ABSTRACT
Many species of Eudyptes penguin have shown substantial population declines and in response, there have been efforts to identify the key demographic parameters. Here, we present the demographic parameters of one of the least well known and the least abundant species of crested penguin, the endangered Fiordland crested penguin Eudyptes pachyrhynchus. A population study incorporating mark–recapture, nest occupancy and breeding success was conducted over 16 years at several sites in the northern half of the range of the species. Survival probabilities were calculated using standard Cormack–Jolly–Seber models and the Burnham Live and Dead model. The annual probability of true survival for banded birds and apparent survival for birds with transponders were both estimated at 89% during their adult years, which is similar to that reported for Eudyptes penguin species inhabiting more southerly latitudes. Annual juvenile survival was assessed for Fiordland crested penguins until their first return at 77%. The mean breeding success (0.61 ± 0.02 chicks/pair) was higher than is observed for other crested penguin species, except the southern rockhopper penguin, which may be due to having lower A-egg ejection rates and higher rates of fledging two chicks per pair. Breeding success was related to the niche of predators present.
Introduction
Eudyptes penguins (Aves: Spenisciformes) range from the sub-Antarctic regions (60°S) to the temperate regions (c.43°S) throughout the Southern Ocean. The seven Eudyptes species have some similar morphological characteristics, such as yellow maxillary crests and some shared biological traits such as the laying of two asymmetrical eggs, reversed hatching, almost always raising only one chick successfully and a breeding cycle with fixed incubation shifts and guarding by the male only. Many of the species have shown substantial population declines during the twentieth and twenty-first centuries (Borboroglu & Boersma Citation2013). To identify the reasons for the decline and the management actions that could be implemented to halt the decline, there have been recent efforts to estimate the key demographic parameters of many of the Eudyptes species (e.g. Guinard et al. Citation1998; Baylis et al. Citation2013; Dehnhard et al. Citation2013; Horswill et al. Citation2014).
New Zealand is home to four of the crested penguin species, including three endemic species (Fiordland crested penguin, Snares crested penguin Eudyptes robustus and erect-crested penguin Eudyptes sclateri). Of the seven Eudyptes penguins, the least studied are the three New Zealand endemic species, with little information on population trends, and breeding and foraging behaviour. The Fiordland crested penguin is one of the more temperate living of the Eudyptes species, breeding at latitudes from 43.5°S to 46.8°S. Despite many breeding colonies being on mainland South Island, there are considerable gaps in our knowledge of the species. It breeds along the South Westland and Fiordland coastlines on the South Island and outlying islands, as well as on Stewart Island/Rakiura just to the south of the South Island. Fiordland crested penguins nest in dense rainforest, dense scrub, caves and rocky shorelines (Mattern Citation2013). Although nests are generally located several metres apart, chicks tend to congregate together (‘crèche’) during the post-brooding period (Warham Citation1974).
In the 1990s, the population was estimated at 2500–3000 breeding pairs (McLean et al. Citation1997), although this is considered an under-estimate (Mattern Citation2013). Nevertheless, it is one of the world’s least abundant penguin species. The status of the Fiordland crested penguin in New Zealand is Nationally Endangered, and it has been assessed by IUCN/BirdLife International as Vulnerable (BirdLife International Citation2012; Robertson et al. Citation2012; respectively).
Limited data on foraging ecology suggests that the Fiordland crested penguin is a demersal forager while breeding and would therefore be limited to the continental shelf (van Heezik Citation1989, Citation1990). The species commences breeding in the austral winter months and chicks begin fledging in November. The breeding behaviour of the Fiordland crested penguin was first studied at one mainland site in South Westland during 1966–1971 (Warham Citation1974). Subsequent studies focused on specific aspects of breeding biology, including brood reduction (Phillipson Citation1991; St Clair Citation1992; McLean Citation2000), nest and mate fidelity (St Clair et al. Citation1999), parent–offspring recognition (Studholme Citation1994), reproductive endocrinology (McQueen et al. Citation1998) and genetic monogamy (McLean et al. Citation2000).
There is some evidence that the Fiordland crested penguin has a declining population trajectory (e.g. St Clair et al. Citation1999; Robertson et al. Citation2012). At a number of breeding sites, the species encounters a range of conditions that may negatively affect adult and chick survival, and be the cause of the current decline. For example, birds breeding on the mainland and on some islands face the threat of egg and chick predation by introduced pests such as stoats, rodents, dogs and cats. In contrast, other populations occur on islands free of introduced mammalian predators. If we are to understand how the demographic characteristics of the species may drive the population trajectory and to identify whether management should focus on adult survival and/or breeding productivity, we need estimates of survival probabilities, breeding success and age of first breeding.
Warham (Citation1974) was able to determine hatching success, chick survival and the age of first breeding. St Clair et al. (Citation1999) calculated return rates of banded adult birds to a colony on Taumaka Island, part of the Open Bay Islands off the South Westland coastline (), of between 53% and 83% during six breeding seasons. Although these values can be considered as minimum survival estimates, we need more robust and precise data from multiple sites over longer periods of time.
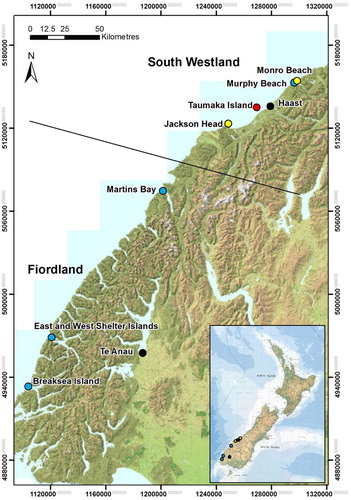
Figure 1. Location of the two sites that were used for both the mark–recapture study and nest/chick monitoring (yellow dots) and the five additional nest/chick monitoring sites (blue dots). The island of Taumaka (red dot), the towns of Haast and Te Anau (black dots) and the regions of South Westland and Fiordland (black line) are shown for geographic reference.
Therefore, alongside efforts to detect the numerical trend in nest numbers (as a proxy for the population trend) at sites in South Westland and Fiordland, a study was established in the early 1990s at two sites in South Westland to determine the demographic parameters. We document for the first time a complete picture of the demographic parameters of the species in the northern part of its range, which should fill a gap that has been identified by multiple authors in recent years (e.g. Croxall et al. Citation2012; Lewison et al. Citation2012; Mattern Citation2013).
Methods
Mark–recapture study sites and marking methods
A mark–recapture study was established at two sites on mainland South Westland, South Island—an area within the Jackson Head colony (43°58″S, 168°36″W) and the entire colony at Monro Beach (43°41″ S, 169°15″W) (). Annually within the mark–recapture sites, adult Fiordland crested penguins were captured in mid to late July (pre-breeding period), and in the first half of November (later stage of the post-brood period). Both adults and fully-grown chicks were captured. From 1994 to 1997, birds at both sites were marked with a stainless-steel flipper band. From 1998 onwards, glass-encased Trovan transponders were used, with the transponder inserted between the shoulder blades. The insertion point was not glued after the transponder was inserted. Birds at Jackson Head were marked with a transponder until 2005, whereas at Munro Beach, adults were marked with a flipper band until 2000, and chicks were marked until 2004 with a transponder (). At Jackson Head, 55 banded birds that were recaptured in 1998 and 1999 were also marked with a transponder.
Table 1. Number of Fiordland crested penguins tagged as adults and as chicks with bands, transponders and both marking methods at Jackson Head and Monro Beach.
Mark and recapture effort occurred annually from 1994 until 2005. There was no search effort between 2006 and 2009, but a final check for the presence of marked birds was made in July 2010 when all birds marked as chicks would be of a breeding age. Both study sites were searched on at least two and up to six separate occasions in July and November by at least two people, with searches being a few days apart so as to capture as many different birds as possible. All birds were recaptured to check for bands and/or transponders because neither an automated gateway system nor a transponder on an extended wand was practical because birds use multiple entry/exit points and they readily move off the nest when approached.
Adult birds were sexed using bill depth with all individuals with a bill depth > 24 mm being assigned as male. It is acknowledged that this would discriminate all males and only 90% of all females, but as both bill length and depth were not measured for all adults, the more discriminatory index using both measurements could not be used (Murie et al. Citation1991).
Survival analysis
Analysis of the capture–recapture data was carried out using MARK (White & Burnham Citation1999; White Citation2008). The terms ‘capture’ and ‘recapture’ are used here to denote, respectively, those birds caught and banded or that had transponders inserted for the first time and those birds previously marked with a band and/or transponder and recaptured at the study site. The notation used and parameters investigated in this analysis follow the convention set by Lebreton et al. (Citation1992). Due to the known effects of bands on penguins (Saraux et al. Citation2011, Dann et al. Citation2014), the survival of birds with bands and transponders was analysed separately.
To analyse the survival of birds with bands, the small number of dead recoveries allowed us to use the Burnham Live and Dead model (Burnham Citation1993). This model allows a joint analysis of band recovery data on dead animals and open capture–recapture data on animals released and alive. In the band recovery models one can estimate survival (S), reporting (r) or recovery (f) probabilities, whereas in the open capture–recapture models one can estimate apparent survival (Φ) and recapture probabilities (p) (Burnham Citation1993). The effect of marking method (band only, transponder only and band + transponder, ‘Method mark’) on the rate of survival was investigated for adults marked as adults using a Burnham Live and Dead analysis.
The analysis of survival of birds with transponders was made using standard Cormack–Jolly–Seber models where:
Φi = Probability that a bird alive at i is alive at i + 1;
Pi = Probability that a bird at risk of capture at i is captured at i + 1.
A general model that was fully time-dependent for Φ and P was taken as the starting point and used as the basis for all goodness-of-fit analyses (i.e. Φ (time) P(time)). Time in this model refers to an encounter in any 1 year. The most parsimonious recapture P models were identified (following the methodology of Lebreton et al. Citation1992), by examining the influence of time-dependent P on both age classes (‘Year marked’), constant P on both age classes, and time-dependent P in sub-adult birds only. Age and sex structure (i.e. ‘Age marked’*sex*Year marked) was introduced into Φ by distinguishing between adults and sub-adults (pooling juveniles [birds of the year] and immatures [birds captured before first breeding]), and between male and female birds. A two-age class structure, adult or sub-adult, was selected because sub-adult survival in many crested penguin species is known to be lower than adult survival (e.g. Guinard et al. Citation1998; Dehnhard et al. Citation2013, Citation2014; Horswill et al. Citation2014, Citation2016). We then examined the influence of time-dependent Φ on all four age/sex classes, constant Φ on all four age/sex classes, and time-dependent Φ in sub-adult birds only. The use of the latter model was based on the grounds that sub-adult Φ is lower than adult Φ in penguins, and is more sensitive to annual variation in environmental conditions (Ballerini et al. Citation2009; Emmerson & Southwell Citation2011; Dehnhard et al. Citation2014). Birds often disperse more in their first year than subsequently, and so we also investigated the significance of transience on the population by allocating sub-adult individuals in their first year after capture a separate survival or age probability using the same methodology as Perret et al. (Citation2003).
Corrected Akaike’s information criterion (AICc) in MARK was used to select the most parsimonious model from a set of candidate models (Cooch & White Citation2001). This procedure was preferred over the use of a likelihood ratio test to test between two nested models, because some of the analyses were conducted using non-nested models. For one model to be selected above another, Anderson and Burnham (Citation1999) recommended that the difference between their respective Akaike’s information criterion (ΔAICc) should be < 2. If ΔAICc is between 1 and 2 then these models should be considered similar, and any subsequent inference should be based on the subset of models unless parameterisation in the closely related models is significantly different (Posada & Buckley Citation2004). The logit link function was used throughout the modelling procedure. As this function in MARK is liable to having incorrect parameter counts, all parameters were recounted manually.
The goodness of fit for models of the banding and transponder data analysis was assessed in absolute terms using a parametric bootstrap approach available in programme MARK using 100 replicates. To deal with the issues of validity of the bootstrapped goodness of fit when sampling unequal time intervals (Gimenez et al. Citation2005), we created a general model in which each year (even those not sampled) was included in the model, but in which P was set at zero in those years in which the site was not visited.
The data sets of banded and transpondered birds were tested for over-dispersion using the median c-hat procedure. In addition, the goodness of fit of the transponder model was evaluated in a series of tests using the program UCARE. TEST2 and TEST3 (including TEST3.Sm and TEST3.SR) were used to assess the first two assumptions of the Cormack–Jolly–Seber model (Burnham et al. Citation1987; Lebreton et al. Citation1992) and TEST2.Ct was used to evaluate recapture heterogeneity (i.e. temporary emigration) (Pradel Citation1993; Choquest et al. Citation2001).
Site fidelity
We assessed fidelity of birds to their natal colony and of adult birds to a single colony using a number of survey methods. As well as detecting marked birds at the two study sites, there was also an opportunity to detect banded birds at an additional nest/chick monitoring site in South Westland (Murphy Beach, which is located between Munro Beach and Jackson Head, ). In addition, as the Jackson Head mark–recapture study area (400 m in length) encompassed only part of the colony, the birds present in July in an adjacent 300 m section were also captured and checked for bands and transponders in 2001, 2003 and 2010.
Nest/chick monitoring
To estimate breeding success, breeding pairs and chicks were counted at the two mark–recapture study sites and at five additional nest/chick monitoring sites (). One of the nest/chick monitoring sites was in South Westland (Murphy Beach) and four sites were in Fiordland (Martins Bay, East Shelter Island, West Shelter Island and Breaksea Island). In order to have at least 10 nests per monitoring site, some sites consisted of two or three spatially distinct monitoring plots, but the individual plot data were combined into one site data set. One nest/chick monitoring site was on a predator-free island, two monitoring sites were on islands with the only predator being the native rail weka (Gallirallus australis) and the other four monitoring sites were on mainland South Island where weka is not present but where many introduced predators such as stoats, ferrets, dogs and cats may be present.
At each monitoring site, breeding pairs were counted during the mid part of the incubation period (around mid-August) and chicks were counted during the latter part of the post-brood period (around early November). Counts were made at the three South Westland monitoring sites in 17 years during 1990–2009 and at four Fiordland monitoring sites for a period of 4 years during 1994–1998 or 1996–2000 and then for a further 4 years during 2006–2009. The number of breeding pairs (not number of chicks per nest) was also counted annually at three South Westland monitoring sites during the brood phase (mid-September) from 1990 until 1998.
The success of the breeding attempt during incubation was calculated as the ratio of breeding pairs found in August (incubation stage) to breeding pairs in September (brood stage). Mean breeding success was calculated as the ratio of breeding pairs found in August to chick counts in November (post-brood stage).
We place a small level of caution around breeding success, which is because of concerns around the accuracy of the chick count at a few of the nest/chick monitoring sites. This is because at these sites, there is potential for chicks produced by pairs nesting within the monitoring plot to crèche in areas outside the monitoring plot (so reducing the estimate of breeding success) and for chicks produced by pairs nesting outside the monitoring plot to crèche inside the monitoring plot (so increasing breeding success).
As two of the nest/chick monitoring sites in South Westland were also the sites where birds were tagged, some banded birds could be detected during the incubation and brood nest checks, and in most cases, the band combination could be recorded from a suitable distance (we did not capture nesting birds because of the risk of birds abandoning nests). As well as increasing the number of recapture events, the collection of this information allowed for additional demographic parameters such as age of first return and age at first breeding to be detected.
Means are expressed with ± standard error of the mean, unless otherwise indicated.
Results
Performance of marking methods
Thirty of the 55 Fiordland crested penguins marked with a band and a transponder were never recaptured. Of the other 25 birds, none lost their band and one bird’s transponder, which was inserted in 1999 and detected in 2004, was not detected by the scanner in 2010. This gives a transponder failure rate of 4% at c.10–12 years after insertion. Of eight banded birds recaptured in 2010, that had been wearing their bands for 12–16 years, none showed any signs of physical damage to the flipper or feathers, although two bands were 1–2 mm open (and these were adjusted).
Survival
The mark–recapture data set included 859 individuals and 1416 recapture events. In the first year of the study (1994), 271 birds were marked, and in subsequent years, between 8 and 84 new individuals were captured and marked and between 1 and 96 marked birds were captured (). A total of 249 adult Fiordland crested penguins marked as adults (43%) and 19 penguins marked as chicks (7%) were recaptured. Apart from the two mark–recapture study sites, recapture events also included live birds located at other beaches on the South Island’s West Coast and seven dead birds that were either found at the study colonies, other West Coast beaches, other sites in New Zealand or in southeast Australia.
Table 2. The total number of Fiordland crested penguins marked and recaptured at Jackson Head and Monro Beach in each year between 1994 and 2005 and in 2010.
The bootstrap goodness-of-fit tests indicated that, for the observed deviance in the full parameter model, the probability of a deviance, as large as or greater than the observed value, was approximately 0.65 for the model of banded birds and 0.63 for the model of the birds with transponders. Hence, we assumed an adequate goodness of fit. The logistic regression estimates of median c-hat (1.06 and 1.012) indicated that these models do not suffer from a substantial degree of over-dispersion.
When the RELEASE goodness-of-fit tests were run on the full parameter Cormack–Jolly–Seber model (transponder model, time-dependent Φ and P) using UCARE, the models passed TEST2 and TEST2.Ct (trap dependence statistic). The data set failed TEST3, but passed TEST3.Sm, indicating that transience was not a substantial problem. This was also demonstrated by the fact that models that incorporated transience did not give lower AICc values than comparable models that did not account for transience. On closer examination, it was found that the data passed TEST3.SR, indicating that survival probabilities were not cohort-dependent, and indeed this is what the model selection determined.
The analysis of all 637 banded birds (582 birds with bands and 55 banded birds up to the point they also received a transponder) using the Burnham Live and Dead analysis found there was no discernible difference between years or sites (i.e. the model S(Age marked) p(Age Marked) r(.) f(.) performed best). True survival was 89% for adults marked as adults and 86% for adults marked as chicks (). Models that incorporated sex as a variable in S or p performed poorly, suggesting that there is no discernible sexual difference in survival or in the probability of recapture.
Table 3. True survival probabilities and likelihood of recapture and reporting for adult Fiordland crested penguins marked with bands for the most parsimonious model S(Age marked) p(Age marked) r(.) f(.).
The analysis on 221 Fiordland crested penguins marked with transponders showed that the four most likely models had issues with parameterisation and identifiability, and for this reason, we used the model-averaging approach implemented in MARK to get realistic and justifiable estimates of apparent survival and error (White et al. Citation2001). Apparent annual survival was 89 ± 1% for adults marked as adults (n = 141 birds) and 77 ± 2% for adults marked as chicks (n = 80), and annual apparent survival probability of immatures until first return to the colony was 77 ± 2%.
A comparison of apparent survival for adults with the three marking methods found the best model to be one that assumes that birds with differing marking methods have different probabilities of survival, of being recaptured each year and of being reported dead (with transponder birds giving inestimable probabilities), but having similar immigration probabilities. Apparent survival for the three groups of mark type varied between 83% for banded birds and 88% for birds with transponders only ().
Table 4. Apparent survival probabilities for adult Fiordland crested penguins marked as adults with three marking methods for the most parsimonious model S(Method marked) p(Year marked) r(Method marked*year mark) f(.).
Lifespan
For a mark–recapture study over 17 years, the oldest known Fiordland crested penguin was 11 years of age. Of 242 unknown aged adults, 28 birds were recaptured 10+ years after the first capture, with one bird being recaptured 16 years after first being banded and two birds being recaptured 17 years after first being banded as adults.
Age at detected return and breeding
Of the 274 Fiordland crested penguin chicks banded or implanted with a transponder, 17 were re-sighted alive. In addition, four birds banded as chicks were found dead, with two at the Jackson Head mark–recapture site and two that were found washed ashore dead in southeastern Australia.
Fifteen marked chicks were first detected back in the natal colony between 1 and 6 years after fledging (mean 2.9 ± 0.4 years). Two birds banded as chicks were found breeding at 4 and 7 years of age. Both birds were first found at 1 and 2 years of age in the colony before being observed breeding.
Site fidelity
Only 19 of the 274 Fiordland crested penguins marked as chicks were re-sighted and of these, 18 were re-sighted at their natal colony and one (5%) was sighted paired up but without an egg at the other mark–capture study site (60 km away) 2 years after fledging. The birds marked as adults at the Jackson Head mark–recapture site also showed strong fidelity to the site where they were captured. In July 2001, only one of 67 birds captured in an area adjacent to the Jackson Head mark–recapture site was marked. Only this marked bird was found there during a second survey in the adjacent area in 2003 and no marked birds were found there in 2010.
There were only two records of a bird being captured in both mark–recapture study sites: the chick described above, and a second bird found at Jackson Head in 1998 and 2000. In 2003, this second bird was found first at Monro Beach and then 7 days later at Jackson Head. No banded Fiordland crested penguins were observed at the Murphy Beach nest/chick monitoring site, which is located between the two mark–recapture study sites.
Breeding success
At the three nest/chick monitoring sites in South Westland over 8 years, the mean success of the breeding attempt during incubation (breeding pairs during brood stage/breeding pairs during incubation stage) was 0.82 ± 0.03%. The mean breeding success (chicks/breeding pairs) at all seven nest/chick monitoring sites in South Westland and Fiordland over 8–17 years was 0.61 ± 0.02 (n = 168 counts), and ranged annually at each site between 0.00 and 1.00.
The mean breeding success for the seven monitoring sites varied between 0.42 and 0.90. Breeding success was significantly higher at the one predator-free island than at three of the four mainland sites, and at one of the two islands with weka only (F6,141 = 6.92, p < 0.001, Tukey post hoc test) (). Breeding success on one of the two islands with weka was also significantly higher than one of the four monitoring sites on the mainland (P = 0.0246).
Table 5. Mean breeding success of Fiordland crested penguins during 1990–2009 at one predator-free island, two islands with weka only and four mainland sites that are outside the range of the weka but have introduced predators.
Discussion
Survival
Robust estimates of survival of penguins generally come from studies where a high proportion of the birds in the study colony are marked and there is regular scanning of breeding and moulting areas for the presence of marked birds throughout the periods when birds are ashore. The only method that can achieve this without significant disturbance to most penguin species is to use an automatic gateway system that records the passage of birds with implanted transponders. This is demonstrated in the survival estimates provided by Dehnhard et al. (Citation2013, Citation2014) for southern rockhopper penguins (Eudyptes chrysocome), by Clarke et al. (Citation2003) and Dugger et al. (Citation2006) for Adélie penguins (Pygoscelis adeliae) and by Le Bohec et al. (Citation2007, Citation2008) for king penguins (Aptenodytes patagonicus).
Our study started in 1994, when banding and recapture of birds to read bands was the standard mark–recapture technique for penguins, and although later in the study we used transponders, we still had to rely on recapturing birds because an automated gateway system and a reader on an extendable wand are both impractical for this species. To avoid disturbing nesting birds, we captured birds in the mark–recapture study sites only during the pre-breeding period and the late post-brood period. We tried to increase the likelihood of capturing new birds and recapturing marked birds by searching study sites on a few days during each occasion. Although this search effort was lower than some other mark–recapture studies of penguin species, our study provides the first robust estimate of the survival of Fiordland crested penguins at two breeding colonies on mainland South Island.
Most survival analyses of penguins have calculated apparent survival and have not accounted for rates of immigration and emigration. In this study, we used the recoveries of dead birds to estimate a probability of true survival, which incorporates a rate of migration. The true survival was 89% for banded birds during their adult years. The same probability was also estimated for apparent survival of adult birds with transponders. This was higher than a return rate of 71% of banded adults in an earlier study that monitored 20–35 nests for six breeding seasons (range 53%–83%) (St Clair et al. Citation1999). In comparison, estimated adult apparent survival for other crested penguin species was 84%–96% for southern rockhopper penguins in the Falkland Islands (transponders) (Dehnhard et al. Citation2013) and 83% at Campbell Island (transponders) (Morrison et al. Citation2016), 84% for northern rockhopper penguins (bands) (Guinard et al. Citation1998) and a mean of 89% over 7 years for macaroni penguins (transponders) (Horswill et al. Citation2014). Although it is useful to compare apparent survival across the Eudyptes species, there are likely biases in this comparison, due to likely differences in how the tag type affects survival in each species and differences in natal and breeding site fidelity.
There were no significant inter-annual differences in apparent survival rates of banded birds. In contrast, other multi-year studies of southern rockhopper penguins and macaroni penguins showed inter-annual differences, with survival rates differing by up to 12% among years (Dehnhard et al. Citation2014; Horswill et al. Citation2014).
As for other penguin species such as southern rockhopper penguins and macaroni penguin (Dehnhard et al. Citation2013; Horswill Citation2015, respectively), modelling of the Fiordland crested penguin data revealed no effect of sex on survival.
Juvenile survival
Analysis of banded Fiordland crested penguins indicated that the survival of juvenile birds was lower than that for adult birds, which agrees with the literature for other penguin species (e.g. Guinard et al. Citation1998; Dehnhard et al. Citation2013, Citation2014; Horswill et al. Citation2014; Morrison et al. Citation2016). In Fiordland crested penguins, first-year birds tend to moult during January (Warham Citation1974), and no 1-year-old birds were sighted in any of the years when the nest/chick monitoring and the mark–recapture work were undertaken (P. van Klink and G. Newton pers. comm.). Therefore, for our study, it was only possible to assess juvenile survival for birds until their first return, and so the estimated probability of 77% is likely to comprise a lower level of survival during the first year and a higher level during subsequent years (see Guinard et al. Citation1998; Dehnhard et al. Citation2014).
Implications associated with different marking methods
Our analysis of survival assumed that tags are not lost because our set of 25 recaptured and double-marked Fiordland crested penguins had a 0% band loss rate and had a 0% transponder failure rate for 1–12 years after implementation and a 4% transponder failure rate at 12 years after insertion. In a study of southern rockhopper penguins on the Falkland Islands with transponders where glue was placed on the insertion point, the failure rate 3 months after insertion was 1% (one in 90 penguins) (M. Poisbleau, pers. comm.). However, studies of macaroni penguin chicks and little penguins (Eudyptula minor) using transponders detected failure rates of 4% and 5%, which was suspected to be due primarily to transponders being lost soon after being injected (C. Horswill pers. comm. and Dann et al. Citation2014, respectively).
Although studies assessing the effects of bands on various aspects of penguin breeding and foraging behaviour have found inconsistent results (e.g. Petersen et al. Citation2005; Boersma & Rebstock Citation2009, Citation2010; Hampton et al. Citation2010; Le Maho et al. Citation2011), a variety of studies on different penguin species, including our study, have detected that banded birds have a 4%–16% lower survival level than birds with transponders only (Dugger et al. Citation2006; Saraux et al. Citation2011; Dann et al. Citation2014; this study). This difference may be most crucial because of all the demographic parameters, adult survival contributes the most to the rate of population growth in long-lived seabirds (Wooller et al. Citation1992; Sæther and Bakke Citation2000).
Age at first breeding
In our study, the age at which Fiordland crested penguins started breeding could only be determined for banded birds because we did not wish to disturb nesting birds to check for the presence of a transponder. Warham (Citation1974) reported that one male bird was 5 years old before there was strong evidence that it had bred. During nest counts in which the bands on nesting birds were able to be read, the two youngest birds we found breeding were 4 years and 7 years of age, respectively. Hence the age at which the Fiordland crested penguin first breeds appears similar to congeneric species such as northern and southern rockhopper penguins, which is estimated to be at 4–5 years of age (Guinard et al. Citation1998; Dehnhard et al. Citation2014; Horswill et al. Citation2014).
Site fidelity
Whether chicks return to their natal colony to moult, prospect and breed is not well known in penguins, particularly in species such as Fiordland crested penguins that only breed in small colonies. In Spheniscus penguins, 5%–9% of African penguins and 3% of Humboldt penguins (Spheniscus humboldti) tagged as chicks returned to breed at their natal colony (Whittington et al. Citation2005; Simeone & Wallace Citation2014, respectively). In a southern rockhopper penguin colony of 5500 birds during a 2-year period, the 1st-year birds had very high survival rates, indicating that the rate of emigration of juveniles from the colony was low or nil (Dehnhard et al. Citation2014). This also seems to be the case for the Fiordland crested penguin, with only one of 19 recaptured Fiordland crested penguin chicks not found at its natal colony.
In our study, we also showed that adult Fiordland crested penguins had high fidelity to the site where they were first recaptured, with only one of 696 birds marked at one of the mark–recapture sites being found in an adjacent area of the breeding colony and only one bird marked as an adult was found at another mark–recapture or nest/chick monitoring site used in this study. Indeed, the species appears to have high nest fidelity, with detailed observational work by St Clair et al. (Citation1999) recording over a 6-year period that banded birds had a mean of 74% fidelity to one particular nest.
Breeding frequency
Only 43% of birds marked as adults were ever recaptured and the Burnham Live and Dead analysis of the banding data indicated that permanent emigration or temporary migration (e.g. due to skipping a breeding season) may account for 22% of the variability in the survival estimate. The detailed observational study by St Clair et al. (Citation1999) also estimated that for banded birds monitored at 46 nests over six breeding seasons, annually 19% of birds known to be alive were not sighted during the year. In comparison, studies of southern rockhopper penguins and macaroni penguins using automatic detection systems detected zero and very low rates of birds having a sabbatical year (i.e. skipped breeding or moulting, and then returned to breed the following breeding season) (Dehnhard et al. Citation2013; Horswill et al. Citation2016, respectively). This apparent difference in the breeding frequency between the Fiordland crested penguin and the southern rockhopper and macaroni penguins may be real, or it may be an artefact of the differing effort of detection employed. It is potentially more likely to be an artefact of the method as Horswill et al. (Citation2014) found that the annual recapture rate for macaroni penguins was best explained by the number of days per year that the automatic detection system was operational and the number of days per year when manual recapture was instead used.
Lifespan
The oldest Fiordland crested penguin in this study was ≥ 19 years (17 years plus 2 years of immaturity) and when last sighted was in the colony during the prospecting period. This is not unexpected as the similarly sized Magellanic penguin (Spheniscus magellanicus) has banded individuals that are ≥ 30 years of age (Boersma et al. Citation2013).
Breeding success
Warham (Citation1974) reported that over three breeding seasons at Jackson Head, a mean of 79% of pairs hatched at least one egg. This corresponds with the values observed in this study at three mainland sites, including at Jackson Head (a mean of 82%). Warham (Citation1974) also gives a breeding success (chicks close to fledging/breeding pair) of 0.64 and rounds it down to 0.50 to account for chicks that die before they actually fledge. This is also similar to the mean breeding success of 0.61 over 8–17 years for colonies in South Westland and Fiordland detected in this study.
The mean breeding success of the Fiordland crested penguin over its northern and central range (0.61 chicks/pair) is higher than is observed for Snares crested penguins, northern rockhopper penguins and macaroni penguins at Marion Island and South Georgia, and for southern rockhopper penguins at Staten Island, Macquarie Island and Marion Island (Marchant & Higgins Citation1990; Crawford et al. Citation2003; Wilson et al. Citation2010; Pütz et al. Citation2013; British Antarctic Survey unpublished data). However, it is lower than reported for southern rockhopper penguin in the Falkland Islands, where the mean breeding success for a number of colonies located across the archipelago during 1995–2009 was 0.74 chicks/pair (Baylis et al. Citation2013). The determinates of breeding success in Eudyptes species are not well understood and are likely to be complex (e.g. Baylis et al. Citation2012). The higher breeding success detected for Fiordland crested penguin and southern rockhopper penguin than for the larger macaroni, royal (Eudyptes schlegeli) and erect-crested penguins may be related to the former species having lower A-egg ejection rates and higher rates of fledging two chicks per pair compared with the latter species (Williams Citation1995; McLean Citation2000; Poisbleau et al. Citation2008; Demongin et al. Citation2010). Extrinsic factors, such as marine productivity, sea and air temperatures, rainfall, wind frequency and/or speed and on-land and at-sea predation pressures, some of which may be quite site-specific, are also likely to be involved (Warham Citation1974; Demongin et al. Citation2010; Horswill Citation2015; Morrison Citation2015; Saraux et al. Citation2016).
This study identified that breeding success in Fiordland crested penguin was related to the niche of predators present, being significantly higher at one predator-free island than at three of four mainland sites (with stoats, dogs, cats, etc. but no weka) and at one of two predator-free islands with weka. The result suggests that introduced predators may have a greater effect on breeding success than the weka. The relationship for other species of penguin that breed on mainland New Zealand is not clear cut. For yellow-eyed penguins (Megadyptes antipodes), breeding success is not higher on predator-free islands compared with sites with various combinations of predators (Seddon et al. Citation2013), and a review of terrestrial management actions identified that although trapping effort for rodents, mustelids and cats was not correlated with population growth rate, trapping did provide ancillary benefits (Busch & Cullen Citation2009). A study of the effect of predator control at little penguin colonies on the West Coast of the South Island showed no significant differences in breeding success between colonies with or without mustelid control during the breeding season (Wilson et al. Citation2013).
Conclusions
The Fiordland crested penguin has similar rates for many demographic parameters as other crested penguin species that inhabit more southerly latitudes, including age at first breeding, natal site fidelity and adult survival. However, adult Fiordland crested penguins may have comparatively low rates of fidelity to one breeding site and/or higher rates of sabbatical years. The Fiordland crested penguin also has a much higher breeding success than most other Eudyptes species, even at sites where there are introduced and/or native predators. As the foraging ecology of the species is not well understood, it is difficult to understand how much the temperate marine location of the species is the reason for these interspecific differences. With the key demographic parameters now documented for the Fiordland crested penguin, there is an opportunity to model them to determine population trends and to identify which of the life history processes most strongly drive the population trajectory. This study has also identified that there are still considerable knowledge gaps, including of the demography of the species at sites within its southern range, its foraging ecology and the effects of various native and introduced predators on breeding success.
Acknowledgements
We thank all the people involved in this long-term programme, including everyone who undertook the nest/chick counts and mark–recapture monitoring. For the work in South Westland, we especially recognise the work of Martin Abel, Paul van Klink, Megan Hieatt, Abi Fay, Glen Newton, Karen Mayhew, Gareth Hopkins, Rebecca Wilson and Kath Morris. We also wish to thank Graeme Taylor and John Lyall for their technical input and guidance of the programme, as well as their help in the field. Graeme Elliott also provided useful insights of the data set. Helen Peat kindly supplied unpublished British Antarctic Survey data. Cielle Stephens created . A review by Kyle Morrison helped to improve an early draft of the manuscript. Referees Nina Dehnhard and Kyle Morrison helped to provide much better clarity. Associate editor: Associate Professor Jim Briskie.
The raw data are available on request to the corresponding author.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Paul Scofield http://orcid.org/0000-0002-7510-6980
References
- Anderson DR, Burnham KP. 1999. Understanding information criteria for selection among capture-recapture or ring recovery models. Bird Study. 46:S14–S21. doi: 10.1080/00063659909477227
- Ballerini T, Tavecchia G, Olmastroni S, Pezzo F, Focardi S. 2009. Nonlinear effects of winter sea ice on the survival probabilities of Adélie penguins. Oecologia. 161:253–265. doi: 10.1007/s00442-009-1387-9
- Baylis AMM, Wolfaardt AC, Crofts S, Pistorius PA, Ratcliffe N. 2013. Increasing trend in the number of southern rockhopper penguins (Eudyptes c. chrysocome) breeding at the Falkland Islands. Polar Biology. 36:1007–1018. doi: 10.1007/s00300-013-1324-6
- Baylis AMM, Zuur AF, Brickle P, Pistorius PA. 2012. Climate as a driver of population variability in breeding gentoo penguins Pygoscelis papua at the Falkland Islands. Ibis. 154:30–41. doi: 10.1111/j.1474-919X.2011.01179.x
- BirdLife International. 2012. Eudyptes pachyrhynchus. The IUCN Red List of Threatened Species 2012: e.T22697776A38927872. http://dx.doi.org/10.2305/IUCN.UK.2012-1.RLTS.T22697776A38927872.en. Downloaded on 12 September 2016.
- Boersma PD, Frere E, Kane O, Pozzi LM, Pütz K, Raya Rey A, Rebstock GA, Simeone A, Smith J, van Buren A, Yorio P, Borboroglu PG. 2013. Magellanic penguin. In: Borboroglu PG, Boersma PD, editors. Penguins: natural history and conservation. Seattle: University of Washington Press; p. 232–263.
- Boersma PD, Rebstock GA. 2009. Flipper bands do not affect foraging-trip duration of Magellanic penguins. Journal of Field Ornithology. 80:408–418. doi: 10.1111/j.1557-9263.2009.00248.x
- Boersma PD, Rebstock GA. 2010. Effects of double bands on Magellanic penguins. Journal of Field Ornithology. 81:195–205. doi: 10.1111/j.1557-9263.2010.00277.x
- Borboroglu PG, Boersma PD, editors. 2013. Penguins: natural history and conservation. Seattle: University of Washington Press.
- Burnham KP. 1993. A theory for combined analysis of ring recovery and recapture data. In: Lebreton JD, North P, editors. Marked individuals in bird population studies. Basle: Birkhauser Verlag; p. 199–213.
- Burnham KP, Anderson D, White GC, Brownie C, Pollock KH. 1987. Design and analysis methods of fish survival experiments based on capture-recapture. American Fisheries Society Monographs No. 5.
- Busch J, Cullen R. 2009. Effectiveness and cost-effectiveness of yellow-eyed penguin recovery. Ecological Economics. 68:762–776. doi: 10.1016/j.ecolecon.2008.06.007
- Choquest R, Reboulet AM, Pradel R, Lebreton JD. 2001. UCARE User’s Guide. Version 3.1. Montpellier, Centre d’Ecologie Fonctionelle et Evolutive, Centre National de la Recherche Scientifique.
- Clarke J, Emmerson LM, Townsend A, Kerry KR. 2003. Demographic characteristics of the Adélie penguin population on Bechervaise Island after 12 years of study. CCAMLR Science. 10:53–74.
- Cooch E, White G. 2001. Using MARK: a gentle introduction. 2nd ed. [cited 2007 June 18] Available from: http://canuck.dnr.cornell.edu/mark/
- Crawford RJM, Cooper J, Dyer BM. 2003. Population of the macaroni penguin Eudytpes chrysolophus at Marion Island, 1994/95–2002/03, with information on breeding and diet. African Journal of Marine Science. 25:475–486. doi: 10.2989/18142320309504036
- Croxall JP, Butchart SHM, Lascelles B, Stattersfield AJ, Sullivan B, Symes A, Taylor P. 2012. Seabird conservation status, threats and priority actions: a global assessment. Bird Conservation International. 22:1–34. doi: 10.1017/S0959270912000020
- Dann P, Sidhu LA, Jessop R, Renwick L, Healy M, Dettmann B, Baker B, Catchpole EA. 2014. Effects of flipper bands and injected transponders on the survival of adult little penguins Eudyptula minor. Ibis. 156:73–83. doi: 10.1111/ibi.12122
- Dehnhard N, Poisbleau M, Demongin L, Ludynia K, Lecoq M, Masello JF, Quillfeldt P. 2013. Survival of rockhopper penguins in times of global climate change. Aquatic Conservation and Marine Freshwater Ecosystems. 23:777–789.
- Dehnhard N, Poisbleau M, Demongin L, Ludynia K, Quillfeldt P. 2014. High juvenile survival and return rate in southern rockhopper penguins but no effect of individual pre-fledging traits. Ibis. 156:548–560. doi: 10.1111/ibi.12167
- Demongin L, Poisbleau M, Strange IJ, Quillfeldt P. 2010. Effects of severe rains on the mortality of southern rockhopper penguin (Eudyptes chrysocome) chicks and its impact on breeding success. Ornitologia Neotropical. 21:439–443.
- Dugger KM, Ballard G, Ainley DG, Barton KJ. 2006. Effects of flipper bands on foraging behavior and survival of Adélie penguins (Pygoscelis adeliae). Auk. 123:858–869. doi: 10.1642/0004-8038(2006)123[858:EOFBOF]2.0.CO;2
- Emmerson L, Southwell C. 2011. Adélie penguin survival: age structure, temporal variability and environmental influences. Oecologia. 167:951–965. doi: 10.1007/s00442-011-2044-7
- Gimenez O, Choquet R, Lamor L, Scofield P, Fletcher D, Lebreton JD, Pradel R. 2005. Efficient profile-likelihood confidence intervals for capture-recapture models. Journal of Agricultural, Biological and Environmental Statistics. 10:184–196. doi: 10.1198/108571105X46462
- Guinard E, Weimerskirch H, Jouventin P. 1998. Population changes and demography of the northern rockhopper penguin on Amsterdam and Saint Paul Islands. Colonial Waterbirds. 21:222–228. doi: 10.2307/1521909
- Hampton SL, Ryan PG, Underhill LG. 2010. The effect of flipper banding on the breeding success of African penguins Spheniscus demersus at Boulders Beach, South Africa. Ostrich. 80:77–80. doi: 10.2989/OSTRICH.2009.80.2.2.829
- van Heezik YM. 1989. Diet of the Fiordland crested penguin during the post-guard phase of chick growth. Notornis. 36:151–156.
- van Heezik YM. 1990. Diets of yellow-eyed, Fiordland crested, and little blue penguins breeding sympatrically on Codfish Island, New Zealand. New Zealand Journal of Zoology. 17:543–548. doi: 10.1080/03014223.1990.10422952
- Horswill C. 2015. The relative importance of opposing drivers in determining population change in macaroni penguins Eudyptes chrysolophus. Published PhD thesis, University of Glasgow, Glasgow, UK. 166p.
- Horswill C, Matthiopoulos J, Green JA, Meredith MP, Forcada J, Peat H, Preston M, Trathan PN, Ratcliffe N. 2014. Survival in macaroni penguins and the relative importance of different drivers: individual traits, predation pressure and environmental variability. Journal of Animal Ecology. 83:1057–1067. doi: 10.1111/1365-2656.12229
- Horswill C, Ratcliffe N, Green JA, Phillips RA, Trathan PN, Matthiopoulos J. 2016. Unravelling the relative roles of top– down and bottom–up forces driving population change in an oceanic predator. Ecology. 97:1919–1928. doi: 10.1002/ecy.1452
- Le Bohec C, Durant JM, Gauthier-Clerc M, Stenseth NC, Park YH, Pradel R, Gremillet D, Gendner JP, Le Maho Y. 2008. King penguin population threatened by Southern Ocean warming. Proceedings of the National Academy of Sciences. 105:2493–2497. doi: 10.1073/pnas.0712031105
- Le Bohec C, Gauthier-Clerc M, Gremillet D, Pradel R, Bechet A, Gendner JP, Le Maho Y. 2007. Population dynamics in a long-lived seabird: I. Impact of breeding activity on survival and breeding probability in unbanded king penguins. Journal of Animal Ecology. 76:1149–1160. doi: 10.1111/j.1365-2656.2007.01268.x
- Lebreton JD, Burnham KP, Clobert J, Anderson KR. 1992. Modelling survival and testing biological hypotheses using marked animals: a unified approach with case studies. Ecological Monographs. 62:67–118. doi: 10.2307/2937171
- Le Maho Y, Saraux C, Durant JM, Viblanc VA, Gauthier-Clerc M, Yoccoz NG, Stenseth NC, Le Bohec C. 2011. An ethical issue in biodiversity science: the monitoring of penguins with flipper bands. Comptes Rendus Biologies. 334:378–384. doi: 10.1016/j.crvi.2011.04.004
- Lewison R, Oro D, Godley BJ, Underhill L, Bearhop S, Wilson RP, Ainley D, Arcos JM, Boersma PD, Borboroglu PG, et al. 2012. Research priorities for seabirds: improving conservation and management in the 21st century. Endangered Species Research. 17:93–121. doi: 10.3354/esr00419
- Marchant S, Higgins PJ, editors. 1990. Handbook of Australian, New Zealand and Antarctic birds. Volume 1. Part A. Melbourne: Oxford University Press.
- Mattern T. 2013. Fiordland penguin. In: Borboroglu PG, Boersma PD, editors. Penguins: natural history and conservation. Seattle: University of Washington Press; p. 153–167.
- McLean IG. 2000. Breeding success, brood reduction and the timing of breeding in the Fiordland crested penguin (Eudyptes pachyrhynchus). Notornis. 47:57–60.
- McLean IG, Abel M, Challies CN, Heppelthwaite S, Lyall J, Russ RB. 1997. The Fiordland crested penguin (Eudyptes pachyrhynchus) survey, stage V: mainland coastline, Bruce Bay to Yates Point. Notornis. 44:37–47.
- McLean IG, Kayes SD, Murie JO, Davis LS, Lambert DM. 2000. Genetic monogamy mirrors social monogamy in the Fiordland crested penguin. New Zealand Journal of Zoology. 27:311–316. doi: 10.1080/03014223.2000.9518240
- McQueen SM, Davis LS, Young G. 1998. The reproductive endocrinology of Fiordland crested penguins Eudyptes pachyrhynchus. Emu. 98:127–131. doi: 10.1071/MU98013
- Morrison KW. 2015. Factors affecting the population dynamics of eastern rockhopper penguins (Eudyptes chrysocome filholi) on Campbell Island, New Zealand. Published PhD thesis, Massey University, Palmerston North, New Zealand. 206p.
- Morrison KW, Armstrong DP, Battley FP, Jamieson SE, Thompson DR. 2016. Predation by New Zealand sea lions and brown skuas is causing the continued decline of an eastern rockhopper penguin colony on Campbell Island. Polar Biology. First Online. doi:10.1007/s00300-016-1996-9
- Murie JO, Davis LS, McLean IG. 1991. Identifying the sex of Fiordland crested penguins by morphometric characters. Notornis. 38:233–238.
- Perret N, Pradel R, Miaud C, Grolet O, Joly P. 2003. Transience, dispersal and survival rates in newt patchy populations. Journal of Animal Ecology. 72:567–575. doi: 10.1046/j.1365-2656.2003.00726.x
- Petersen SL, Branch GM, Ainley DG, Boersma PD, Cooper J, Woehler EJ. 2005. Is flipper banding of penguins a problem? Marine Ornithology. 33:75–79.
- Phillipson SM. 1991. Aspects of the brood reduction process of the Fiordland crested penguin. Unpublished Masters thesis. Lincoln, New Zealand: Lincoln University.
- Poisbleau M, Demongin L, Strange IJ, Otley H, Quillfeldt P. 2008. Aspects of the breeding biology of the southern rockhopper penguin Eudyptes c. chrysocome and new consideration on the intrinsic capacity of the A-egg. Polar Biology. 31:925–932. doi: 10.1007/s00300-008-0431-2
- Posada D, Buckley TR. 2004. Model selection and model averaging in phylogenetics: advantages of Akaike information criterion and bayesian approaches over likelihood ratio tests. Systematic Biology. 53:793–808. doi: 10.1080/10635150490522304
- Pradel R. 1993. Flexibility in survival analysis from recapture data: handling trap-dependence. In: Lebreton JD, North PM, editors. Marked individuals in the study of bird populations. Basle: Birkhäuser Verlag; p. 29–37.
- Pütz K, Raya Rey A, Otley H. 2013. Southern rockhopper penguin. In: Borboroglu PG, Boersma PD, editors. Penguins: natural history and conservation. Seattle: University of Washington Press; p. 113–129.
- Robertson HA, Dowding JE, Elliott GP, Hitchmough RA, Miskelly CM, O’Donnell CFJ, Powlesland RG, Sagar PM, Scofield RP, Taylor GA. 2012. Conservation status of New Zealand birds, 2012. New Zealand Threat Classification Series 4. Wellington, Department of Conservation.
- Sæther BE, Bakke Ø. 2000. Avian life history variation and contribution of demographic traits to the population growth rate. Ecology. 81:642–653. doi: 10.1890/0012-9658(2000)081[0642:ALHVAC]2.0.CO;2
- Saraux C, Chiaradia A, Salton M, Dann P, Viblanc VA. 2016. Negative effects of wind speed on individual foraging performance and breeding success in little penguins. Ecological Monographs. 86:61–77.
- Saraux C, LeBohec C, Durant JM, Viblanc VA, Gauthier-Clerc M, Beaune D, Park Y-H, Yoccoz NG, Stenseth NC, LeMaho Y. 2011. Reliability of flipper-banded penguins as indicators of climate change. Nature. 469:203–206. doi: 10.1038/nature09630
- Seddon PJ, Ellenberg U, van Heezik Y. 2013. Yellow-eyed penguin. In: Borboroglu PG, Boersma PD, editors. Penguins: natural history and conservation. Seattle: University of Washington Press; p. 91–110.
- Simeone A, Wallace RS. 2014. Evidence of philopatry and natal dispersal in Humboldt penguins. Emu. 114:69–73. doi: 10.1071/MU13021
- St Clair CC. 1992. Incubation behaviour, brood patch formation and obligate brood reduction in Fiordland crested penguins. Behavioral Ecology and Sociobiology. 31:409–416. doi: 10.1007/BF00170608
- St Clair CC, McLean IG, Murie JO, Phillipson SM, Studholme BJS. 1999. Fidelity to nest site and mate in Fiordland crested penguins. Marine Ornithology. 27:37–41.
- Studholme BJS. 1994. Parent-offspring recognition in the Fiordland crested penguin (Eudyptes pachyrhynchus). New Zealand Natural Sciences. 21:27–36.
- Warham J. 1974. The Fiordland crested penguin Eudyptes pachyrhynchus. The Ibis. 116:1–27. doi: 10.1111/j.1474-919X.1974.tb00220.x
- White GC. 2008. Program mark website assessed at [cited 2010 March 20]. Available from: http://welcome.warnercnr.colostate.edu/~gwhite/mark/mark.htm
- White GC, Burnham KP. 1999. Program MARK: survival estimation from populations of marked animals. Bird Study. 46:S120–138. doi: 10.1080/00063659909477239
- White GC, Burnham KP, Anderson DR. 2001. Advanced features of program mark. In: Field R, Warren RJ, Okarma H, Sievert PR, editors. Wildlife, land, and people: priorities for the 21st century. Proceedings of the Second International Wildlife Management Congress. Bethesda, Maryland, USA: The Wildlife Society; p. 368–377.
- Whittington PA, Randall RM, Crawford RJM, Wolfaardt AC, Klages NTW, Randall BM, Bartlett PA, Chesselet YJ, Jones R. 2005. Patterns of immigration to and emigration from breeding colonies by African penguins. African Journal of Marine Science. 27:205–213. doi: 10.2989/18142320509504079
- Williams TD. 1995. The penguins: Spheniscidae. Bird families of the world. Oxford: Oxford University Press.
- Wilson JW, Burle MH, Cuthbert R, Stirnemann RL, Ryan PG. 2010. Breeding success of northern rockhopper penguins (Eudyptes moseleyi) at Gough Island, South Atlantic Ocean. Emu. 110:137–141. doi: 10.1071/MU09095
- Wilson KJ, Lane R, Braidwood J, Columbus A, Davies I. 2013. Cars, dogs and mustelids: mortality of little penguins on the West Coast, South Island, New Zealand. In Agnew P. Proceedings of the 8th Oamaru Penguin Symposium 2012. New Zealand Journal of Zoology. 40:236–248. p. 248. doi: 10.1080/03014223.2012.741069
- Wooller RD, Bradley JS, Croxall JP. 1992. Long-term population studies of seabirds. Trends in Ecology and Evolution. 7:111–114. doi: 10.1016/0169-5347(92)90143-Y
