizard (Lacerta vivipara). Behav Ecol. 19:163–168. doi: 10.1093/beheco/arm119
- Cowan P, Clout M. 2000. Possums on the move: activity patterns, home ranges, and dispersal. In: Montague TL, editor. The brushtail possum: biology, impacts and management of an introduced marsupial. Lincoln: Manaaki Whenua Press; p. 24–34.
- Cowan PE. 1989. Denning habits of common brushtail possums, Trichosurus vulpecula, in New Zealand lowland forest. Australian Wildlife Research. 16:63–78. doi: 10.1071/WR9890063
- Cowan PE. 2000a. Factors affecting possum re-infestation—implications for management. Science for Conservation 144. Wellington: Department of Conservation.
- Cowan PE. 2000b. Responses of common brushtail possums (Trichosurus vulpecula) to translocation on farmland, southern North Island, New Zealand. Wildlife Res. 28:277–282. doi: 10.1071/WR99097
- Cowan PE. 2005. Brushtail possum. In: King CM, editor. The handbook of New Zealand mammals. 2nd ed. Auckland: Oxford University Press; p. 56–80.
- Cowan PE, Brockie RE, Smith RN, Hearfield ME. 1997. Dispersal of juvenile brushtail possums, Trichosurus vulpecula, after a control operation. Wildlife Res. 24:279–288. doi: 10.1071/WR96005
- Cowan PE, Rhodes DS. 1993. Electric fences and poison buffers as barriers to movements and dispersal of brushtail possums (Trichosurus vulpecula) on farmland. Wildlife Res. 20:671–686. doi: 10.1071/WR9930671
- Cowan PE, Waddington DC. 1990. Suppression of fruit production of the endemic forest tree, Elaeocarpus dentatus, by introduced marsupial brushtail possums, Trichosurus vulpecula. New Zeal J Bot. 28:217–224. doi: 10.1080/0028825X.1990.10412310
- Crawley MC. 1973. A live-trapping study of Australian brush-tailed possums, Trichosurus vulpecula Kerr, in the Orongorongo Valley, Wellington, New Zealand. Australian J Zool. 21:75–90. doi: 10.1071/ZO9730075
- Efford M. 1998. Demographic consequences of sex-biased dispersal in a population of brushtail possums. J Anim Ecol. 67:503–517. doi: 10.1046/j.1365-2656.1998.00222.x
- Efford M. 2000. Possum density, population structure, and dynamics. In: Montague TL, editor. The brushtail possum: biology, impacts and management of an introduced marsupial. Lincoln: Manaaki Whenua Press; p. 47–61.
- Efford M, Warburton B, Spencer N. 2000. Home-range changes by brushtail possums in response to control. Wildlife Res. 27:117–127. doi: 10.1071/WR99005
- Efford MG, Cowan PE. 2004. Long-term population trend of the brushtail possums Trichosurus vulpecula in the Orongorongo Valley, New Zealand. In: Goldingay RL, Jackson SM, editors. The biology of Australian possums and gliders. Chipping Norton, New South Wales: Surrey Beatty; p. 471–483.
- Gilmore DP. 1969. Seasonal reproductive periodicity in the male Australian brush-tailed possum (Trichosurus vulpecula). Journal of Zoology. 157:75–98. doi: 10.1111/j.1469-7998.1969.tb01690.x
- Ji W, Sarre SD, White PCL, Clout MN. 2004. Population recovery of common brushtail possums after local depopulation. Wildlife Res. 31:543–550. doi: 10.1071/WR02048
- Montague TL, editor. 2000. The brushtail possum: biology, impacts and management of an introduced marsupial. Lincoln: Manaaki Whenua Press.
- Pech R, Byrom A, Anderson D, Thomson C, Coleman M. 2010. The effects of poisoned and notional vaccinated buffers on possum (Trichosurus vulpecula) movements: minimising the risk of bovine tuberculosis spread from forest to farmland. Wildlife Res. 37:283–292. doi: 10.1071/WR09161
- Pekelharing CJ. 1970. Cementum deposition as an age indicator in the brush-tailed possum, Trichosurus vulpecula Kerr (Marsupialia). Australian J Zool. 18:71–76. doi: 10.1071/ZO9700071
- Shaw AK, Kokko H. 2014. Mate finding, Allee effects and selection for sex-biased dispersal. J Anim Ecol. 83:1256–1267. doi: 10.1111/1365-2656.12232
- Stamps JA. 2006. The silver spoon effect and habitat selection by natal dispersers. Ecol Lett. 9:1179–1185. doi: 10.1111/j.1461-0248.2006.00972.x
- Sutherland WJ, Gill JA, Norris K. 2002. Density-dependent dispersal in animals: concepts, evidence, mechanisms and consequences. In: Bullock JM, Kenward RE, Hails RS, editors. Dispersal ecology. Oxford: Blackwell; p. 134–151.
- Sweetapple P, Nugent G. 2009. Possum demographics and distribution after reduction to near-zero density. New Zeal J Zool. 36:461–471. doi: 10.1080/03014223.2009.9651478
- Travis JMJ, Mustin K, Barton KA, Benton TG, Clobert J, Delgado MM, Dytham C, Hovestadt T, Palmer SCF, Van Dyck H, Bonte D. 2012. Modelling dispersal: an eco-evolutionary framework incorporating emigration, movement, settlement behaviour and the multiple costs involved. Methods Ecol Evol. 3:628–641. doi: 10.1111/j.2041-210X.2012.00193.x
- Ward GD. 1984. Comparison of trap- and radio-revealed home ranges of the brush-tailed possum (Trichosurus vulpecula Kerr) in New Zealand lowland forest. New Zeal J Zool. 11:85–92. doi: 10.1080/03014223.1984.10428231
- Ward GD. 1985. The fate of young radio-tagged common brushtail possums, Trichosurus vulpecula, in New Zealand lowland forest. Australian Wildlife Research. 12:145–150. doi: 10.1071/WR9850145
- Wood SN. 2011. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J R Stat Soc (B). 73:3–36. doi: 10.1111/j.1467-9868.2010.00749.x