ABSTRACT
Taxonomy lies at the heart of species conservation, yet many large New Zealand orthopterans remain undescribed. Among New Zealand’s anostostomatid wētā, Hemiandrus (ground wētā) is the most speciose genus but also the most poorly characterised and thus most in need of taxonomic and ecological work. Here we redescribe H. maculifrons and describe two new species of ground wētā previously encompassed by the specific name Hemiandrus maculifrons: Hemiandrus luna sp. nov. and H. brucei sp. nov. We also describe a morphologically similar and related species, Hemiandrus nox sp. nov.
http://zoobank.org/urn:lsid:zoobank.org:pub:71EA0879-A2F9-46B2-A105-E97A9AB25061
http://zoobank.org/References/71EA0879-A2F9-46B2-A105-E97A9AB25061
Introduction
Many species of wētā (Insecta: Orthoptera: Anostostomatidae and Rhaphidophoridae) are abundant in New Zealand forests, but many are undescribed (Brockie Citation1992; Johns Citation1997; Fitness et al. Citation2015). New Zealand Anostostomatidae (true wētā) comprises three main lineages, giant and tree wētā (Deinacrida White, Citation1842; Hemideina White, Citation1846), tusked wētā (Anisoura Ander, Citation1938, Motuweta Johns, Citation1997) and ground wētā (Hemiandrus Ander, Citation1938) (Johns Citation1997; Trewick & Morgan-Richards Citation2004; Trewick & Morgan-Richards Citation2005). Together, they comprise some 60 species that occupy diverse habitats from lowland forest to the alpine zone. Among them, the ground wētā are putatively the most speciose but also the most poorly characterised. Additional taxonomic and ecological work on Hemiandrus is needed to test the reported diversity of this genus. There are currently 11 species described to date (Johns Citation1997, Citation2001; Jewell Citation2007; Taylor-Smith et al. Citation2013); however, dozens of ‘tag-names’ exist (Johns Citation2001; Jewell Citation2007) indicating entities that may be a separate species or populations of uncertain taxonomic rank (Leschen et al. Citation2009). In addition, an estimated 10 undescribed species of Hemiandrus are endemic to Australia (Johns Citation1997), although molecular data indicate that at least some of these are not part of a monophyletic clade with the New Zealand Hemiandrus (Pratt et al. Citation2008).
Ground wētā are found throughout the North and South Islands, as well as on numerous offshore islands. Most species have restricted ranges but H. maculifrons has a range that extends across both main islands and is considered to be the most widespread ground wētā and New Zealand anostostomatid. It has been reported throughout much of the South Island and in the North Island in Taranaki, the Kaweka Ranges, Tongariro National Park (Johns Citation2001), the western Tararua Ranges (Spurr & Berben Citation2004), the Orongorongo Valley (Moeed & Meads Citation1985) and on the Coromandel Peninsula (Chappell et al. Citation2014a, Citation2014b).
Pratt et al. (Citation2008) found that H. maculifrons comprises multiple clades separated by high genetic distances. Further genetic and morphological analyses have shown that H. maculifrons is not a single species (BLTS, MMR, SAT, Massey University, unpubl. data). Here we redescribe H. maculifrons and introduce two new species previously encompassed by the specific name H. maculifrons: Hemiandrus luna sp. nov. and Hemiandrus brucei sp. nov. The morphological similarity of these three species explains why they were previously treated as a single taxon. We also describe a sympatric, morphologically similar close relative that is easily mistaken for a species of the H. maculifrons species complex: Hemiandrus nox sp. nov. These four Hemiandrus species are found in primary native forest throughout New Zealand where they are often sympatric with one another and with other ground wētā species.
Materials and methods
Taxonomic methods
Specimens were examined from a variety of sources: Department of Conservation (DOC) pitfall traps (Sinclair & Stringer Citation2003), New Zealand Arthropod Collection (NZAC), Auckland Museum (AMNZ) and the Phoenix Lab insect collection at Massey University, Palmerston North. All specimens were dry or preserved in 70%–95% ethanol. Specimens were examined using an Olympus SZX7 Zoom Stereomicroscope with an attached SC100 digital camera.
We distinguished the specimens described herein from all other Hemiandrus species based on adult female morphology (see ‘key to species’ in Discussion), the presence of microsetae on the entire fourth segment of the maxillary palps, the number of tibial spines and the shape of the ninth abdominal tergite (see Johns Citation2001). One hundred and eighty-two female wētā and 73 male wētā (total 255) were measured and examined for their morphology. Males were assessed to be adults based on the presence of dark, sclerotised hooks (falci) beneath the ninth abdominal tergite, whereas females were assessed to be adults based on the shape of the subgenital plate and the sclerotisation of the ovipositor (Cary Citation1981). Body length (partial body length: length from frons to distal margin of metanotum), head width, head length, pronotum length, pronotum width, femur width, femur length, ovipositor length and subgenital plate length measurements were made using digital callipers. The hind tibial spines were counted on each leg of each individual and then averaged for each of superior prolateral spines, superior retrolateral spines and inferior spines. The stridulatory pegs of the inner hind femur and the first three abdominal tergites on the right hand side of each wētā were counted. Photographs of male terminalia, female ovipositors, mid and hind tibiae, and pronota were captured using the Olympus Image Analysis Software. Photographs of heads and whole specimens were captured using a Canon EOS 40D digital camera. Specimens from additional locations were examined to assess the distributions of each species and distributions were mapped with the software ArcMap from ArcGIS 10.1 (ESRI).
Abbreviations
Prefixes: GW is used for ground wētā in the Phoenix Lab collection (Massey University, Palmerston North); DOCORD, DOC pitfall specimens; AMNZ, Auckland Museum specimens; NZAC, New Zealand Arthropod Collection specimens; FD is used for a single specimen collected by Eric Edwards (DOC) from Fiordland; MONZ, Museum of New Zealand Te Papa Tongarewa.
Anatomy ( in Taylor-Smith et al. Citation2013): BL, partial body length; HW, head width; HL, head length; PL, pronotum length; PW, pronotum width; FW, femur width; FL, femur length; OV, ovipositor length; SG, subgenital plate length; T1, first abdominal tergite; T2, second abdominal tergite; T3, third abdominal tergite; T7, seventh abdominal tergite; T8, eighth abdominal tergite; T9, ninth abdominal tergite; T10, tenth abdominal tergite; MP3, third segment of the maxillary palps; MP4, fourth segment of the maxillary palps; MP5, fifth segment of the maxillary palps.
Area codes (Crosby et al. Citation1976): SD, Marlborough Sounds; MB, Marlborough; NN, Nelson; BR, Buller; NC, North Canterbury; MC, Mid Canterbury; WD, Westland; FD, Fiordland; OL, Otago Lakes; CO, Central Otago; DN, Dunedin; SL, Southland; TK, Taranaki; BP, Bay of Plenty; GB, Gisborne; HB, Hawke’s Bay; RI, Rangitikei; WN, Wellington; ND, Northland; AK, Auckland; WO, Waikato; CL, Coromandel; TO, Taupo; WI, Whanganui.
Hemiandrus species descriptions
Class InsectaOrder OrthopteraSuborder EnsiferaSuperfamily StenopelmatoideaFamily Anostostomatidae Saussure (1859)Genus Hemiandrus Ander (1938)
Type species: Hemiandrus furcifer Ander (Citation1938)
Redescription of Hemiandrus maculifrons
Hemiandrus maculifrons was originally described by Walker (Citation1869). Johns (Citation1997) synonymised two described Hemiandrus species, H. maori (Pictet and Saussure Citation1893) and H. gracilis (Salmon, Citation1950), with H. maculifrons.
The holotype for H. maculifrons (Libanasa (?) maculifrons) (Walker, Citation1869) is from an unknown New Zealand location and is damaged, discoloured and lacking diagnostic features. Walker’s species description could be applied to multiple ground wētā species. However, Walker gave the length of this specimen as 7.5 lines (approximately 16 mm). Only some populations of the species with the southern-most geographic distribution of this complex have such a large body size, indicating that the species he described was the southern-most species.
Pictet & Saussure (Citation1893) based their description on specimens found near Mt Cook (Hutton Citation1897), which falls within the range of the southern-most species. Furthermore, they state that the ovipositor is the length of the hind femur and that two small spines are positioned approximately midway on the inferior side of the hind tibiae (). These features are consistent with the species with southern-most geographic distribution. Salmon (Citation1950) also based his description on species collected from Southland, Westland, Fiordland and Mt Arthur, which are all localities with populations of the southern species of H. maculifrons. Therefore, although H. maculifrons is a species complex, all three of these species descriptions appear to have described the same species of this complex. Because of this, the southern-most species of this complex will retain the name H. maculifrons while the others will be given new names.
Figure 1. Inferior hind tibial spines. From left to right: Hemiandrus maculifrons, Hemiandrus luna sp. nov., Hemiandrus brucei sp. nov., Hemiandrus nox sp. nov. Hemiandrus maculifrons usually has two spines, but sometimes more and, in a rare case, only a single spine. Hemiandrus nox sp. nov. has no articulated spines on the inferior side of the hind tibiae. Hemiandrus luna sp. nov. usually has only a single spine, while Hemiandrus brucei sp. nov. usually has one or two spines.

Diagnosis
A small to medium-sized ground wētā found in forests of the South Island, New Zealand, with the following traits: head and body mostly brown with some pale areas including large lateral and dorsal pale patches on the pronotum; the three apical segments of the maxillary palps with fine microsetae; mid tibiae with four spines along the inferior retrolateral angle (excluding apical spine); hind tibiae with two to four inferior articulated spines (rarely one); male subgenital plate short, with a flat or weakly concave apical margin; adult male cerci blunt; male T9 bilobed with acute lobes; females with a very long, gently curved ovipositor.
Description
Size
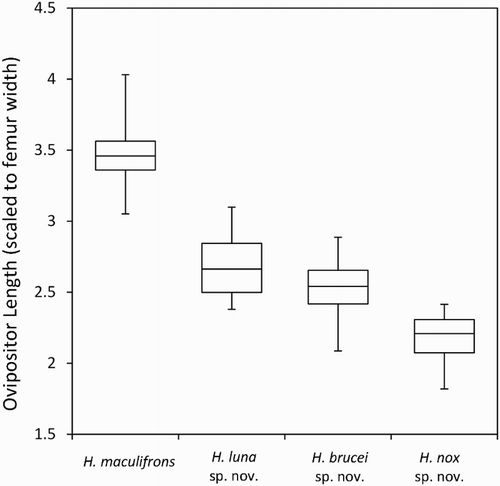
Adult male (n = 15): PL 3.82–4.76 mm; PW 3.70–4.73 mm; FL 10.53–13.93 mm; FW 2.80–3.66 mm; HL 4.84–5.99 mm; HW 3.43–4.26 mm; BL 7.20–8.94 mm; SG 1.91–2.61 mm; SG/PL 0.43–0.65; FL/HW 2.98–3.54. Adult female () (n = 63): PL 3.40–5.54 mm; PW 3.53–5.55 mm; FL 9.89–15.70 mm; FW 2.72–4.13 mm; HL 5.06–6.96 mm; HW 3.36–4.88 mm; BL 6.88–10.93 mm; OV 9.58–15.10 mm; OV/FW 3.05–4.03; FL/HW 2.80–3.47. See .
Figure 2. Representative female individuals of each of the four ground wētā species described here. A, Hemiandrus maculifrons; B, Hemiandrus luna sp. nov.; C, Hemiandrus brucei sp. nov.; D, Hemiandrus nox sp. nov. Scale bar = 10 mm.

Table 1. Body measurements (in mm), spine numbers and peg numbers for both sexes of each of the four species (re)described here. Mean and range (in brackets) are reported.
Head
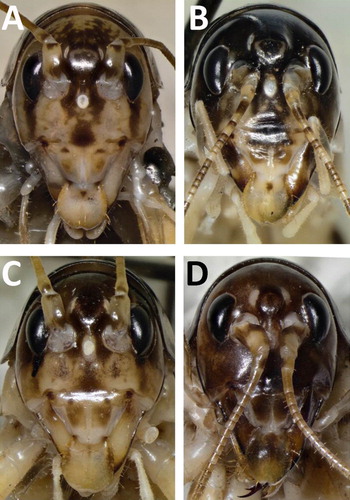
(A). Shiny, darker brown on top with faint pale dorsal midline; gena cream or mottled cream and brown; frons and clypeus mottled, rugose; labrum cream and/or brown, setose; mandibles cream and brown, dark distally; scape, pedicel and antennomeres cream and light brown; antennae longer than body; flagella proximally smooth (12–14 antennomeres), distal antennomeres covered by short fine microsetae; eyes black; vertex with raised subtriangular fastigium; ocellar spots white; maxillary palps cream with bulbous apices and with widely spaced setae and covered by short, fine microsetae (MP5 100% pilose, MP4 100%, MP3 c. 50%).
Thorax
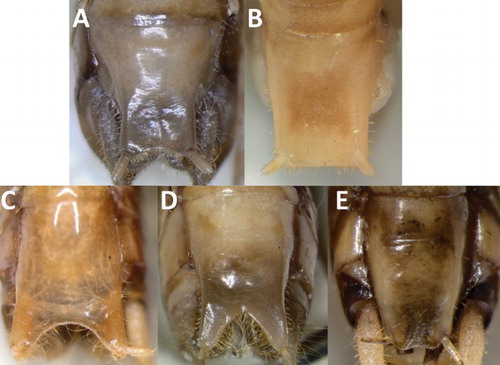
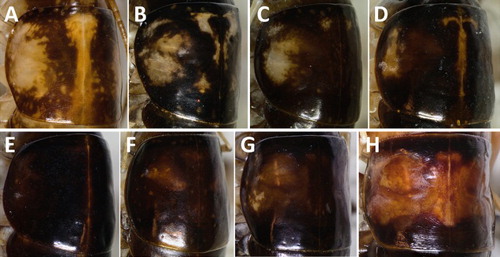
Pronotum, mesonotum and metanotum brown with a faint pale dorsal mid-line which extends from the head to the abdominal tergites; pronotum shiny and smooth dorsally, rugose ventrally, approximately as long as wide, large lateral pale patches and the dorsal area mottled with large pale patches (). Thoracic sterna cream and/or light brown; each with two blunt spines which are long and thin on the prosternum and short and wide on the meso- and metasterna (figure 5A and 5B [ventral aspects] in Taylor-Smith et al. Citation2013).
Figure 4. Patterning of the pronotum varies both within and between species. A, Hemiandrus maculifrons; B, Hemiandrus luna sp. nov. (South Island); C, Hemiandrus luna sp. nov. (North Island); D, Hemiandrus brucei sp. nov.; E, Hemiandrus brucei sp. nov.; F, Hemiandrus nox sp. nov.; G, Hemiandrus ‘madisylvestris’; H, Hemiandrus fiordensis.
Legs
Long (hind femora 2.8 to 3.5 times head width); coxae and trochanters cream; fore and mid coxae with spinous lobes; femora cream at base becoming brown with cream spots; tibiae brown with cream spots; fore tibiae lacking tympanum. Fore tibiae with 13 cream articulated spines with brown tips arranged as follows: four apicals, one positioned medially on superior prolateral angle, none on superior retrolateral angle, four along the inferior prolateral angle, four along the inferior retrolateral angle. Mid tibiae with 17 cream articulated spines with brown tips arranged as follows: four apicals, two along the superior prolateral angle, three along the superior retrolateral angle, four along the inferior prolateral angle, four along the inferior retrolateral angle (). Hind tibiae with 30–47 spines arranged as follows: two pairs of short apical spines, one pair of long apical spines, one pair of long subapical spines, 11–18 fixed spines along the superior prolateral angle, 10–17 fixed spines along the superior retrolateral angle, two to four (usually two, rarely one) small articulated spines on the ventral side of the tibiae (). Tarsi cream, setose, four-segmented, first segment with foot-pad divided into two, other segments with a single foot-pad. The first segment sparsely setose, segments two and three range from bare to pilose, fourth is pilose with some setae. The inner side of the hind femora with 0–66 small pegs.
Figure 5. Inferior mid tibial spines. From left to right: Hemiandrus maculifrons, Hemiandrus luna sp. nov., Hemiandrus brucei sp. nov., Hemiandrus nox sp. nov. Spines are positioned in pairs with a single apical pair and four pairs spaced along the inferior mid tibiae. Hemiandrus nox sp. nov. lacks a proximal spine along the retrolateral angle leaving a single unpaired spine (arrow). Left tibiae shown here.

Abdomen
Abdominal tergites brown with some pale spots, paler laterally; T1 with 4–71 stridulatory pegs; T2 with 22–66 stridulatory pegs; T3 with 17–63 stridulatory pegs; pleural membrane light brown or cream with stridulatory pegs; sternites cream.
Males
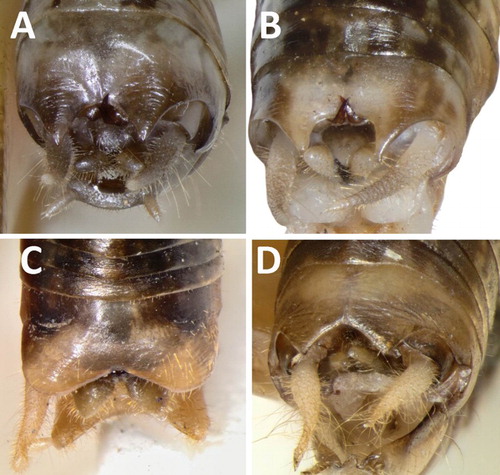
Ninth abdominal tergite (T9) paler than other tergites, apical margin with two acute lobes (); T10 with two dark hooks usually positioned between the lobes of T9 but sometimes hidden beneath; subgenital plate short (mean 2.23 mm, 43%–65% of pronotum length) with a flat or weakly concave distal margin (); styles short; conical paraprocts; cerci blunt, cream, setose.
Females
T7 simple or with a small median lobe; T8 simple or with a small median lobe; T9 bilobed or slightly notched; cerci cream, long, pointed, with long setae; ovipositor gently curved, very long (mean 12.03 mm), 3.1–4.0 times hind femur width (); subgenital plate triangular; seventh abdominal sternite with paired pits situated anteriorly either side of the mid line.
Voucher specimens
Adult female, collected February 2014 from Lewis Pass (BR) (−42.324°, 172.1283°), BL Taylor-Smith and NB Smith (MONZ AI.033208; GW1021). Adult male, collected February 2014 from Lewis Pass (BR) (−42.3786°, 172.2776°), BL Taylor-Smith and NB Smith (MONZ AI.033209; GW1011).
Distribution
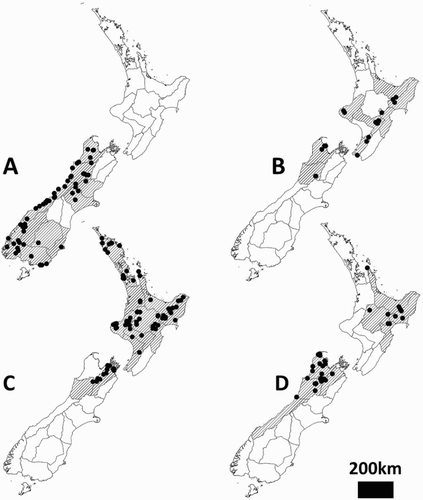
NN, BR, NC, MC, WD, FD, OL, CO, DN, SL, FD (see A).
Figure 9. Species distributions and the Crosby regions (Crosby et al. Citation1976) in which each species is found (hatched areas). A, Hemiandrus maculifrons; B, Hemiandrus luna sp. nov.; C, Hemiandrus brucei sp. nov.; D, Hemiandrus nox sp. nov. Black dots are sampling sites.
Material examined
NN: Mt Arthur (GW670; GW790, DOCORD055928); Denniston (GW758, GW864, GW895); Flora Saddle (GW788, GW694); Te Kuha (GW1081); Mt Owen (GW214); Tasman Wilderness Area (GW215, GW217). BR: Rolleston Track (GW872, GW873, GW874, GW875, GW876, GW877); Lewis Pass (GW461, GW462A, GW462B, GW463A, GW463B, GW1011, GW1017, GW1018, GW1019, GW1020, GW1021, GW1022, GW1025); Kaiata (GW920, GW921, GW922M, GW922F, GW923, GW924F, GW925F); Mt Sewell Track (GW1062); Braeburn Track (DOCORD048036); Paparoa Ranges (GW147, GW152, NZAC). WD: Awatuna (GW824, GW825, GW826, GW827, GW828, GW829, GW830, GW831, GW832, GW835, GW836, GW837, GW839, GW840, GW861); Franz Josef (GW150); Haast (GW259, NZAC); Mahitahi River (NZAC); Franz Josef (NZAC03015718); Barrier Valley (NZAC03015719); Karangarua River (NZAC); Hunts Beach (NZAC); Callery Gorge Track (NZAC); Wanganui River (NZAC); Ross (NZAC); Lake Paringa (NZAC); Waita River (AMNZ5339); Ōkaritō (AMNZ5726). MC: Arthur’s Pass (GW146, GW898, GW908, GW909A, GW909B, GW910M, GW910B, GW911, GW912, GW913F, GW914F, GW915F, GW915M); Craigieburn Forest Park (GW130, GW133); Methven (NZAC); Lake Marion (GW942, GW943); Mt Algidus (NZAC). CO: Piano Flat (NZAC). DN: Leith Saddle Track (NZAC03015729). OL: Eglinton Valley (GW564); Hollyford Valley (NZAC03015713). FD: Sinbad Gully (FD4); Te Anau (GW28); Kepler Track (GW141, GW142); Wet Jacket Arm (NZAC03015714); Bowen Falls (NZAC03015716); Secretary Island (NZAC03015717); Homer Saddle (NZAC03015721); Lake Hauroko (NZAC); Dean Burn (NZAC); Borland Saddle (NZAC); West Arm, Manapouri (NZAC); Hollyford Valley (NZAC). SL: Forest Hill (GW1099); Waipohatu Stream (GW119, NZAC03015715); Takitimu Range (GW198, GW201, GW202); Tautuku, Catlins (GW32A); Haldane Reserve (GW40).
Species description: Hemiandrus luna sp. nov.
Diagnosis
A small ground wētā found in forests of the North Island and northern South Island, New Zealand, with the following traits: head and body mostly brown with some pale areas including large lateral and dorsal pale patches on the pronotum; the three apical segments of the maxillary palps with fine microsetae; mid tibiae with four spines along the inferior retrolateral angle (excluding apical spine); hind tibiae with a single inferior articulated spine (rarely two); male subgenital plate short, with a flat or weakly concave apical margin; adult male cerci blunt; male T9 bilobed with acute lobes; females with a long, gently curved ovipositor.
Description
Size
Adult male (n = 9): PL 3.80–4.55 mm; PW 3.61–4.53 mm; FL 9.67–10.91 mm; FW 2.85–3.44 mm; HL 5.16–5.73 mm; HW 3.41–3.74 mm; BL 6.76–7.82 mm; SG 1.80–2.35 mm; SG/PL 0.42–0.55; FL/HW 2.77–2.95. Adult female () (n = 24): PL 4.04–5.34 mm; PW 4.15–5.17 mm; FL 10.69–12.31 mm; FW 3.04–3.97 mm; HL 5.63–6.54 mm; HW 3.86–4.43 mm; BL 7.36–11.05 mm; OV 8.52–10.80 mm; OV/FW 2.34–3.10; FL/HW 2.66–3.02.
Head
(B). Shiny, darker brown on top with faint pale dorsal midline; gena cream or mottled cream and brown; frons and clypeus mottled, rugose; labrum cream and/or brown, setose; mandibles cream and brown, dark distally; scape, pedicel and antennomeres cream and light brown; antennae longer than body; flagella proximally smooth (9–12 antennomeres), distal antennomeres covered by short fine microsetae; eyes black; vertex with raised subtriangular fastigium; ocellar spots white; maxillary palps cream with bulbous apices and with widely spaced setae and covered by short, fine microsetae (MP5 100% pilose, MP4 100%, MP3 c. 50%).
Thorax
Pronotum, mesonotum and metanotum brown with a faint pale dorsal mid-line which extends from the head to the abdominal tergites; pronotum shiny and smooth dorsally, rugose ventrally, approximately as long as wide, large lateral pale patches and the dorsal area with little to extensive mottling and pale patches (). Thoracic sterna cream and/or light brown; each with two blunt spines which are long and thin on the prosternum and short and wide on the meso- and metasterna.
Legs
Long (hind femora 2.7 to 3.0 times head width); coxae and trochanters cream; fore and mid coxae with spinous lobes; femora cream at base becoming brown with cream spots; tibiae brown with cream spots; fore tibiae lacking tympanum. Fore tibiae with 13 cream articulated spines with brown tips arranged as follows: four apicals, one positioned medially on superior prolateral angle, none on superior retrolateral angle, four along the inferior prolateral angle, four along the inferior retrolateral angle. Mid tibiae with 17 cream articulated spines with brown tips arranged as follows: four apicals, two along the superior prolateral angle, three along the superior retrolateral angle, four along the inferior prolateral angle, four along the inferior retrolateral angle (). Hind tibiae with 31–41 spines arranged as follows: two pairs of short apical spines, one pair of long apical spines, one pair of long subapical spines, 11–16 fixed spines along the superior prolateral angle, 11–15 fixed spines along the superior retrolateral angle, one (rarely two) small articulated spine on the ventral side of the tibiae (). Tarsi cream, setose, four-segmented, first segment with foot-pad divided into two, other segments with a single foot-pad. The first segment sparsely setose, segments two and three range from bare to pilose, fourth is pilose with some setae. The inner side of the hind femora with 10–55 small pegs.
Abdomen
Abdominal tergites brown with some pale spots, paler laterally; T1 with 18–54 stridulatory pegs; T2 with 26–68 stridulatory pegs; T3 with 10–63 stridulatory pegs; pleural membrane light brown or cream with stridulatory pegs; sternites cream and/or light brown.
Males
Ninth abdominal tergite (T9) paler than other tergites, apical margin with two acute lobes (); T10 with two dark hooks usually positioned between the lobes of T9 but sometimes hidden beneath; subgenital plate short (mean 2.13 mm, 42%–55% of pronotum length) with a flat or weakly concave distal margin (); styles short; conical paraprocts; cerci blunt, cream, setose.
Females
T7 simple or with a small median lobe; T8 simple or with a small median lobe; T9 bilobed or slightly notched; cerci cream, long, pointed, with long setae; ovipositor gently curved, long (mean 9.35 mm), 2.3–3.1 times hind femur width (); subgenital plate triangular; seventh abdominal sternite with paired pits situated anteriorly either side of the mid line.
Type data
Holotype: adult female, collected February 2014 from Lewis Pass (BR) (−42.3786°, 172.2776°), BL Taylor-Smith and NB Smith (MONZ AI.033202; GW1015). Paratype: adult male, same collection data as holotype (MONZ AI.033203; GW1012).
Distribution
TK, BP, GB, HB, RI, WN, NN, BR (see B).
Additional material examined
TK: Dawson Falls (DOCORD036029, NZAC03015712); Pouakai Hut (NZAC03015707). RI: Ruahine Ranges (GW552, GW553, GW661, GW662, GW916B, GW932, NZAC03015708, NZAC03015711). BP: Huiarau Range (NZAC03015705). GB: Maungapohatu Road (DOCORD042006, DOCORD044515); Waikaremoana (GW465, GW747, GW750, GW752, DOCORD042025, DOCORD044954). HB: Makahu Saddle (DOCORD057216). WN: Kahuterawa Valley (GW759, GW761, GW815, GW817); Herepai Hut, Tararua Range (GW611); Rimutaka Range (NZAC03015730). NN: Abel Tasman National Park (GW900); Rameka (GW100, GW102, GW104, GW105, GW565, GW567, GW568, GW569, GW570, GW571, GW573, GW574, GW575, GW577, GW578); Cobb Lookout (DOCORD042191, DOCORD042392); Canaan Road (DOCORD043636). BR: Lewis Pass (GW23, GW143, GW1010, GW1013, GW1014, GW1016).
Etymology
From Luna, the Roman goddess of the moon in reference to the nocturnal habit of Hemiandrus species.
Comments
On the South Island, H. luna sp. nov. has extensive mottling of the pronotum ().
Species description: Hemiandrus brucei sp. nov.
Diagnosis
A small ground wētā found throughout forests of the North Island and northern South Island, New Zealand, with the following traits: head and body mostly brown with some pale areas sometimes including small lateral and sometimes small dorsal pale patches on the pronotum; the three apical segments of the maxillary palps with fine microsetae; the mid tibiae with four spines along the inferior retrolateral angle (excluding apical spine); hind tibiae with a single inferior articulated spine (rarely two); male subgenital plate long, with deep V- or U-shape apical margin. Adult male cerci blunt; male T9 bilobed with obtuse, curved lobes; females with a long, gently curved ovipositor.
Description
Size
Adult male (n = 33): PL 3.56–4.74 mm; PW 3.73–4.89 mm; FL 9.54–13.12 mm; FW 2.74–3.86 mm; HL 4.22–5.61 mm; HW 3.04–3.85 mm; BL 6.13–9.18 mm; SG 2.38–3.50 mm; SG/PL 0.66–0.80; FL/HW 2.95–3.50. Adult female () (n = 69): PL 3.98–6.05 mm; PW 4.15–5.91 mm; FL 10.19–14.38 mm; FW 3.00–4.48 mm; HL 4.95–6.68 mm; HW 3.42–4.69 mm; BL 6.76–10.45 mm; OV 7.79–11.55 mm; OV/FW 2.09–2.89; FL/HW 2.81–3.23.
Head
(C). Shiny, darker brown on top with faint pale dorsal midline; gena cream or mottled cream and brown; frons and clypeus mottled, rugose; labrum cream and/or brown, setose; mandibles cream and brown, dark distally; scape, pedicel and antennomeres cream and light brown; antennae longer than body; flagella proximally smooth (11–13 antennomeres), distal antennomeres covered by short fine microsetae; eyes black; vertex with raised subtriangular fastigium; ocellar spots white; maxillary palps cream with bulbous apices and with widely spaced setae and covered by short, fine microsetae (MP5 100% pilose, MP4 100%, MP3 c. 50%).
Thorax
Pronotum, mesonotum and metanotum brown with a faint pale dorsal mid-line which extends from the head to the abdominal tergites; pronotum shiny and smooth dorsally, rugose ventrally, approximately as long as wide, with or without small lateral pale patches and the dorsal area with or without pale patterning around midline and anterior edge (). Thoracic sterna cream and/or light brown; each with two blunt spines which are long and thin on the prosternum and short and wide on the meso- and metasterna.
Legs
Long (hind femora 2.8 to 3.5 times head width); coxae and trochanters cream; fore and mid coxae with spinous lobes; femora cream at base becoming brown with cream spots; tibiae brown with cream spots; fore tibiae lacking tympanum. Fore tibiae with 13 cream articulated spines with brown tips arranged as follows: four apicals, one positioned medially on superior prolateral angle, none on superior retrolateral angle, four along the inferior prolateral angle, four along the inferior retrolateral angle. Mid tibiae with 17 cream articulated spines with brown tips arranged as follows: four apicals, two along the superior prolateral angle, three along the superior retrolateral angle, four along the inferior prolateral angle, four along the inferior retrolateral angle (). Hind tibiae with 29–48 spines arranged as follows: two pairs of short apical spines, one pair of long apical spines, one pair of long subapical spines, 10–20 fixed spines along the superior prolateral angle, 10–18 fixed spines along the superior retrolateral angle, usually one or two small articulated spines on the ventral side of the tibiae (). Tarsi cream, setose, four-segmented, first segment with foot-pad divided into two, other segments with a single foot-pad. The first segment sparsely setose, segments two and three range from bare to pilose, fourth is pilose with some setae. Inner side of the hind femora with 0–96 small pegs.
Abdomen
Abdominal tergites brown, paler laterally; T1 with 18–80 stridulatory pegs; T2 with 23–75 stridulatory pegs; T3 with 15–75 stridulatory pegs; pleural membrane light brown or cream with stridulatory pegs; sternites cream.
Males
Ninth abdominal tergite (T9) paler than other tergites, apical margin with two widely-spaced, obtuse lobes (); T10 with two dark hooks usually positioned beneath T9 but sometimes exposed; subgenital plate long (mean 2.82 mm, 66%–80% of pronotum length) with a deep V- to U-shaped distal margin (); styles short; paraprocts wide and irregularly shaped; cerci blunt, cream, setose.
Females
T7 simple or with a small median lobe; T8 simple or with a small median lobe; T9 bilobed or slightly notched; cerci cream, long, pointed, with long setae; ovipositor gently curved, long (mean 9.44 mm), 2.1–2.9 times hind femur width (); subgenital plate triangular; seventh abdominal sternite with paired pits situated anteriorly either side of the mid line.
Type data
Holotype: adult female, collected March 2012 from Kahuterawa Valley (WN) (−40.47190°, 175.61417°), BL Taylor-Smith (MONZ AI.033206; GW805). Paratype: adult male, same collection data as holotype (MONZ AI.033207; GW770).
Distribution
ND, AK, WO, CL, BP, GB, TO, TK, RI, WI, WN, HB, SD, MB, BR (see C).
Additional material examined
ND: Manginangina Reserve (GW64); Herekino Forest (GW726; NZAC); Puketi (GW49A, GW49B1, GW49B2); Opononi (GW44); Ngaiotonga Reserve (GW464); Omahuta Kauri Reserve (NZAC); Waipoua Forest (AMNZ6952, AMNZ6953). AK: Henderson, Auckland (GW74); Waiwera (GW617); Waimauku (NZAC); Matuku Reserve (NZAC); Mataitai Forest (AMNZ63158). WO: Maungatautari (GW169, GW172). CL: Coromandel (GW234, GW247A); Moehau Range (NZAC). BP: Manganuku Bridge (GW4); Wairata (GW21, GW700, GW703); Moanui (GW665, DOCORD058871); Tauranga (GW937); Opotiki (GW944A, GW944M, GW944B); Matawai (DOCORD059825); Whinray Scenic Reserve (DOCORD059901, DOCORD059910). GB: Waikaremoana (GW68, GW735, GW738, GW739, GW740, GW741, GW742, GW744, GW745, GW746, GW751, GW753, GW90; DOCORD041704; DOCORD041753, DOCORD044915, DOCORD044954, DOCORD041971); Orangihikoia (DOCORD041545); Rahui Island (DOCORD041565); Huiarau Summit (DOCORD041773, DOCORD044648); Maraunui (DOCORD041909, DOCORD044579); Waterworks, Tarewa (DOCORD042535); Rakauroa Scenic Reserve (DOCORD057496; DOCORD057517; DOCORD058076); Tarndale (DOCORD058229, DOCORD058292); Kokomuka Road (DOCORD058470; DOCORD058489); Mangaraukokore Stream (DOCORD058679). HB: Little’s Clearing (DOCORD057109); William Hartree Memorial Scenic Reserve (DOCORD057383); Kaweka Forest Park (DOCORD057244, DOCORD057711, GW666). TO: Owhango (DOCORD036082); Raurimu (GW218, GW219, GW220); Pureora (GW126, GW548, GW549, GW550, GW551, GW624, GW625, GW627, GW628, GW630, GW631, GW633); Opepe (GW109, GW195F, GW196); Whirinaki (DOCORD049157, DOCORD056341, DOCORD058622, DOCORD058698, DOCORD059035, DOCORD059093, DOCORD059375, DOCORD059522, DOCORD069122); Tarawera (DOCORD043429). TK: Moki (DOCORD047457, DOCORD059262); Ohauora (GW222, GW223, GW224, GW225); Lucy’s Gully (GW229, GW239, GW777, GW778); Dawson Falls (GW893, GW893F); Lake Mangamahoe (GW919); Jackson’s Lookout, Mt Taranaki (DOCORD034986); York Road, Mt Taranaki (GW472); Whangamomona Saddle (DOCORD036045); Meeting of the Waters Scenic Reserve (DOCORD047178; DOCORD058722); Makino East (DOCORD047268; DOCORD059075); Tangarakau Stream (DOCORD047639); Umutekai Conservation Area (DOCORD058944); Paparata (DOCORD058981); Waitewhena Forest (AMNZ5207). RI: Tieke Kainga (DOCORD036275); Paengaroa (DOCORD040810). WI: Atene (GW554, GW557, GW558, GW559, GW636). WN: Kahuterawa Valley (GW760, GW765, GW766, GW767, GW768, GW769, GW771, GW772, GW773, GW774, GW775, GW797, GW798, GW799, GW800, GW801, GW802, GW803, GW804, GW806, GW807, GW808, GW809, GW810, GW811, GW812, GW813, GW814, GW819). SD: Opouri (DOCORD048177A, DOCORD047862, DOCORD048066); Esson's Valley (DOCORD043166, DOCORD043192, DOCORD047818, DOCORD048349); Shakespeare Bay (DOCORD048131); Mt Ronga (DOCORD048151); Pukaka Valley (DOCORD069989); Pelorus Bridge (GW93). MB: Kahikatea Flat (DOCORD047748); Wairau Valley (DOCORD047997, DOCORD048585, DOCORD049283); Lake Chalice (DOCORD053402); Pine Valley (DOCORD053516, DOCORD070103); Silverstream (DOCORD070159); Red Hills Track (GW1026). BR: Lake Rotoroa (DOCORD048695).
Etymology
Named in memory of Bruce Edwin Smith, grandfather of Briar Taylor-Smith and supporter of the sciences.
Comments
Hemiandrus brucei sp. nov. has a high level of morphological variation. Specimens from East Cape and Northland appear to be slightly larger with no or little patterning on their pronota. Males within these populations appear to have variable terminalia with the distal margins of their subgenital plates ranging from V-shaped to U-shaped. Hemiandrus brucei sp. nov. specimens examined from all other locations had more extensive patterning on their pronota and males had U-shaped subgenital plates.
Species description: Hemiandrus nox sp. nov.
Diagnosis
A small ground wētā found in forests of the North and South Islands, New Zealand, with the following traits: head and body dark brown with cream and brown clypeus; sometimes with small pale patches on the pronotum; the three apical segments of the maxillary palps with fine microsetae; mid tibiae with three spines along the inferior retrolateral angle (excluding apical spine); hind tibiae with no inferior articulated spines; male subgenital plate short, narrower towards apex, with a flat or slightly U-shaped apical margin that may extend beyond the styles; adult male cerci pointed; male T9 bilobed with each lobe slightly pointed; females with a medium-length, strongly curved ovipositor with dark patches at its base.
Description
Size
Adult male (n = 16): PL 4.23–4.73 mm; PW 4.13–4.42 mm; FL 9.54–11.46 mm; FW 3.12–3.7 mm; HL 4.57–5.25 mm; HW 2.84–3.38 mm; BL 6.96–7.42 mm; SG 1.71–2.24 mm; SG/PL 0.39–0.47; FL/HW 3.12–3.38. Adult female () (n = 26): PL 4.25–5.43 mm; PW 3.99–5.24 mm; FL 10.02–12.41 mm; FW 3.10–4.08 mm; HL 4.82–6.29 mm; HW 3.00–3.97 mm; BL 7.13–9.30 mm; OV 6.81–8.55 mm; OV/FW 1.82–2.41; FL/HW 2.98–3.45.
Head
(D). Shiny, dark with faint pale dorsal midline; gena sometimes slightly paler brown; frons and clypeus rugose, brown but clypeus with white mottling; labrum brown, sometimes also cream, setose; mandibles brown, darker distally; scape, pedicel and antennomeres light brown; antennae longer than body; flagella proximally smooth (six to ten antennomeres), distal antennomeres covered by short fine microsetae; eyes black; vertex with raised subtriangular fastigium; ocellar spots white; maxillary palps cream with bulbous apices and with widely spaced setae and covered by short, fine microsetae (MP5 100% pilose, MP4 100%, MP3 c. 50%).
Thorax
Pronotum, mesonotum and metanotum dark brown with a faint pale dorsal mid-line which extends from the head to the abdominal tergites; pronotum shiny and smooth dorsally, rugose ventrally, approximately as long as wide, usually paler laterally and the dorsal area sometimes with small pale spots (). Thoracic sterna cream and/or light brown; each with two blunt spines which are long and thin on the prosternum and short and wide on the meso- and metasterna.
Legs
Long (hind femora 3.0 to 3.5 times head width); coxae and trochanters cream; fore and mid coxae with spinous lobes; femora cream at base becoming brown with cream spots; tibiae brown with cream spots; fore tibiae lacking tympanum. Fore tibiae with 13 cream articulated spines with brown tips arranged as follows: four apicals, one positioned medially on superior prolateral angle, none on superior retrolateral angle, four along the inferior prolateral angle, four along the inferior retrolateral angle. Mid tibiae with 16 cream articulated spines with brown tips arranged as follows: four apicals, two along the superior prolateral angle, three along the superior retrolateral angle, four along the inferior prolateral angle, three along the inferior retrolateral angle (). Hind tibiae with 30–41 spines arranged as follows: two pairs of short apical spines, one pair of long apical spines, one pair of long subapical spines, 12–17 fixed spines along the superior prolateral angle, 10–16 fixed spines along the superior retrolateral angle, no small articulated spine on the ventral side of the tibiae (). Tarsi cream, setose, four-segmented, first segment with foot-pad divided into two, other segments with a single foot-pad. The first segment sparsely setose, segments two and three range from bare to pilose, fourth is pilose with some setae. The inner side of the hind femora with no small pegs.
Abdomen
Abdominal tergites dark brown with some pale spots, paler laterally; T1 with 15–25 stridulatory pegs; T2 with 16–25 stridulatory pegs; T3 with 11–25 stridulatory pegs; pleural membrane dark brown with stridulatory pegs; sternites cream and/or light brown.
Males
Ninth abdominal tergite (T9) paler than other tergites, apical margin with two obtuse slightly pointed lobes (); T10 with two dark hooks positioned beneath T9; subgenital plate short (mean 2.01 mm, 39%–47% of pronotum length), narrower towards apex, with a flat or slightly U-shaped distal margin that may extend beyond the styles (); styles short; blunt cylindrical paraprocts; cerci cream, sharp, setose.
Females
T7 simple or with a small median lobe; T8 with a small median lobe; T9 slightly notched; cerci cream, long, pointed, with long setae; ovipositor very curved, dark patches at base (), medium length (mean 7.63 mm), 1.8–2.4 times hind femur width (); subgenital plate triangular; seventh abdominal sternite with paired pits situated anteriorly either side of the mid line.
Type data
Holotype: adult female, collected February 2014 from Kahurangi National Park (NN) (−40.6522°, 172.46°), BL Taylor-Smith and NB Smith (MONZ AI.033204; GW996). Paratype: adult male, same collection data as holotype (MONZ AI.033205; GW995).
Distribution
CL, BP, GB, HB, TO, MB, NN, BR, WD (see D).
Additional material examined
CL: Coromandel (GW796); Moehau Range (NZAC03015735). BP: Moanui (GW664); Wairata (GW918). GB: Waikaremoana (GW94A, GW734); Rakauroa Scenic Reserve (DOCORD057496); Whakapunake (DOCORD046185). TO: Waipapa Loop Track, Pureora (NZAC03015736, NZAC03015737); Whirinaki (DOCORD059109). HB: Bellbird Bush (DOCORD055762); Cache’s Bush (DOCORD056594). NN: Huia Cave (DOCORD055878B); Mt Haidinger (GW212); Denniston (GW865, GW896A, GW896B, GW896C, GW899); Paturau River (GW993, GW999, GW1000, GW1002); Flora Carpark (GW689, GW690, GW691, GW692, GW695, GW786); Mt Arthur (DOCORD055928); Brooklyn Valley (DOCORD056506); Harwoods Hole (AMNZ64052); Cobb Valley (GW95); Lake Hanlon (AMNZ5206); Ōpārara Basin (AMNZ5338). MB: Lake Chalice (DOCORD070144); Wairau Valley (DOCORD049001). BR: Lake Rotoroa (DOCORD048529B, DOCORD048677, DOCORD049133, DOCORD049316B, DOCORD049316C, DOCORD048529A, DOCORD048529C, DOCORD048733, GW33BB, GW33BS); St Arnaud (GW10A, GW10BB, GW10BS, GW46, GW481); Awakiri Valley (GW76); Braeburn Track (DOCORD048036); Lewis Pass (GW1023); Maruia Saddle Road (DOCORD049048, DOCORD049368); Porika Track (DOCORD048416, DOCORD048716); Shenandoah Saddle (DOCORD048755, DOCORD049266, DOCORD048518). WD: Awatuna (GW834).
Etymology
From Nox, the Roman goddess of the night in reference to the nocturnal habit of Hemiandrus species.
Comments
This species is found in primary native forest in sympatry with the three other species described here and is most abundant in northwest Nelson. This species is probably the tag-named entity H. ‘alius’ (Johns Citation2001), although Hemiandrus nox sp. nov. is found on both the North and South Islands while H. ‘alius’ has only been recorded on the South Island. Based on mtDNA sequence data H. nox sp. nov. is phylogenetically sister to the H. maculifrons complex (Pratt et al. Citation2008, ).
Discussion
Taxonomic knowledge of New Zealand invertebrates has significant gaps (Buckley et al. Citation2015). The species described here illustrate one reason for gaps—cryptic diversity, which requires molecular analyses in order to delimitate species boundaries. Limited numbers of adult specimens in insect collections also hampers taxonomy. Another species within this complex is likely in northwest Nelson; however, the collection of more adult specimens is required before the description can be written. The four species (re)described here can be distinguished from one another by head and body colour, patterning of the pronotum, mid tibial and hind tibial spines, male terminalia size and shape, female and ovipositor length (see key). Two South Island species, Hemiandrus fiordensis (Salmon, Citation1950) and H. ‘madisylvestris’ (Johns, Citation2001), are the most similar to the species described here but both are larger and have little pronotal patterning (). Hemiandrus fiordensis can be distinguished from the four species in this description by the absence of spots on its hind femora (Jewell Citation2007).
Key to species
Acknowledgements
We are grateful for the help provided by Clare Allen, Ollie Ball, Paul Barret, Gareth Boyt, Andrew Blayney, Selina Brown, Marianna Bulgarella, I. Buunk, Esta Chappell, J. Clayton-Green, R. Coker, Lorraine Cook, Cindy Coreman, Amelia Corin, Steve Corin, Jess Costal, T. de Cruz, Jon de Vries, Robyn Dewhurst, Edwina Dowle, John Early, H. Edmonds, Eric Edwards, Entecol, Jo Fitness, Gillian Gibb, George Gibbs, Shona Gibbs, Aaron Gillespie, Julia Goldberg, Chris Golding, R. Goudsward, C. Green, Melissa Griffin, Darryl Gwynne, Marty Haigh, Grace Hall, Tracy Harris, M. Henderson, J. Hiscock, Rod Hitchmough, J. Hoare, Matt Irwin, Tony Jewell, Peter Johns, Stefanie König, Bailey Langley, P. Lei, Mike Lusk, K. Mahlfeld, Graeme Matamata, Jay McCartney, Paul McGahan, Tarsha McKean, Trish McLenachan, Ian Millar, Niki Murray, Shelley Myers, R. Nicol, Shaun Nielsen, C. O'Donnell, Mike Ogle, Kane O’Keeffe, Christina Painting, B.H. Patrick, Renae Pratt, Dhahara Ranatunga, J. Rearson, Adele Reweti, Bridget Reweti, Birgit Rhode, Christina Rowe, Dave Seldon, Helen Sharpe, Pete Shaw, Robert Silberbauer, A. Smart, Noel Smith, Regan Smith, Roxy Smith, Snail Team DOC, Ian Stringer, J. Tansell, Mike Thorsen, Bianca Trewick, Edward Trewick, John Trewick, Leo Trewick, P. Van Veen, Frans van Wyngaarden, Mike Wakelin, Cleland Wallace, Troy Watson, Cilla Wehi, N. Willans, Angus Wilson, George Wilson, Rob Wilson. We are grateful for the support and contributions from the Phoenix Lab (www.evolves.massey.ac.nz).
Associate Editor: Dr Christina Painting.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ander K. 1938. Diagnosen neuer Laubheuschrecken. Opuscula Entomologica. 3:50–56.
- Brockie RE. 1992. A living New Zealand forest. Auckland: Bateman.
- Buckley TR, Krosch M, Leschen RA. 2015. Evolution of New Zealand insects: summary and prospectus for future research. Austral Entomology. 54:1–27. doi: 10.1111/aen.12116
- Cary PRL. 1981. The biology of the weta Zealandosandrus gracilis (Orthoptera: Stenopelmatidae) from the Cass region. Unpublished MSc thesis. Christchurch, New Zealand: University of Canterbury.
- Chappell EM, Webb DS, Brown AJ, Tonkin JD. 2014a. A preliminary survey of altitudinal variation in two ground wētā species, Hemiandrus maculifrons (Walker) and Hemiandrus pallitarsis (Walker) (Orthoptera: Anostostomatidae). New Zealand Entomologist. 38:1–6. doi: 10.1080/00779962.2014.922234
- Chappell EM, Webb DS, Tonkin JD. 2014b. Notes on sexual size dimorphism, sex ratio and movements of adult ground weta Hemiandrus maculifrons (Walker) (Orthoptera: Anostostomatidae). New Zealand Entomologist. 37:83–92. doi: 10.1080/00779962.2013.856377
- Crosby TK, Dugdale JS, Watt JC. 1976. Recording specimen localities in New Zealand: an arbitrary system of areas and codes defined. New Zealand J Zool. 3:69. doi: 10.1080/03014223.1976.9517903
- Fitness J, Morgan-Richards M, Ball O, Godfrey RJA, Trewick SA. 2015. Improved resolution of cave weta diversity (Orthoptera: Rhaphidophoridae): ecological implications for Te Paki, Far North, New Zealand. New Zealand J Zool. 42:1–16. doi: 10.1080/03014223.2014.983939
- Hutton F. 1897. The Stenopelmatidae of New Zealand. Transactions and proceedings of the New Zealand Institute. 29:208–242.
- Jewell T. 2007. Two new species of Hemiandrus (Orthoptera : Anostostomatidae) from Fiordland National Park, New Zealand. Zootaxa. 1542:49–57.
- Johns PM. 1997. The Gondwanaland weta: Family Anostostomatidae (formerly in Stenopelmatidae, Henicidae or Mimnermidae): nomenclatural problems, world checklist, new genera and species. Journal of Orthoptera Research. 6:125–138. doi: 10.2307/3503546
- Johns PM. 2001. Distribution and conservation status of ground weta, Hemiandrus species (Orthoptera: Anostostomatidae). Science for conservation 180, New Zealand Department of Conservation, Wellington, New Zealand.
- Leschen RAB, Buckley TR, Hoare R. 2009. The use of tag-names in New Zealand taxonomy. New Zealand Entomologist. 32:85–87. doi: 10.1080/00779962.2009.9722180
- Moeed A, Meads MJ. 1985. Seasonality of pitfall trapped invertebrates in three types of native forest, Orongorongo Valley, New Zealand. New Zealand J Zool. 12:17–53. doi: 10.1080/03014223.1985.10428264
- Pictet A, Saussure H. 1893. De quelques orthoptères nouveaux. Mitteilungen der Schweizerischen Entomologischen Gesellschaft. 8:293–318.
- Pratt RC, Morgan-Richards M, Trewick SA. 2008. Diversification of New Zealand weta (Orthoptera: Ensifera: Anostostomatidae) and their relationships in Australasia. Philos T R S B. 363:3427–3437. doi: 10.1098/rstb.2008.0112
- Salmon JT. 1950. A revision of the New Zealand wetas Anostostominae (Orthoptera: Stenopelmatidae). Dominion Museum Records of Entomology. 1:121–177.
- Saussure HD. 1859. Revue et Magasin de Zoologie. 2:209.
- Sinclair L, Stringer I. 2003. Invertebrate conservation with computer-based tools. Science poster number 60, New Zealand Department of Conservation, Wellington, New Zealand.
- Spurr EB, Berben H. 2004. Assessment of non-target impact of 1080-poisoning for vertebrate pest control on weta (Orthoptera: Anostostomatidae and Rhaphidophoridae) and other invertebrates in artificial refuges. New Zealand J Ecol. 28:63–72.
- Taylor-Smith BL, Morgan-Richards M, Trewick SA. 2013. New Zealand ground wētā (Anostostomatidae: Hemiandrus): descriptions of two species with notes on their biology. New Zealand J Zool. 40:314–329. doi: 10.1080/03014223.2013.804422
- Trewick SA, Morgan-Richards M. 2004. Phylogenetics of New Zealand’s tree, giant and tusked weta (Orthoptera: Anostostomatidae): evidence from mitochondrial DNA. Journal of Orthoptera Research. 13:185–196. doi: 10.1665/1082-6467(2004)013[0185:PONZTG]2.0.CO;2
- Trewick SA, Morgan-Richards M. 2005. After the deluge: mitochondrial DNA indicates Miocene radiation and Pliocene adaptation of tree and giant weta (Orthoptera: Anostostomatidae). J Biogeogr. 32:295–309. doi: 10.1111/j.1365-2699.2004.01179.x
- Walker F. 1869. Catalogue of the specimens of Dermaptera Saltatoria and supplement to the Blattariae in the collection of the British Museum. London: British Museum.
- White A. 1842. Gray’s zoological miscellany 78.
- White A. 1846. Voyage of the H.M.S. Erebus and Terror. Zoology, Insects, 9.