ABSTRACT
Only four species of freshwater Nematomorpha (Gordiida) and one undetermined Gordius sp. are known from New Zealand. During recent surveys in the South Island we discovered a new species belonging to the genus Gordionus, a genus new to New Zealand. Scanning electron microscopic observations of seven collected specimens revealed that the species is clearly morphologically distinct from all other members of the genus and is characterised by the presence of an extensive row of large bristles on the ventral side of the posterior end in males. This row is much larger than in any known Gordionus species. Analysis of the mitochondrial CO1 gene and of the nuclear 18S rRNA gene showed that the new Gordionus specimens form a monophyletic clade, but with partly extensive internal genetic difference. This indicates that cryptic species might be present, for although there are minor variations in cuticular areoles, other morphological characters indicate little difference between specimens.
LSID urn:lsid:zoobank.org:pub:E6E436C0-9032-4D4B-B08C-1C7B6948291A
Introduction
Of about 360-plus freshwater gordiid (Nematomorpha) species known globally, only five species in three genera are known to occur in New Zealand (Poinar Citation1991, Citation2009; Schmidt-Rhaesa Citation2008, Citation2013; see Townsend Citation1970 and Zervos Citation1989 for two further records of undetermined gordiids). The species include: Gordius dimorphus Poinar, Citation1991; Gordius paranensis Camerano, 1892; Parachordodes diblastus (Örley Citation1881); Euchordodes nigromaculatus Poinar, Citation1991; and one further, but undetermined, Gordius species (Schmidt-Rhaesa Citation2008; Schmidt-Rhaesa et al. Citation1998, Citation2000). Due to its varied topography, consisting of several islands and sharp mountain peaks, and the fact that this region had remained isolated for a very long time, New Zealand does possess a unique diversity of animals. Additionally, the country is known to possess a number of insect species that are considered to be adequate hosts for several species of nematomorphs (see Poinar Citation1991; Poulin Citation1995; Winterbourn Citation2005; Winterbourn & Pohe Citation2016). Therefore, it is expected that a broader and more thorough sampling may reveal new species of nematomorphs in New Zealand.
During the course of a recent study of nematomorph genetic diversity and population structure (Tobias et al. Citation2017), a comprehensive sampling of free-living, post-parasitic nematomorphs was conducted in a variety of montane and subalpine locations in the Central Otago region of New Zealand’s South Island. Altogether, this study yielded 87 specimens of adult nematomorphs. A thorough scanning electron microscopic (SEM) investigation of the collected material revealed the presence of seven specimens, which are described herein as the first Gordionus species from New Zealand (Parachordodes diblastus had been described as Gordius diblastus by Örley Citation1881 and then named Gordionus diblastus by Poinar Citation1991; but this species clearly belongs to the genus Parachordodes, see Schmidt-Rhaesa Citation2008). We also present mitochondrial and nuclear ribosomal DNA sequence data for this species, information that is rare for the genus.
Materials and methods
The seven Gordionus specimens were collected by hand in their free-living adult stage of small, first order streams in the cushionfields and tussock grasslands of three mountainous areas in the Central Otago region of the South Island: the Rock and Pillar Range (RP) (45.433755 S, 170.076145 E; 1319 m); the Old Man Range (OM) (45.344442 S, 169.199390 E; 1588 m); and the Ida Range (ID) (44.925604 S, 170.263658 E; 1148 m) between March and May 2016 (). The material was fixed in 70% ethanol overnight at 4 °C. Subsequently, the specimens were observed by light microscopy for body colour patterns, and the body lengths and diameters measured. Approximately 5 mm long sections of the anterior, mid-body and posterior region of each specimen were excised and stored in 70% ethanol for SEM, with the rest of the specimen stored at 4 °C in 95% ethanol for molecular work. Material for SEM was dehydrated in an increasing ethanol series, critical point dried and coated with gold in a sputter coater. Observations were made using a LEO SEM 1524. Digital images were taken.
Figure 1. Collection sites in Otago, South Island, New Zealand. ID, Ida Range; OM, Old Man Range; RP, Rock and Pillar Range.
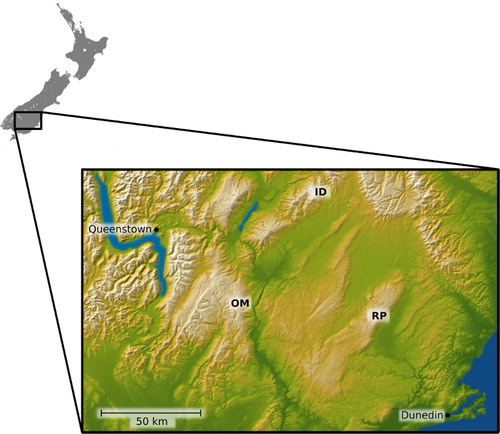
Specimens were deposited in the collections of the Otago Museum in Dunedin, New Zealand (abbreviated as OMNZ) and of the Zoological Museum in Hamburg, Germany (abbreviated as ZMH).
Genomic DNA from all nematomorphs was extracted using a modified Chelex method (Walsh et al. Citation1991). For each hairworm, about 5 mm section was removed and the cuticle was sliced longitudinally with a scalpel. The internal tissue was then scraped out and placed in 300 µl of an aqueous 5% Chelex 100 (BioRad) solution containing 0.2 mg/ml of proteinase K (Roche). Extractions were incubated at 55 °C overnight and then at 94 °C for 10 min to deactivate the proteinase K. Tubes were then centrifuged at 14,000 rpm for 10 min to pellet Chelex resin and undegraded cuticle. The supernatant was kept and stored at 4 °C.
Amplification of 709 bp or 592 bp partial fragments of the mitochondrial gene cytochrome c oxidase subunit 1 (CO1) were amplified using the primers HHW-cox1-F2(5′-TTTCWACYAATCAYAARGAYATTGG-3′) and HHW-cox1-R2(5′-TWACTTCAGGRTGACCAAAAAAYC-3′) (Hanelt et al. Citation2015), or the primers NZHW_CO1_F (5′-DTTYTGRTCTGCHTTTTTRGG-3′) and NZHW_CO1_R (5′-RAAAAAWGAWGTDTTAAYATTTCG-3′) (Tobias et al. Citation2017), respectively. The nuclear rDNA region containing a partial sequence of the 3′ end of the 18S rRNA gene, internal transcribed spacer 1, 5.8S rRNA gene, internal transcribed spacer 2, and a partial sequence of the 5′ end of the 28S rRNA were amplified using the primers HHW-ITS-F2 (5′-CACGCGCGCTACACTGAA-3′) and HHW-ITS-R2(5′-GGCGCTGGACTCTTCCTATT-3′) (Hanelt et al. Citation2015). Polymerase chain reactions (PCRs) were performed in a final volume of 15 µL, comprising 3 µL of MyTaq Red 5× reaction buffer (Bioline), 0.75–1.5 µl of 10 µM stock (75–150 pmol) of each primer, 0.2 µl (1U) of MyTaq Red DNA Polymerase (5U/µL) and 3 µL of extracted nematomorph genomic DNA. Products were purified of remaining dNTPs and oligonucleotides using the ExoSap method. Sanger sequencing by capillary electrophoresis was performed by Genetic Analysis Services, Department of Anatomy, University of Otago (Dunedin, New Zealand) or Macrogen Inc. (Seoul, Republic of Korea).
All sequence data editing and aligning was performed using Geneious v8.1.8 (http://www.geneious.com; Kearse et al. Citation2012). All sequences were trimmed with an error probability limit of 0.05, inspected by eye for incorrect base calls and ambiguities, and then edited manually. Alignments of the partial CO1 and partial 18S sequences were made using the MAFFT algorithms (Katoh & Stanley Citation2013). Sequences were inspected further to check for obvious incorrect base calls. CO1 pairwise genetic distance was calculated with MEGA v7 (Kumar et al. Citation2016) using the Kimura 2-parameter measure of genetic divergence (Kimura Citation1980). For phylogenetic analyses, model selection and optimal partitioning schemes were determined using PartitionFinder v2.0.0 (pre-release 14) (Lanfear et al. Citation2012). For CO1, the optimal partitioning scheme consisted of one partition containing all codon positions. Phylogenetic tree building was conducted using MrBayes v3.2.6 (Ronquist & Huelsenbeck Citation2003) and RAxML v8 (Stamatakis Citation2014), performed remotely using the CIPRES Science Gateway (Miller et al. Citation2010). For Bayesian analyses of 18S and CO1, PartitionFinder selected the models SYM + I + Γ and GTR + I + Γ, respectively. Two runs, each consisting of four Markov chains, were run for 10,000,000 generations and were sampled every 1,000. A chain heating parameter of 0.04 was used. The first 2,500 trees (25%) were discarded as burn-in and posterior probabilities were obtained from a majority-rule consensus. For maximum likelihood (ML), the RAxML default model of GTR+Γ was used for all markers. Bootstrap support values were obtained using the rapid bootstrap method with 1,000 replicates. Nematomorph reference sequences used in phylogenetic analyses were obtained from GenBank and can be found in . GenBank accession numbers for the new specimens are:
KY172682, KY172691, KY172700, KY172714, KY172743, KY172744, KY172758, KY172774, KY172779, KY172788, KY172814, KY172815.
Table 1. GenBank accession numbers of nematomorph sequences used for phylogenetic analyses.
Results
Taxonomic account
Class Nematomorpha Vejdovsky, 1886
Order Gordiida Vejdovsky, 1886
Family Chordodidae May, 1919
Genus Gordionus Müller, 1926
Gordionus maori Yadav, Tobias and Schmidt-Rhaesa, 2017
Material examined . Holotype (male, OMNZ IV85078), paratype (female, OMNZ IV85079) and five further specimens (four males: ZMH V13406, ZMH V13407, OMNZ IV85077, OMNZ IV85080 and one female: ZMH V13408).
Molecular results
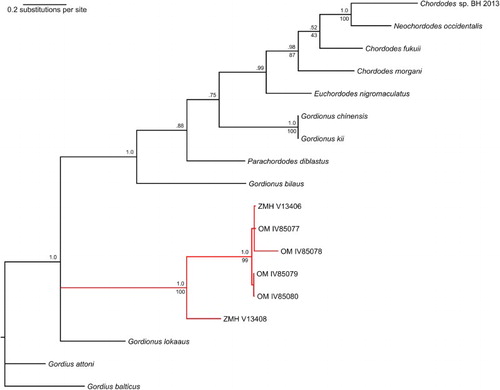
Phylogenetic analyses of both the CO1 and 18S genes demonstrate that the specimens of Gordionus maori n. sp. form a monophyletic clade (). This clade is well separated from other Chordodidae as well as from other Gordionus species, indicating that the genus is likely polyphyletic. As shown in the CO1 tree, ZMH V13408 is significantly divergent from the other members of the species (). Mean K2P genetic distance between ZMH V13408 and the other specimens was 0.205, compared to a mean of 0.03 among the remaining specimens alone.
Figure 2. Composite 18S maximum likelihood (ML)/Bayesian inference phylogenetic tree of Gordionus maori specimens and selected nematomorph reference sequences. Bayesian posterior probabilities shown above nodes; ML bootstrap support values shown below nodes. The branch containing the specimens of Gordionus maori n. sp. is marked in red. The labels of the specimens are their accession numbers (see text).
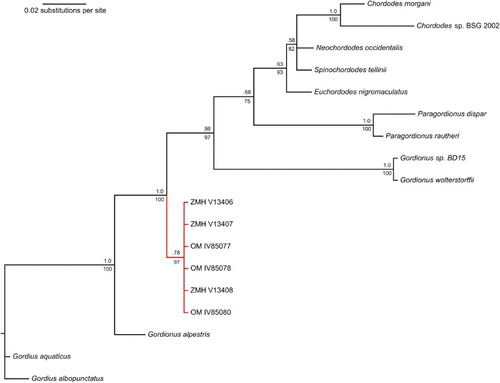
Figure 3. Composite CO1 maximum likelihood (ML)/Bayesian inference phylogenetic tree of Gordionus maori specimens and selected nematomorph reference sequences. Bayesian posterior probabilities shown above nodes; ML bootstrap support values shown below nodes. The branch containing the specimens of Gordionus maori n. sp. is marked in red. The labels of the specimens are their accession numbers (see text).
Morphological description
The seven specimens share similarities as well as smaller differences.
Males are between 58 and 140 mm long (58, 71, 81, 129, 140 mm; mean value 95.8 mm; holotype 81 mm), whereas females are 149 mm (paratype) and 81 mm (ZMH V13408) mm long. The diameter of males is between 0.22 (holotype) and 0.42 mm (0.22, 0.3, 0.4, 0.42, 0.30 mm; mean value 0.33 mm) and that of females is 0.5 mm (paratype) and 0.35 mm (ZMH V13408). The body colour varies between pale yellowish brown and light brown. A black collar in the head region, as present in most other Gordionus species, is not present. Only the female paratype (OMNZ IV85079) was dark tan on both ends.
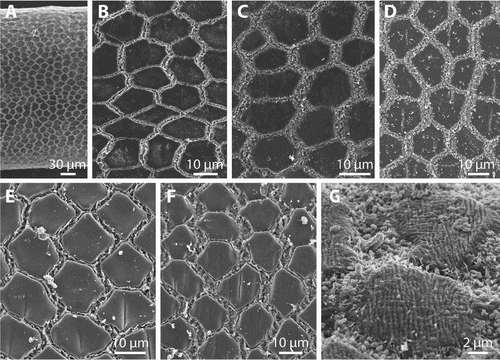
Cuticle in the midbody region of all specimens contained a single type of flat areoles (A–F). These are variable in shape and size, but mostly have a polygonal appearance (B–F). The surface of the areoles is either smooth (ZMH V13406, ZMH V13407, OMNZ IV85077, OMNZ IV85078) (B, C, D) or has a few distinct furrows (OMNZ IV85079, ZMH V13408; E, F), which are oriented perpendicular to the longitudinal axis of the animal. In specimen ZMH V13407 the smooth areoles appear to have a very fine striped pattern under high magnification (G). Areoles are separated by an interareolar groove. The width of this groove varies, the measured maximal width being between 3.0 and 6.0 µm. The groove is filled with short bristles that barely extend over the areolar surface (C, D). The bristles were evident in specimens ZMH V13407, OMNZ IV85077, OMNZ IV85078, OMNZ IV85079 and OMNZ IV85080, but in specimens ZMH V13406 and ZMH V13408 unidentifiable material in the groove obscured them (B, E, F). In specimen OMNZ IV85080 the small bristles partly extend onto the areolar surface. Higher magnification of specimen ZMH V13407 showed that the interareolar bristles have different sizes and varied between 1 and 2 µm (G).
Figure 4. Gordionus maori n. sp. A, Overview of the cuticle of ZMH V13407; B–F, pattern of areoles and interareolar spaces in specimens ZMH V13406 (B), ZMH V13407 (C), paratype OMNZ IV85079 (D) and ZMH V13408 (E–F); G, fine structure of areoles and interareolar structures in ZMH V13407.
The posterior end of males is bilobed, but the ventral cloacal opening, which lies slightly anterior of where the tail lobes start to separate from each other, was covered with debris in all male specimens and could not be observed. The region around the cloacal opening and the inner side of the tail lobes has a smooth cuticle (A–C). The region of the smooth cuticle is bordered in most specimens by a row of bristles (A–D). Anterior to the cloacal opening, both sides of the row almost meet and leave only a small stretch of cuticle without bristles in between (A, C). The rows are up to about 310 µm long. Posteriorly, they extend onto about two-thirds of the tail lobes (A–D). The length of the bristles decreases towards the anterior and posterior end of the rows (B–D). In the centre, at about the level of the cloacal opening, they reach their maximal length of between 27.3 and 36.1 mm (n = 4). Apically, some bristles have a few short branches (A–B). In the region posterior of the cloacal opening, and extending onto the inner side of the tail lobes, are short but slender spines (B–C). These could be observed only in the holotype, because the region is obscured by debris in the other specimens. The spines decrease in size towards the posterior end. On the posterior tip of the tail lobes are some very short processes. These processes are present in the smooth region of the cuticle as well as between the areoles (D). Where present, the areoles on the posterior end are more elevated than elsewhere on the body and are almost semicircular in shape (D). Adhesive warts (as present in other Gordionus species) were not observed, possibly because investigated pieces of the posterior end were too short.
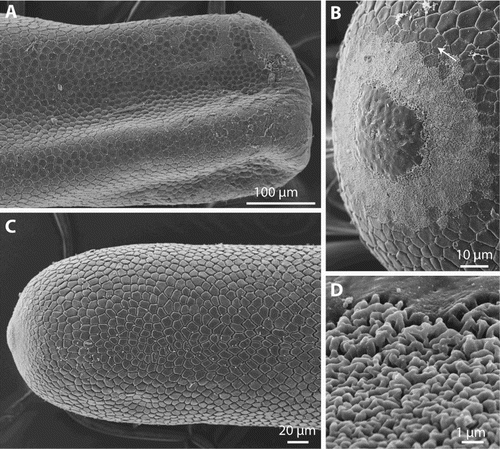
In the female specimen HW_086 the cuticular structure of the anterior end (C) did not have the usual areolar pattern, but was smooth (B) and from 5.5 to 20.0 µm (measured from the tip) there is a ring of altered cuticle (B) composed of a spongiform network of elevated cords (D).
The female posterior end, as observed in the paratype, showed no peculiar features, with areoles extending along the entire posterior end (A).
Figure 5. Gordionus maori n. sp. A–C, Ventral view of posterior end of ZMH V13406 (A), ZMH V13407 (B) and OMNZ IV85078 (holotype) (C). Encircled is the region of the cloacal opening, pbr indicates the precloacal rows of bristles; D, lateral view of a row of very large bristles in the holotype (OMNZ IV85078).
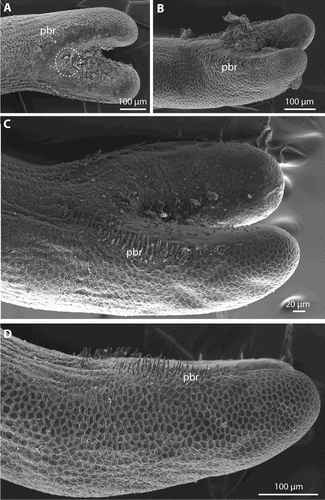
Figure 6. Gordionus maori n. sp. A–B, Magnification of precloacal bristles (pbr), encircled are some examples of apically branching bristles. ZMH V13407 (A), holotype (OMNZ IV85078) (B); C, spines (arrows) on the inside of the tail lobes in holotype; D, hemispherical areoles and short bristles (arrows) on the tips of the tail lobes in ZMH V13407.
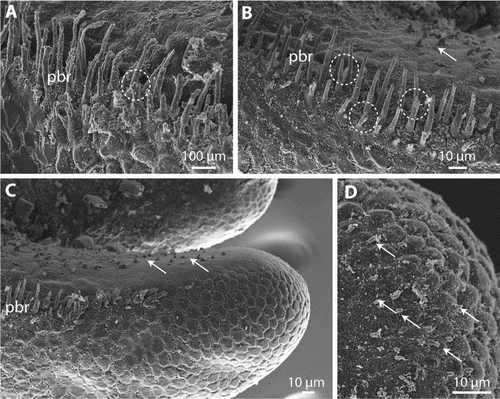
Figure 7. Gordionus maori n. sp. A, Posterior end of female specimen OMNZ IV85079 (paratype); B–D, anterior end in female specimen ZMH V13408, showing the changing pattern between smooth tip of the animal, region of alternate cuticular structure and transition to areoles. D shows the fine structure of the intermediate area.
Etymology. The name respectfully refers to the indigenous inhabitants of New Zealand, the Māori.
Type locality. Rock and Pillar Range (45.433755 S, 170.076145 E; 1319 m), South Island, New Zealand: one male, collected by Zachary Tobias and Brenah M. Hearne on 26 March 2016.
Further localities . Three specimens from type locality (ZMH V13406, ZMH V13407, OMNZ IV85077). Old Man Range (45.344442 S, 169.199390 E; 1588 m) (paratype, OMNZ IV85079) and the Ida Range (44.925604 S, 170.263658 E; 1148 m) (ZMH V13408, OMNZ IV85080). Specimens were collected by one of the authors, Zachary Tobias and his fiancée Brenah M. Hearne on 26 March 2016 (Rock and Pillar Range), 4 May 2016 (Old Man Range) and 14 May 2016 (Ida Range).
Type depositories. Specimens were deposited in the Otago Museum (Dunedin, New Zealand) and in the Zoological Museum Hamburg (Germany) under the following accession numbers: holotype (Otago Museum IV85078), paratype (Otago Museum IV85079). Further non-type material: ZMH V13406, ZMH V13407, OMNZ IV85077, OMNZ IV85080, ZMH V13408.
Differential diagnosis. The investigated New Zealand specimens fit quite well into the descriptions of the genus Gordionus and still represent a unique species. Gordionus is a wellknown genus, consisting of about 59 valid species, which are mainly distributed in Europe, Asia, and North and South America (see Begay et al. Citation2012; Schmidt-Rhaesa Citation2013; Ernst et al. Citation2016), but none have been reported from Oceania or Australasia regions until now. The cuticle contains only one type of areole, with or without some knob- or bristle-like interareolar structures. The male posterior end is similar to other genera (Paragordionus, Parachordodes and Beatogordius) and bears paired rows or fields of bristles anterolateral of the cloacal opening and spines in the region posterior to the cloacal opening (see Schmidt-Rhaesa Citation2002, Citation2013). In a single known case (G. sinepilosus Schmidt-Rhaesa, Hanelt and Reeves, Citation2003) the precloacal rows of bristles are lacking (Schmidt-Rhaesa et al. Citation2003). In comparison with other Gordionus species, the rows of bristles in G. maori appear more extensive, include very long bristles and extend posteriorly distinctly further onto the tail lobes. Therefore the expression ‘precloacal bristles’ is not precise in this context, but it expresses homology to the shorter rows of bristles in other Gordionus species. Bristles in other Gordionus species may also be apically branched. The shape of the postcloacal spines in the New Zealand specimens corresponds to that in other Gordionus species. Usually, there are short spines around the cloacal opening in Gordionus specimens; these could not be observed in the New Zealand specimens because the region was covered with debris. Nevertheless, the mentioned differences make it certain that the New Zealand specimens represent a new species.
Additionally, the structure of the anterior end of specimen HW_086 has not been observed in any other gordiid specimen. Whether this is a constant character of the species could not be confirmed, because only one anterior end was investigated.
Further remarks. Analysis of DNA sequence data revealed that, although all investigated specimens form a monophyletic clade, there is considerable genetic distance between specimen ZMH V13408 and the other specimens. Nevertheless, we could not detect clear morphological differences between specimen ZMH V13408 and the other specimens. Because ZMH V13408 is a female and, as in most nematomorph species, the posterior end of the female in Gordionus species does not contain any reliable diagnostic characters, only the cuticular structure can be used for comparison. The interareolar space of specimen ZMH V13408 appears to have no bristles, but there were some cuticular structures in the interareolar space of this specimen. The appearance of these interareolar structures is comparable to specimen ZMH V13406. It is not clear whether bristles are really absent or are modified in an artificial way, possibly due to desiccation or other preparation procedures. Due to the lack of clear morphological differences, we hesitate to consider specimen ZMH V13408 as a distinct species, although this possibility exists based on the genetic difference.
The K2P CO1 genetic distances between ZMH V13408 and the other specimens is much greater than intraspecific distances observed in studies of other hairworm species, and are more in agreement with interspecific distances (Chiu et al. Citation2011; Hanelt et al. Citation2015). Chiu et al. (Citation2011) found intraspecific distances of no greater than 0.019 among 39 specimens of Chordodes formosanus, and distances greater than 0.165 when comparing to the congeneric Chordodes japonensis. In a study revealing cryptic species within Gordius robustus, Hanelt et al. (Citation2015) demonstrated intraspecific distances of less than 0.026 and interspecific distances between 0.080 and 0.243.
Morphological variability of cuticular characters is still little understood in nematomorphs. Variation of areolar shapes has been documented; for example, for Gordionus violaceus (Baird, 1853) and G. wolterstorffii (Camerano, 1888) (Schmidt-Rhaesa Citation2001) and also for G. chinensis (Villot, 1874) (Schmidt-Rhaesa & Sato Citation2009). Unpublished CO1 data make it possible that G. violaceus is a species without much variation, but G. wolterstorffii is a species with a broad range of variation (A. Schmidt-Rhaesa, University of Hamburg, unpubl. data).
The position of G. maori in our two phylogenetic trees confirms that Gordionus is polyphyletic as found by Begay et al. (Citation2012) and Efeykin et al. (Citation2016). From the morphological perspective, no characters can be named that unequivocally support a monophyly of this genus (Schmidt-Rhaesa Citation2002). According to our molecular results, species of Gordionus are placed basally within the Chordodidae and it can be inferred that several other genera (Parachordodes, Beatogordius, Paragordionus) and the subfamily Chordodinae evolved from Gordionus-like ancestors.
Acknowledgements
We thank Robert Poulin (Otago, New Zealand) for his support and impact on the project. AKY participated as an Overseas Research Associate of Department of Biotechnology, Govt. of India at University of Hamburg. Renate Walter (Hamburg) kindly assisted in SEM work. Associate Editor: Dr Christina Painting.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Aleshin VV, Milyutina IA, Kedrova OS, Vladychenskaya NS, Petrov NB. 1998. Phylogeny of Nematoda and Cephalorhyncha derived from 18S rDNA. Journal of Molecular Evolution. 47:597–605. doi: 10.1007/PL00006416
- Begay AC, Schmidt-Rhaesa A, Bolek MG, Hanelt B. 2012. Two new Gordionus species (Nematomorpha: Gordiida) from the southern Rocky Mountains (USA). Zootaxa. 3406:30–38.
- Blaxter ML, De Ley P, Garey JR, Liu LX, Scheldeman P, Vierstraete A, Vanfleteren JR, Mackey LY, Dorris M, Frisse LM, Vida JT. 1998. A molecular evolutionary framework for the phylum Nematoda. Nature. 392:71–75. doi: 10.1038/32160
- Bleidorn C, Schmidt-Rhaesa A, Garey JR. 2002. Systematic relationships of Nematomorpha based on molecular and morphological data. Invertebrate Biology. 121:357–364. doi: 10.1111/j.1744-7410.2002.tb00136.x
- Bolek MG, Szmygiel C, Kubat A, Schmidt-Rhaesa A, Hanelt B. 2013. Novel techniques for biodiversity studies of gordiids and description of a new species of Chordodes (Gordiida, Nematomorpha) from Kenya, Africa. Zootaxa. 3717:23–38. doi: 10.11646/zootaxa.3717.1.2
- Chiu MC, Huang CG, Wu WJ, Shiao SF. 2011. A new horsehair worm, Chordodes formosanus n. sp. (Nematomorpha: Gordiida) from Hierodula mantids of Taiwan and Japan with redescription of a closely related species, Chordodes japonensis. Zookeys. 160:1–22. doi: 10.3897/zookeys.160.2290
- Efeykin BD, Schmatko VY, Spiridonov SE. 2016. Comparative phylogenetic informativity of single ribosomal cluster regions in freshwater horsehair worms (Gordiacea, Nematomorpha). Biology Bulletin. 43:34–41. doi: 10.1134/S1062359016010040
- Ernst CM, Hanelt B, Buddle CM. 2016. Parasitism of ground beetles (Coleoptera: Carabidae) by a new species of hairworm (Nematomorpha: Gordiida) in Arctic Canada. J Parasitol. doi:10.1645/15-863.
- Hanelt B, Schmidt-Rhaesa A, Bolek MG. 2015. Cryptic species of hairworm parasites revealed by molecular data and crowdsourcing of specimen collections. Molecular Phylogenetics and Evolution. 82:211–218. doi: 10.1016/j.ympev.2014.09.010
- Harkins C, Shannon R, Papeş M, Schmidt-Rhaesa A, Hanelt B, Bolek MG. 2016. Using gordiid cysts to discover the hidden diversity, potential distribution, and new species of gordiids (phylum Nematomorpha). Zootaxa. 4088:515–530. doi: 10.11646/zootaxa.4088.4.3
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution. 30:772–780. doi: 10.1093/molbev/mst010
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, et al. 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 28:1647–1649. doi: 10.1093/bioinformatics/bts199
- Kimura M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution. 16:111–120. doi: 10.1007/BF01731581
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Molecular Biology and Evolution. doi: 10.1093/molbev/msw054
- Lanfear R, Calcott B, Ho SY, Guindon S. 2012. PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Molecular Biology and Evolution. 29:1695–1701. doi: 10.1093/molbev/mss020
- Looney C, Hanelt B, Zack RS. 2012. New records of nematomorph parasites (Nematomorpha: Gordiida) of ground beetles (Coleoptera: Carabidae) and camel crickets (Orthoptera: Rhaphidophoridae) in Washington State. Journal of Parasitology. 98:554–559. doi: 10.1645/GE-2929.1
- Miller MA, Pfeiffer W, Schwartz T. 2010. “Creating the CIPRES Science Gateway for inference of large phylogenetic trees”. Proceedings of the Gateway Computing Environments Workshop (GCE), 14 November 2010, New Orleans, LA; p. 1–8.
- Örley L. 1881. On hair-worms in the collection of the British Museum. Annals and Magazine of Natural History. 8:325–332. doi: 10.1080/00222938109487466
- Poinar GO. 1991. Hairworm (Nematomorpha: Gordioidea) parasites of New Zealand wetas (Orthoptera: Stenopelmatidae). Canadian Journal of Zoology. 69:1592–1599. doi: 10.1139/z91-223
- Poinar GO. 2009. Phylum Nematomorpha, horsehair worms, gordian worms. In: DP Gordon, editor. New Zealand inventory of biodiversity. Vol. 1. Christchurch: Canterbury University Press; p. 491–497.
- Poulin R. 1995. Hairworms (Nematomorpha: Gordioidea) infecting New Zealand short-horned grasshoppers (Orthoptera: Acrididae). Journal of Parasitology. 81:121–122. doi: 10.2307/3284023
- Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 19:1572–1574. doi: 10.1093/bioinformatics/btg180
- Sato T, Watanabe K, Fukushima K, Tokuchi N. 2014. Parasites and forest chronosequence: long-term recovery of nematomorph parasites after clear-cut logging. Forest Ecology and Management. 314:166–171. doi: 10.1016/j.foreco.2013.12.004
- Sato T, Watanabe K, Tamotsu S, Ichikawa A, Schmidt-Rhaesa A. 2012. Diversity of nematomorph and cohabiting nematode parasites in riparian ecosystems around the Kii Peninsula, Japan. Canadian Journal of Zoology. 90:829–838. doi: 10.1139/z2012-048
- Schmidt-Rhaesa A. 2001. Variation of cuticular characters in the Nematomorpha: studies on Gordionus violaceus (Baird, 1853) and G. wolterstorffii (Camerano, 1888) from Britain and Ireland. Systematic Parasitology. 49:41–57. doi: 10.1023/A:1010600200665
- Schmidt-Rhaesa A. 2002. Are the genera of Nematomorpha monophyletic taxa? Zoologica Scripta. 31:185–200. doi: 10.1046/j.1463-6409.2002.00073.x
- Schmidt-Rhaesa A. 2008. New data on New Zealand horsehair worms (Nematomorpha, Gordiida). Mitteilungen aus dem Hamburgischen Zoologischen Museum und Institut. 105:5–12.
- Schmidt-Rhaesa A. 2013. Nematomorpha. In: Schmidt-Rhaesa A, editor. Handbook of Zoology. Gastrotricha, Cycloneuralia and Gnathifera. Vol. 1: Nematomorpha, Priapulida, Kinorhyncha and Loricifera. Berlin: De Gruyter; p. 29–145.
- Schmidt-Rhaesa A, Hanelt B, Reeves WK. 2003. Redescription and compilation of Nearctic freshwater Nematomorpha (Gordiida), with the description of two new species. Proceedings of the Academy of Natural Sciences of Philadelphia. 153:77–117. doi: 10.1635/0097-3157(2003)153[0077:RACONF]2.0.CO;2
- Schmidt-Rhaesa A, Sato T. 2009. Gordionus chinensis (Villot, 1874) and Gordionus kii sp.n. (Nematomorpha, Gordiida), new reports of the genus Gordionus in Japan. Species Diversity. 14:61–67.
- Schmidt-Rhaesa A, Thomas F, Poulin R. 1998. Scanning electron microscopy and intraspecific variation in Euchordodes nigromaculatus from New Zealand. Journal of Helminthology. 72:65–70. doi: 10.1017/S0022149X00001000
- Schmidt-Rhaesa A, Thomas F, Poulin R. 2000. Redescription of Gordius paranensis Camerano, 1892 (Nematomorpha), a species new for New Zealand. Journal of Natural History. 34:333–340. doi: 10.1080/002229300299516
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 30:1312–1313. doi: 10.1093/bioinformatics/btu033
- Tobias ZJC, Yadav AK, Schmidt-Rhaesa A, Poulin R. 2017. Intra- and interspecific genetic diversity of New Zealand hairworms (Nematomorpha). Parasitology (online first). doi: 10.1017/S0031182017000233
- Townsend JI. 1970. Records of gordian worms (Nematomorpha) from New Zealand Carabidae. New Zealand Entomologist. 4:98–99. doi: 10.1080/00779962.1970.9722929
- Walsh PS, Metzger DA, Higuchi R. 1991. Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques. 10:506–513.
- Winnepenninckx B, Backeljau T, Mackey LY, Brooks JM, De Wachter R, Kumar S, Garey JR. 1995. 18S rRNA data indicate that Aschelminthes are polyphyletic in origin and consist of at least three distinct clades. Molecular Biology and Evolution. 12:1132–1137.
- Winterbourn MJ. 2005. Dispersal, feeding and parasitism of adult stoneflies (Plecoptera) at a New Zealand forest stream. Aquatic Insects. 27:155–166. doi: 10.1080/01650420500062840
- Winterbourn MJ, Pohe SR. 2016. Feeding and parasitism of adult Stenoperla spp. (Plecoptera: Eustheniidae) in New Zealand. Austral Entomology. doi: 10.1111/aen.12222
- Zervos S. 1989. Stadial and seasonal occurrence of gregarines and nematomorphs in two New Zealand cockroaches. New Zealand Journal of Zoology. 16:143–146. doi: 10.1080/03014223.1989.10423708
