ABSTRACT
The endemic New Zealand ground wētā (Hemiandrus sp. ‘promontorius’) has a Naturally Uncommon conservation status. This is because of the paucity of information on its density and distribution. Here, the biology, density and distribution of a population of this wētā found in and around vineyards in the Awatere Valley, Marlborough was studied. Wētā density was assessed in vineyards, paddocks and shrublands in this valley. Soil moisture, penetration resistance, pH and organic matter were recorded at locations with and without wētā. Wētā density in vineyards was significantly higher than in either paddocks or shrub habitats. In vineyards, the density of this insect was significantly higher under-vines than in the inter-rows. Higher numbers of this wētā were found in moist soils that required lower force to burrow. Females laid an average of 55 eggs between March and April, which hatched in September. These findings highlight the intersection between agriculture and conservation.
Introduction
New Zealand has a diverse wētā fauna consisting of over 140 endemic species in the families Anostostomatidae and Rhaphidophoridae (Macfarlane et al. Citation2010). Many wētā are threatened by predation from introduced mammals, particularly stoats (Mustela erminea Linnaeus, 1758) and rats (Rattus norvegicus [Berkenhout, 1769], R. rattus [Linnaeus, 1758], R. exulans [Peale, 1848]; Towns & Broome Citation2003; Smith et al. Citation2005), as well as habitat degradation. The ‘wētā recovery plan’ was therefore developed to identify and protect those threatened (Sherley Citation1998). This plan categorised the different wētā species according to their conservation status based on the rankings of Molloy & Davis (Citation1994).
However, most wētā species in the genus Hemiandrus Ander, 1938 were classified as having an Indeterminate conservation status because of the paucity of information on their biology, density and distribution (Sherley Citation1998; Johns Citation2001). Hemiandrus comprises approximately 40 species, which are referred to as ‘ground wētā’, but only 11 of these have been described to date (Johns Citation1997, Citation2001; Jewell Citation2007; Taylor Smith et al. Citation2013). Each of the species in this genus is found at specific locations in the North and South Islands of New Zealand, although some species, such as H. maculifrons (Walker, 1869), occur on both islands (Pratt et al. Citation2008; Chappell et al. Citation2012). The habitat preference of some of these ground wētā is partially separated by elevation. For example, H. pallitarsis Walker, 1869 is found at lower altitudes than H. maculifrons (Walker, 1869) (Chappell et al. Citation2015). Studies involving other wētā species, for example Hemideina maori (Pictet & Saussure, 1891), H. thoracica (White, 1842) and H. crassidens (Blanchard, 1851), have also shown that wētā occupying particular habitat ranges are physiologically adapted to those microclimates (Ramlov Citation1999; Bulgarella et al. Citation2014). An understanding of other factors within a habitat that influence wētā distribution will be vital for protecting them, especially when they inhabit agricultural areas.
In the early 2000s, a then unknown species of ground wētā was found causing significant damage to vine buds in the Awatere Valley, Marlborough, leading to direct impact on vine yield (J Brady, Constellation Brands, pers. comm. 2014). Morphological examination and DNA barcoding of specimens collected from some of these vineyards confirmed that they were Hemiandrus sp. ‘promontorius’ (Johns, Citation2001) (; PM Johns, Canterbury Museum, pers. comm. 2015; J Nboyine, Lincoln University, unpubl. data). This species of wētā was first found between Marfells Beach (−41.7255° E, 174.2045° N) and Cape Campbell (−41.7372° S, 174.2760° E), Marlborough. It has a Naturally Uncommon threat status (Townsend et al. Citation2008; Trewick et al. Citation2012). There is no information on its density even at the above two localities (Johns Citation2001). Strategies for mitigating damage by this wētā in affected vineyards are needed; however, such management measures must not be lethal to the population because of its threat status. The success of any wētā management strategy hinges on availability of data on the numbers of this insect currently inhabiting vineyards and their biology. This will ensure to what extent declines in its numbers after adopting any management method can be ameliorated.
This study therefore contributes information on the density, distribution and biology of Hemiandrus sp. ‘promontorius’ () found in and around vineyards in the Awatere Valley. The work: 1. compares the densities of wētā in different habitats (vineyards, paddocks and shrublands) in the Awatere Valley; 2. determines their distribution within vineyards and the factors potentially affecting wētā presence; and 3. describes life history traits such as period of oviposition and sex ratios.
Materials and methods
Study sites and period
The study was conducted in the Awatere Valley, which is south of Blenheim, southeast of the Wairau Plains and north of Cape Campbell, Marlborough. The distance from Cape Campbell to the valley is 53 km. The study took place from 19 May 2014 to 6 November 2015.
This valley has a more extreme climate than most of Marlborough. The total annual rainfall is < 1000 mm and its mean minimum and maximum monthly air temperatures are 0.6 °C and 24.2 °C, respectively. It also has a mean monthly maximum wind speed of 78.3 km/hr (http://www.mrc.org.nz/category/weather-data/awatere-valley-dashwood-weather-data/).
The grape variety in the vineyards used for the study was sauvignon blanc, although wētā can also be found in vine blocks containing other varieties such as pinot noir.
Distribution and density of Hemiandrus sp. ‘promontorius’
Density in different habitats
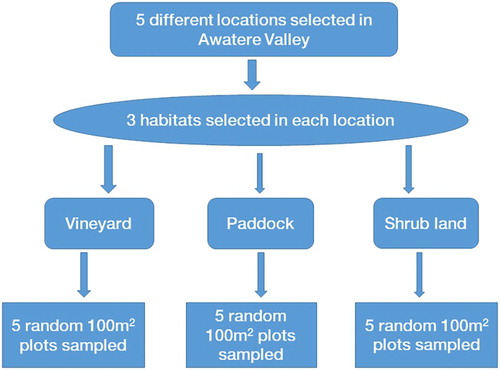
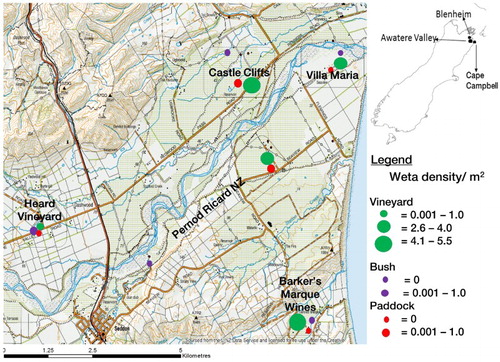
Densities of Hemiandrus sp. ‘promontorius’ in three habitat types (vineyards, paddocks and shrublands) commonly found in the Awatere Valley were estimated in January (summer) and November (spring) 2015, by searching for this insect and its burrows in each habitat. The shrublands were dominated by gorse (Ulex europaeus Linn.), gum tree (Eucalyptus sp. [L’Hèr.]), willow (Salix sp. Linn.), ngaio (Myoporum laetum Forst.), matagouri (Discaria toumatou Raoul) and cabbage tree (Cordyline australis [Forst.]). Five different locations (Castle Cliffs, Barker’s Marque Wines, Pernod Ricard New Zealand, Heard Vineyard and Villa Maria), which were at least 3 km apart, were used. At each of these locations, a single habitat of paddock, shrubland and vineyard were sampled. Thus, a total of 15 sampling sites (i.e. five locations × three habitats) were sampled for this insect during the study.
Within each of the 15 sites, five 100 m2 plots were randomly demarcated and carefully searched for wētā and their burrows (). The presence of the latter was determined by scraping off the top 5 mm soil layer. Grassy/weedy plots within each habitat were searched by clearing the grasses and/or weeds before scraping off the topsoil layer to a depth of 5 mm to expose all burrows present. Three burrows were randomly selected and dug within each plot and the numbers of wētā present were counted.
Wētā counts in each habitat were converted into density (i.e. number of wētā/m2). These data were subjected to randomised complete block analysis of variance (ANOVA) with location as the blocking factor and habitat as the treatment factor.
Distribution and seasonal pattern in vineyards
A stratified sampling method was used to assess the distribution of wētā in vineyards and their density fluctuations in different seasons (). Six vineyard blocks located at Caseys Road, The Favourite and Castle Cliffs (see for vineyard details) were sampled in May (autumn), July (winter), and October (spring) of 2014 and January 2015 (summer).
Table 1. Names and locations of vineyard blocks used to monitor seasonal wētā densities.
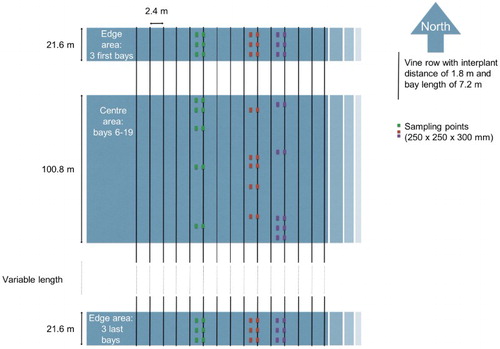
Three vine rows and their adjacent inter-rows were randomly selected for sampling on each of the dates and for each of the six blocks. Vines were planted at inter-vine and inter-row spacing of 1.8 m and 2.4 m, respectively. Within each row, bays were 7.2 m long and comprised four vines. The selected rows, which ran south to north in all vineyard blocks, were divided into ‘edge’ and ‘centre’. The ‘edge’ consisted of the first three complete bays and it was sampled by digging a 250 × 250 × 300 mm (length × breadth × depth) hole in the middle of each bay and its corresponding point in the middle of the inter-row on the east side of the sampled row.
The ‘centre’ consisted of the area between bays 6 and 19 in the row. The under-vines and inter-rows of five randomly chosen bays in this area were sampled by digging as described for the edge. A total of 48 samples (i.e. three rows × 16 samples/row) were taken per vineyard in four seasons and sampling was conducted from the south to north end.
All excavated holes were carefully searched for the presence of wētā and their eggs. This information was considered fundamental to understanding the biology of this pest. Adults were sexed using the general descriptions for male and female ground wētā (Van Wyngaaden Citation1995). The number of females brooding eggs was recorded and numbers of eggs/female were counted. The females and eggs were returned to the soil afterwards.
Data from each season were converted into means of the numbers of wētā/m2 for under-vines, inter-rows, edge and centre, and overall weighted means were calculated. Tests for statistical significances of mean numbers of wētā/m2 at the different dates and sampling positions within vineyard blocks were determined by computing the 95% confidence intervals.
Statistical differences between the numbers of male and female wētā were determined by testing the null hypothesis, H0: the proportion of males = 0.5, using the Minitab 17 statistical program. Chi-squared tests were performed to determine if the sex ratio and proportion of brooding females changed with time.
Measurement of soil properties
The soil variables measured were considered appropriate to the study locations, which were all close together in the Awatere Valley, with negligible variation in slope and altitude. Therefore, the latter variables were not considered.
Volumetric soil moisture
This was measured in each of the vineyard blocks using a Delmhorst KS-D1 Digital Soil Moisture Tester. The meter was calibrated to measure moisture up to 200 mm below the soil surface. The probe of the moisture meter was inserted next to each hole dug to a depth of 50 mm.
Soil pH
The soil pH in the vineyard blocks was measured by collecting soil to a depth of 200 mm. The soil was placed in zipped plastic bags and immediately frozen to stop any chemical or biological processes that could alter the pH. Forty-eight soil samples were collected from each of the six vineyard blocks.
In the laboratory, soil subsamples (> 10 g) were taken from the contents of each bag and emptied into individually labelled plastic trays; they were dried in an oven at 25 °C for 48 h. The soils were then ground and 10 g of each sample were transferred to clean plastic vials. Deionised water (25 mL) was added to the contents of each vial, after which they were left on the laboratory bench for 24 h. The pH of each subsample was measured with an Orion Star A211 pH, mV, ORP and temperature bench-top meter.
Soil organic matter
After pH measurements were taken, the remaining soil was used to determine organic matter content. This was done by weighing 10–20 g of those soils into labelled crucibles and then drying them in an oven at 105 °C for 24 h. The dried soils were cooled in a desiccator for 30 min before they were weighed and then burned in a furnace at 500 °C for 5 h. Organic matter content was computed based on the weight loss after burning (Blakemore et al. Citation1987).
Resistance to soil penetration
This was measured at each of the points sampled in the vineyard blocks using the 3 cm3 cone of the static Eijkelk cone penetrometer. A total of 48 readings were recorded/vineyard block/season. A constant penetration velocity of approximately 30 mm/s (American Society of Agricultural Engineers Citation1998) was used to drive the cone into the soil to a depth of 15 cm. The force required to push it to this depth was recorded in kN.
Data analysis
The statistical software GENSTAT v16.0 was used to perform regressions of relationships between the density of wētā and soil properties. Density was the dependent variable and the soil properties were the independent variables.
Results
Density and distribution of Hemiandrus sp. ‘promontorius’
Density of wētā in different habitats
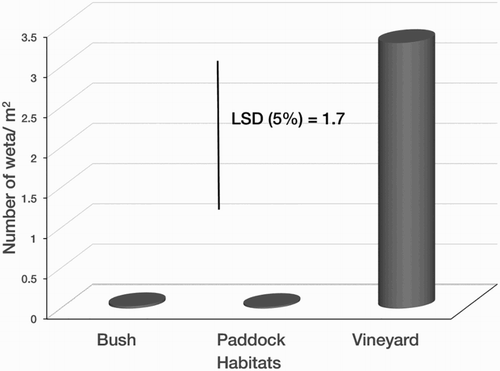
shows the density of wētā in each of the 15 sites sampled. The mean wētā density in January (summer) was not significantly different from that in November (spring). However, habitat significantly affected the density of the insect (P < 0.001). The highest mean density over the two seasons (i.e. summer and spring) was recorded in vineyards (3.3 individuals/m2) while the lowest was in paddocks (0.02 individuals/m2). Mean density in the latter habitat was not significantly different from that of shrublands (0.03 individuals/m2) ().
Distribution and seasonal pattern in vineyards
shows the wētā density at different dates and sampling positions within vineyards. Density was not significantly different between the edge and centre of the vineyards on any date. In May 2014 there was no significant difference between under-vine and inter-row densities. However, the density was significantly higher under-vines than in the inter-row from July 2014 to January 2015. The weighted mean density was significantly higher in January 2015 than in May, July and October 2014.
Table 2. Mean density of Hemiandrus sp. ‘promontorius’ at different locations in six vineyard blocks.
Egg laying and sex ratios
Of 74 adult wētā sexed in October 2014, 47.3% were males and the rest were females. In January 2015, 36.4% of the 77 insects were males and 36.7% (11) of those sexed in March 2015 were males. The observed proportion of males was not significantly different to that of females (P = 0.050, 95% CI for percentage of males = 33.76%–50.02%). The sex ratio in October was not significantly different from that in January ((χ2 = 1.4) and March ((χ2 = 0.59). There was no significant difference in sex ratio between January and March ((χ2 = 0.04).
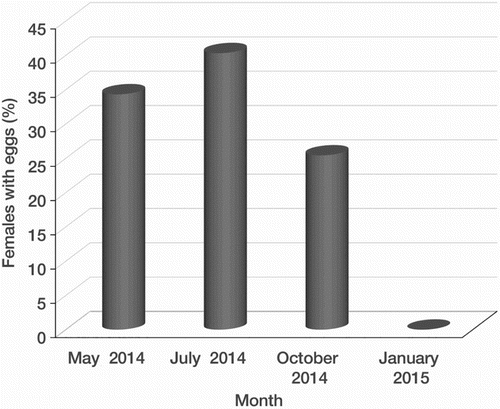
Forty-four of the 149 females collected from May to October 2014 were found brooding eggs, but none was found in January 2015 (). The proportion of brooding females varied significantly with the seasons and was highest in July 2014 (40.3%) and lowest in January 2015 (zero) (χ2 = 10.2; P < 0.01). The proportion of females with eggs in October (25.4%) was not significantly different from that in July (40.3%, χ2 = 1.23) and May (34.3%, χ2 = 0.21), but these were significantly higher than in January 2015. The mean number of eggs per female was 55 ± 5 (n = 68).
Relationship between wētā density and soil properties
There was a significant positive relationship between wētā density and soil moisture (P = 0.018; A). The latter accounted for 44% of the fitted regression model. Wētā density was significantly and inversely related to soil compaction (P = 0.010, R2 = 0.4972; B). Soil moisture content was significantly and inversely related to compaction (P < 0.001, R2 = 0.8116).
Figure 7. Relationship between the density of wētā and soil properties. A, Wētā density versus moisture content (%); B, wētā density versus resistance to soil penetration (kN); C, wētā density versus organic matter (%); D, wētā density versus pH.
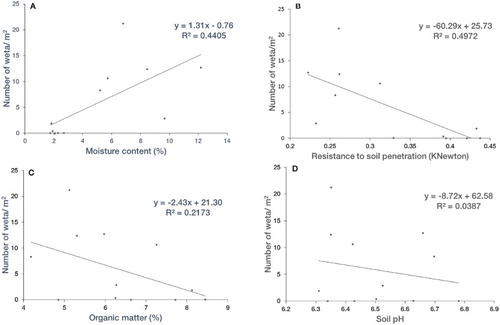
Wētā density was not significantly related to soil organic matter (P = 0.127, R2 = 0.2173) and pH (P = 0.540, R2 = 0.0387) (C–D). Similarly, there was no significant relationship between soil moisture and either organic matter content (P = 0.250, R2 = 0.1299) or pH (P = 0.211, R2 = 0.0098).
Discussion
Density and distribution of wētā
The wētā Hemiandrus sp. ‘promontorius’ was first discovered at Cape Campbell and Marfells Beach, Marlborough (Johns Citation2001). Later, Trewick et al. (Citation2012) reported the existence of populations of this species in other locations. Recently, further searching has revealed a population of this wētā in the Awatere Valley, Marlborough. Of three major habitats sampled here (paddocks, shrublands and vineyards), the density of this wētā was about 100 times higher in vine than in non-vine habitats. Because this species is omnivorous (Johns Citation2001), habitat choice might have been influenced by availability of plant and animal components of its diet. The inter-rows of vineyards were sown and maintained with grasses. Weeds also grew between these grasses and occasionally under the vines where irrigation water sustains their physiological growth and functioning (Jones Citation2004; Cifre et al. Citation2005; Dalley et al. Citation2006) even in the dry summer and autumn. Wētā and other arthropod herbivores therefore have access to plant food throughout the year. The former also preyed on some of the latter (e.g. Collembolla, Coleoptera, Diptera) to further satisfy its animal protein requirement (Wahid Citation1978; Cary Citation1983; Van Wygaarden Citation1995). The year-round availability of food probably contributed to the high wētā numbers in these vineyards. The arid nature of the non-vine habitats, especially in summer and autumn, potentially reduced the availability of food required for their survival. This may have contributed to the low wētā numbers recorded in those habitats.
The mean density of Hemiandrus sp. ‘promontorius’ in vineyards was estimated at 3.0/m2 and 6.4/m2 in the two studies reported herein. The absence of prior information on the density of this insect in the Awatere Valley or elsewhere makes deductions on any population change difficult. But the conversion of these lands from paddocks and/or shrublands (Gillingham Citation2012) to vineyards does not seem to have adversely affected their survival. This is because the estimated population size for this wētā was higher than the 1.8/m2 and 3.0/m2 reported for the ground wētā H. maia Taylor Smith, 2013 and H. electra Taylor Smith, 2013, respectively, in non-agricultural lands (Taylor Smith et al. Citation2013). Given the size of the population and the significant damage on vines, this species can be considered as a pest in the Awatere Valley vineyards.
A population of the same species has recently been observed causing damage in vineyards in the Wairau Valley, Marlborough 63 km northwest of the Awatere Valley (J Brady, Constellation Brands, pers. comm. 2015; PM Johns, Canterbury Museum, pers. comm. 2015). It is most likely that this wētā was present in these two valleys before the vines were planted but their feeding damage to the vines was not noticed initially because their numbers were low. The vines in the Awatere Valley were planted in the late 1980s but economic damage by this insect was first observed in the early 2000s. This probably suggests that their numbers have increased over time, resulting in their feeding damage becoming noticeable. Another species in this genus, Hemiandrus sp. ‘horomaka’ was reported as a pest in apricot orchards at Horotane Valley, Christchurch (Wahid Citation1978). These observations could indicate that the feeding activity of wētā in the genus Hemiandrus makes them potential pests when their native habitat is converted to agricultural land.
In the vineyards studied Hemiandrus sp. ‘promontorius’ was present throughout the year. This indicates that wētā is adapted to the routine seasonal vineyard management practices (Wardle et al. Citation1999; Siqueira et al. Citation2014). Its density was, however, lower in autumn, winter and spring than in summer when most of the individuals recorded were nymphs. Similar density fluctuation has been observed in other univoltine insects in the order Orthoptera (Mariottini et al. Citation2011) and this is an important determinant of the potential threat to agricultural crops by pest species.
In vineyards, wētā density was higher under-vines than in the inter-rows. The former were bare and there was sparse plant debris on the soil surface, unlike the inter-rows that were densely covered with a mixture of grass species (Lolium perenne Linn., Festuca pratensis Huds., Poa annua Linn.). Ground wētā preferentially make their burrows in open ground under shrubs, grasses and trees (Johns Citation2001; Taylor Smith et al. Citation2013). The presence of large areas of bare soil under-vines therefore contributed to the high density of this species. Its density was, however, the same between the edges and centres of vineyards.
This Naturally Uncommon wētā whose habitat has been converted to vineyards should be protected from viticultural practices (e.g. pesticide applications) that can potentially harm it. This will prevent its numbers from declining to a level that will require translocation to protected areas for conservation purposes. The viticulture industry and Department of Conservation (DOC) can work together to achieve this outcome.
Oviposition and sex ratios
In summer, male Hemiandrus exit their burrows and sit on leaves where they use pheromones to attract mates from long distances. At short ranges males attract females by drumming their abdomen onto a substrate (Gwynne Citation2004). After mating, female Hemiandrus sp. ‘promontorius’ begin ovipositing in March and this could extend into May depending on when mating occurred (J Nboyine, pers. obs.). Eggs were seen in vineyards from May to early October without any obvious changes in density. Other ground wētā (e.g. Hemiandrus sp. ‘horomaka’, H. pallitarsis, etc.) have been found brooding eggs at similar periods (Wahid Citation1978; Gwynne Citation2004). The mean number of eggs per female in vineyards was 55. This was higher than the 30 eggs per female reported by Gwynne (Citation2004) for the same species, possibly because observations in the current work were made on field populations rather than captive ones, as used by Gwynne (Citation2004). In the present work, nymphs were first seen in late September for this species and their emergence was estimated to begin at least 5 months after oviposition, though eggs of other ground wētā can hatch after 4 months (Wahid Citation1978; Gwynne Citation2004).
The sex ratio did not differ from autumn, spring and summer. Previous studies of the sex ratio of wētā in the family Anostostomatidae Saussure, 1859 mostly concluded that populations were either male- or female-biased. For example, the tusked wētā Motuwētā riparia Gibbs, 2002 (McCartney et al. Citation2006) and the stone wētā Hemideina maori (Pictet & Saussure, 1891) were female-biased (Joyce et al. Citation2004), while populations of a ground wētā Hemiandrus maculifrons were male-biased (Chappell et al. Citation2014a). These ratios, however, were probably skewed due to sampling error (Wehi et al. Citation2011). This can be avoided when decisions on methods and time of sampling are based on an analysis of species behaviour. For instance, sexually active male and female ground wētā actively exit their burrows for mating during the breeding season and will therefore be easily trapped or sighted during night searches (Gwynne Citation2004; Chappell et al. Citation2014a). Sex ratios estimated with these methods and at such periods are likely to be 50:50. After mating, males continue to exit their burrows and forage actively but the activity of females depends on their degree of maternal care. Those that exhibit maternal care (i.e. species with short ovipositor such as Hemiandrus sp. ‘promontorius’) are mostly occupied tending their eggs and seldom exit their burrow, unlike species that do not show maternal care (i.e. species with long ovipositor; Gwynne Citation2004). Thus, females with a short ovipositor will be less frequently trapped or sighted than the males of the same species and sex ratios will be erroneously skewed in favour of males when estimated during this period.
Relationship between wētā densities and soil properties
The density of wētā was higher at locations with low soil penetration resistance. Soils with high resistance to penetration are difficult to dig and are prone to flooding after precipitation due to reduced infiltration rate (Hamza & Anderson Citation2005). Wētā will therefore require more effort to burrow such areas. Such soils are also less well aerated and this does not support the survival of soil organisms (Lipiec & Stepniewski Citation1995). The wētā may have avoided these conditions. Generally, soils in the inter-rows required higher force of penetration than those under-vines. This was probably caused by farm machinery and by grazing farm animals being used for weed management (Lipiec & Stepniewski Citation1995; Whalley et al. Citation1995; Hamza & Anderson Citation2005).
Soil moisture was another important factor that determined the distribution of the wētā species studied here. Work on another ground wētā species Hemiandrus sp. ‘horomaka’ in apricots also found that it inhabited mainly moist areas (Wahid Citation1978). This could be due to the influence of moisture on the availability of prey and the presence of its desired plant food (Brust & House Citation1990; Chikoski et al. Citation2006; Powell et al. Citation2007; Mariottini et al. Citation2011). In addition, moisture is needed for egg development and hatching in ground wētā (Wahid Citation1978) and in some grasshopper species that lay their eggs in the soil (Mariottini et al. Citation2011). Consequently, newly hatched nymphs do not disperse over long distances but build their burrows at close proximity, resulting in increased density over time.
The density of this insect was not related to either soil organic matter or pH. These parameters were relatively uniform both within and between vineyards. The uniformity of pH values was because of the application of lime to soils (Baath et al. Citation1980) in vineyards prior to their establishment. In a related study, pH had no effect on the density of a soil burrowing cricket Gryllotalpa major Saussure, 1874 (Hill et al. Citation2009). Soil organic matter is also generally uniform for a given crop cover and cultivation practices on a farm (Parton et al. Citation1987; Burke et al. Citation1989). These did not change within and between vineyards, and were therefore not correlated with wētā distribution.
In conclusion, this study provides fundamental information on the density and biology of Hemiandrus sp. ‘promontorius’ in vine and non-vine habitats in the Awatere Valley. The conversion of the habitat of this insect into vineyards has not adversely affected its numbers. Densities were, in fact, higher in vine than non-vine habitats, causing significant economic damage as they fed on vine buds. These findings should assist the DOC and vineyard managers to make informed decisions about the management of this species. Native species becoming agricultural pests after conversion of their natural habitat has been observed in many other taxa (e.g. Lefort et al. Citation2014). However, because wētā are iconic animals, and this particular species is Naturally Uncommon, it is essential to devise management measures that preserve the population while limiting damage to vines. For example, interventions could target only under-vine areas and/or those soils with low compaction and/or high soil moisture. Further work is needed to estimate the population size of this wētā in the Wairau Valley, Marlborough and other locations.
Acknowledgements
The authors gratefully acknowledge Ms Joanne Brady and the entire staff and management of Constellation Brands New Zealand for their support during the study. We are also grateful to Pernod Ricard New Zealand—Seaview Vineyard, Villa Maria, Barker’s Marque Wines—Muritai Vineyard, Treasury Wine Estate—Matua Vineyard and Heard Vineyard for allowing us to search their properties for wētā. Special thanks to Peter M Johns for assisting with morphological identification of the specimens. The suggestions of the anonymous reviewers are greatly appreciated.
Associate Editor: Dr Cor Vink.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- American Society of Agricultural Engineers. 1998. ASAE S313.3, standard soil cone penetrometer, ASAE Standard. 45th ed. St. Joseph: American Society of Agricultural Engineers; p. 820–821.
- Baath E, Berg B, Lohm U, Lundgren B, Lundkvist H, Rosswall T, Soderstrom B, Wiren A. 1980. Effects of experimental acidification and liming on soil organisms and decomposition in a Scots pine forest. Pedobiologia. 20:85–100.
- Blakemore LC, Searle PL, Daly BK. 1987. Methods for chemical analysis of soils. Lower Hutt: New Zealand Soil Bureau.
- Brust GE, House GJ. 1990. Effect of soil moisture, no-tillage and predators on Southern Corn Rootworm (Diabrotica undecimpunctata howardi) survival in corn agro-ecosystem. Agr Ecosyst Environ. 31:199–215. doi: 10.1016/0167-8809(90)90220-8
- Bulgarella M, Trewick SA, Minards NA, Jacobson MJ, Morgan-Richards M. 2014. Shifting ranges of two tree wētā species (Hemideina sp.): competitive exclusion and changing climate. J Biogeogr. 41:524–535. doi: 10.1111/jbi.12224
- Burke K, Yonker CM, Parton WJ, Cole CV, Flach K, Schimel DS. 1989. Texture, climate and cultivation effects on soil organic matter content in U.S. grassland soil. Soil Sci Soc Am J. 53:800–805. doi: 10.2136/sssaj1989.03615995005300030029x
- Cary PRL. 1983. Diet of the ground wētā Zealandosandrus gracilis (Orthoptera: Stenopelmatidae). New Zeal J Zool. 10:295–297. doi:10.1080/03014223.1983.10423918
- Chappell EM, Trewick SA, Morgan-Richards M. 2012. Shape and sound reveal genetic cohesion not speciation in New Zealand orthopteran Hemiandrus pallitarsis despite high mitochondrial DNA divergence. Biol J Linn Soc. 105:169–186. doi: 10.1111/j.1095-8312.2011.01777.x
- Chappell EM, Webb DS, Brown AJ, Tonkin JD. 2015. A preliminary survey of altitudinal variation in two ground wētā species, Hemiandrus maculifrons (Walker) and Hemiandrus pallitarsis (Walker) (Orthoptera: Anostostomatidae). New Zeal Entomol. 38:1–6. doi:10.1080/00779962.2014.922234
- Chappell EM, Webb DS, Tonkin JD. 2014a. Notes on sexual size dimorphism, sex ratio and movements of adult ground wētā Hemiandrus maculifrons (Walker) (Orthoptera: Anostostomatidae). New Zeal Entomol. 37:83–92. doi:10.1080/00779962.2013.856377
- Chikoski JM, Ferguson SH, Meyer L. 2006. Effect of water addition on soil arthropods and soil characteristics in a precipitation-limited environment. Acta Oecol. 30:203–211. doi: 10.1016/j.actao.2006.04.005
- Cifre J, Bota J, Escalona JM, Medrano H, Flexas J. 2005. Physiological tools for irrigation scheduling in grapevine (Vitis vinifera L.). An open gate to improve water-use efficiency? Agr Ecosyst Environ. 106:159–170. doi:10.1016/j.agee.2004.10.005
- Dalley CD, Bernards ML, Kells JJ. 2006. Effect of weed removal timing and row spacing on soil moisture in corn (Zea mays). Weed Technol. 20:399–409. doi: 10.1614/WT-05-098R.1
- Gillingham A. 2012. Soils and regional land use—northern and western South Island [Internet]. Te Ara—the Encyclopedia of New Zealand; [cited 2015 Dec 14]. Available from: http://www.TeAra.govt.nz/en/photograph/17103/-awatere-valley-soils
- Gwynne DT. 2004. Reproductive behaviour of ground wētā (Orthoptera: Anostostomatidae): drumming behaviour, nuptial feeding, postcopulatory guarding and maternal care. J Kansas Entomol Soc. 77:414–428. doi: 10.2317/E-34.1
- Hamza MA, Anderson WK. 2005. Soil compaction in cropping systems. A review of the nature, causes and possible solutions. Soil Till Res. 82:121–145. doi: 10.1016/j.still.2004.08.009
- Hill PSM, Deere JPHH, Fancher J, Howard DR, Tapp JB. 2009. Burrow aggregation of prairie mole cricket, Gryllotalpa major Saussure (Orthoptera: Gryllotalpidae) males is not based on soil microhabitat constraints at lek sites in Oklohama. J Kansas Entomol Soc. 82:122–134. doi: 10.2317/JKES503.21.1
- Jewell T. 2007. Two new species of Hemiandrus (Orthoptera: Anostostomatidae) from Fiordland National Park, New Zealand. Zootaxa. 1542:49–57.
- Johns PM. 1997. The Gondwanaland wētā: family Anostostomatidae (formerly in Stenopelmatidae, Henicidae or Minermidae): nomenclatural problems, world checklist, new genera and species. J Orthoptera Res. 6:125–138. doi: 10.2307/3503546
- Johns PM. 2001. Distribution and conservation status of ground wētā, Hemiandrus species (Orthoptera: Anostostomatidae). Science for Conservation 180. Wellington: New Zealand Department of Conservation.
- Jones HG. 2004. Irrigation scheduling: advantages and pitfalls of plant-based methods. J Exp Bot. 55:2427–2436. doi:10.1093/jxb/erh213
- Joyce SJ, Jamieson IG, Barker R. 2004. Survival of adult mountain stone wētā Hemideina maori (Orthoptera: Anostostomatidae) along an altitude gradient as determined by mark-recapture. New Zeal J Ecol. 28:55–61.
- Lefort M-C, Boyer S, De Romans S, Glare T, Armstrong K, Worner S. 2014. Invasion success of a scarab beetle within its native range: host range expansion versus host-shift. PeerJ. 2:e262. doi: 10.7717/peerj.262
- Lipiec J, Stepniewski W. 1995. Effect of soil compaction and tillage systems on uptake and losses of nutrients. Soil Till Res. 35:37–52. doi: 10.1016/0167-1987(95)00474-7
- Macfarlane RP, Maddison PA, Andrew IG, Berry JA, Johns PM, Hoare RJB, Larivière M-C, Greenslade P, Henderson RC, Smithers CN, Palma RL, Ward JB, Pilgrim RLC, Towns DR, McLellan I, Teulon DAJ, Hitchings TR, Eastop VF, Martin NA, Fletcher MJ, Stufkens MAW, Dale PJ, Burckhardt D, Buckley TR, Trewick SA. 2010. Phylum Arthropoda subphylum Hexapoda: Protura, springtails, Diplura, and insects. In: Gordon DP editor. New Zealand Inventory of Biodiversity Volume Two: Kingdom Animalia: Chaetognatha, Ecdysozoa, Ichnofossils. Christchurch: Canterbury University Press, p. 233–467.
- Mariottini Y, De Wysiecki ML, Lange CE. 2011. Seasonal occurrence of life stages of grasshopper (Orthoptera: Acridoidea) in the Southern Pampas, Argentina. Zool Stud. 50:737–744.
- McCartney J, Armstrong DP, Gwynne DT, Kelly CD, Barker RJ. 2006. Estimating abundance, age structure and sex ratio of a recently discovered New Zealand tusked wētā Motuwētā riparia (Orthoptera, Anostostomatidae), using mark-recapture analysis. New Zeal J Ecol. 30:229–235.
- Molloy J, Davis A. 1994. Setting priorities for the conservation of New Zealand’s threatened plants and animals. 2nd ed. Wellington: Department of Conservation.
- Parton WJ, Schimel DS, Cole CV, Ojima DS. 1987. Analysis of factors controlling soil organic matter levels in Great Plains grasslands. Soil Sci Soc Am J. 51:1173–1179. doi: 10.2136/sssaj1987.03615995005100050015x
- Powell LR, Berg AA, Johnson DL, Warland JS. 2007. Relationships of pest grasshopper populations in Alberta, Canada to soil moisture and climate variables. Agr Forest Meteorol. 144:73–84. doi: 10.1016/j.agrformet.2007.01.013
- Pratt RC, Morgan-Richards M, Trewick SA. 2008. Diversification of New Zealand wētā (Orthoptera: Ensifera: Anostostomatidae) and their relationships in Australasia. Philos T R Soc B. 363:3427–3437. doi: 10.1098/rstb.2008.0112
- Ramlov H. 1999. Microclimate and variation in haemolymph composition in freezing-tolerant New Zealand alpine wētā, Hemideina maori Hutton (Orthoptera: Stenopelmatidae). J Comp Physiol B. 109:224–235.
- Sherley GH. 1998. Threatened wētā recovery plan: Threatened species recovery plan no. 25. Wellington: Department of Conservation.
- Siqueira GM, Silva EFF, Paz-Ferreiro J. 2014. Land use intensification effects in soil arthropod community of an entisol in Pernambuco State, Brazil. Sci World J. 2014:1–3. doi:10.1155/2014/625856
- Smith DHV, Jamieson IG, Peach RME. 2005. Importance of ground wētā (Hemiandrus spp) in stoat (Mustela erminea) diet in small montane valleys and alpine grasslands. New Zeal J Ecol. 29:207–214.
- Taylor Smith BL, Morgan-Richards M, Trewick SA. 2013. New Zealand ground wētā (Anostostomatidae: Hemiandrus): descriptions of two species with notes on their biology. New Zeal J Zool. 40:314–329. doi:10.1080/03014223.2013.804422
- Towns DR, Broome KG. 2003. From small Maria to massive Campbell: forty years of rat eradications from New Zealand islands. New Zeal J Zool. 30:377–398. doi:10.1080/03014223.2003.9518348
- Townsend AJ, de Lange PJ, Duffy CAJ, Miskelly CM, Molloy J, Norton DA. 2008. New Zealand threat classification system manual. Wellington: Department of Conservation.
- Trewick SA, Morris SJ, Johns PM, Hitchmough RA, Stringer IAN. 2012. The conservation status of New Zealand Orthoptera. New Zeal Entomol. 35:131–136. doi: 10.1080/00779962.2012.686318
- Van Wyngaarden F. 1995. The ecology of the Tekapo ground wētā (Hemiandrus new sp.; Orthoptera:Anostostomatidae) and recommendations for the conservation of a threatened close relative [MSc thesis]. Christchurch: University of Canterbury.
- Wahid MB. 1978. The biology and economic impact of the wētā, Hemiandrus sp. (Orthoptera: Stenopelmatidae) in an apricot orchard, Horotane Valley [MSc thesis]. Christchurch: University of Canterbury.
- Wardle DA, Nicholson KS, Bonner KI, Yeates GW. 1999. Effects of agricultural intensification on soil-associated arthropods population dynamics, community structure, diversity and temporal variability over a seven-year period. Soil Biol Biochem. 31:1691–1706. doi: 10.1016/S0038-0717(99)00089-9
- Wehi PM, Nakagawa S, Trewick SA, Morgan-Richards M. 2011. Does predation result in adult sex ratio skew in a sexually dimorphic insect genus? J Evolution Biol. 24:2321–2328. doi:10.1111/j.1420-9101.2011.02366.x
- Whalley WR, Dumitru E, Dexter AR. 1995. Biological effects of soil compaction. Soil Till Res. 35:53–68. doi: 10.1016/0167-1987(95)00473-6