ABSTRACT
Confuga persephone is the only troglobitic planthopper species known for New Zealand to date. We provide a complementary description for this species, including photographs of structures of taxonomic interest, as well as from a living specimen. Information on its habitat and threats is given. We strongly recommend this species to be included on the list of endangered species for New Zealand.
Introduction
Caves comprise subterranean environments mainly formed by dissolution, erosion and/or fractures on rocks such as limestones, dolomites, quartzites, among others (Gines & Gines Citation1992; Gibert et al. Citation1994), as well as by volcanic activity (e.g. White Citation2005). They present total absence of light far from the entrances, which makes direct primary photosynthetic production impossible. Hence, the majority of the organic resources used by cave species are allochthonous (Culver Citation1982; Simon et al. Citation2007; Souza Silva et al. Citation2011); roots from surface vegetation penetrating into cavernous spaces relatively close to the surface represent autochthonous resources for herbivorous invertebrates (Stone et al. Citation2005). Among the several groups that can be found in caves there are species from the Hemiptera suborder Auchenorrhyncha (Hoch Citation1991). Although they are phytophagous, such organisms can be found in caves feeding on roots from epigean vegetation (e.g. Hoch Citation1994). There are species that establish populations both inside and outside caves, being considered as troglophiles (Cavichioli & Takiya Citation2012). However, there are some species that evolved in caves, becoming specialised in such environments and thus losing an ability to survive in epigean habitats (troglobites) (e.g. Hoch & Howarth Citation1989).
Troglobites are usually fragile and vulnerable to environmental changes (Eberhard Citation2001). Many species present small populations and low resilience, and should therefore be protected by specific legislation aiming at their conservation. In the case of the troglobitic Auchenorryncha, there are only few cases of ‘widely’ distributed species (Hoch & Ferreira Citation2013), and most taxa are known only for the type locality or nearby caves. Examples of highly endemic troglobitic Auchenorrhyncha (known for a single cave) are Ibleocixius dunae D’Urso and Grasso, Citation2009 (from a limestone cave in Sicily), Trirhacus helenae Hoch, Citation2013 (from a limestone cave in Croatia) and Ferricixius davidi Hoch and Ferreira, Citation2012 (from one iron ore cave in Brazil), among many others (see also Hoch Citation1994; Hoch & Asche Citation1993; Hoch et al. Citation2012).
Species descriptions prepared decades ago sometimes did not incorporate details of the species morphology that are only accessed using new tools that allow a better visualisation of their structures. Furthermore, previous descriptions that considered only few specimens frequently did not incorporate the description of possible variations of structures that may have taxonomic significance. Accordingly, complementary descriptions can become extremely important, allowing the improvement of knowledge regarding many aspects of the biology of a given species (Sartori Citation2014; Saturnino et al. Citation2015; Oliarinony et al. Citation2016). Additionally, by ‘revisiting’ the location of a species that has been described a long time ago it is possible to verify its conservation status, which may be essential in order to define protection policies that may be needed.
In 1975 R.G. Fennah described a new genus and new species of a troglobitic cixiid from a cave in the region of Nelson, New Zealand: Confuga persephone (Fennah, Citation1975). In the original description, 19 specimens were mentioned, of which seven were adult males, four adult females and eight nymphs. These specimens were collected on four different occasions, although the number of specimens collected on each date was not specified. No information, however, was provided on the biology of the species, its habitat, or its population and conservation status. It was only 40 years after the description of Confuga persephone that researchers from the Center of Studies of Subterranean Biology (CEBS), Brazil, together with cave biologists from New Zealand, were able to return to the type locality of the species. During this visit some exuviae were observed, as well as a single living specimen (an adult female) and three dead males (carcasses), but with their genitalia and tegmina relatively well preserved. Therefore, the objectives of this work is to provide a complementary description of Confuga persephone (including the first ever photograph of a living specimen, as well as high-resolution photographs of structures of taxonomic importance), and to present some observations on the conservation status of this species. Finally, we argue that this species should be considered as Threatened, according to the New Zealand Threat Classification System.
Material and methods
Collecting, preservation, permanent storage
The living female was collected by hand and transferred immediately into a vial containing 96% ethanol. The male carcasses () were also collected manually and gently transferred to dry Eppendorf tubes.
Morphological examination techniques, visualisation
Measurements and examinations of external body features were made from the specimen in ethanol, without further manipulation. To prepare male genitalia for dissection, the genital capsule was removed from the carcasses, macerated for 24 h in 10% KOH at room temperature, washed in water, and transferred to glycerine for storage or to glycerine-jelly for photographs. The description of colouration follows the standard names of the 267 Color Centroids of the NBS/IBCC Color System (Mundie Citation1995).
Habitus photographs
The software Zen 2.3 and the stereomicroscope Zeiss Axio Zoom V16 was used for image capture and measurements (given in millimetres). The images were subsequently processed with Adobe Photoshop CS3. The photographs were taken from both the female specimen preserved in ethanol and the structures (genital parts and tegmina) from the male carcasses.
Depository
CEBS—Centro de Estudos em Biologia Subterrânea; ISLA—Coleção de Invertebrados Subterrâneos da UFLA (Universidade Federal de Lavras), Brazil.
Taxonomy
Cixiidae Spinola, 1839
Confuga Fennah, Citation1975
Type species. Confuga persephone Fennah, Citation1975 (type locality: New Zealand, Nelson Province, Council Cave, east of Takaka).
Original diagnosis (Fennah Citation1975)
The following text was transcribed from the original description (Fennah 1975, p. 379):
Length (male) 4.5 mm, (female) 5.0 mm. Pale yellowish brown, membranous areas creamy white. Tegmina dilute yellowish brown, with brown veins. Vertex 1.5× broader than long, disc of each compartment depressed; frons nearly 1.2× longer than broad, wider at base than at apex (not quite 1.2:1), widest at middle, disc transversely convex except near lateral margins; postclypeus medially finely carinate; antennae with segment 2 2.3× longer than broad, segment 3 with arista about 5× as long as segment. Tegmina with Sc + R and Cu1 and union of claval veins at about same level, but venation a little variable.
Complementary description
Length (female) 4.8 mm (). Colouration (preserved specimen): vivid yellow (82), contrasting with some areas that are strong yellowish brown (74). Living specimen colouration as in .
Figures 1–6. (1) Adult female, dorsal view; (2) adult female, left lateral view; (3) frons and clypeus, ventral view; (4) head, pronotum and mesonotum, dorsal view; (5) frons and antennae (scape, pedicel, arista missing), frontal view; (6) head and prothorax, left lateral view.
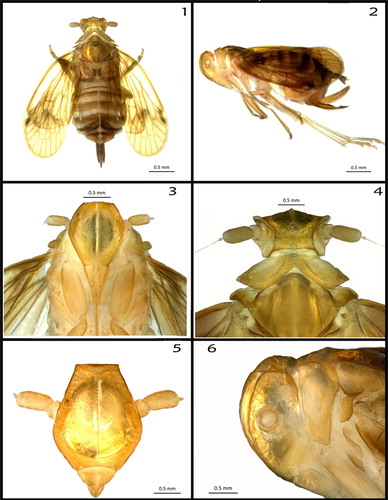
Head (Fig. 1–6). Vertex (): 1.4 times wider (0.466 mm) than long (0.337 mm). Frons (): 1.9 times longer (1.641 mm) than wide (0.862 mm), being wider in the middle, with a convex transverse disc in the middle (symmetric). Postclypeus and anteclypeus (): medially 1.8 times longer (0.677 mm) than wide (0.367 mm) with strong lines (carinae). Antennae (): first segment (scape) wider than long; second segment (pedicel) long and cylindrical, with many sensory setae; bearing two flagella, both sharing the same ovoid base; one of the flagella is tapered from half its length and the other is typical (without any tapering). Eyes/ocelli (): strongly reduced compound eyes (0.193 mm—longer length), median, frontal, ocellus absent, lateral ocelli rudimentary, unpigmented. Ovipositor: (length: 1.232 mm); anal segment: (length: 0.576 mm).
Thorax (): pronotum () tricarinate with median carina barely visible, quite depigmented and almost translucent. Mesonotum () large, as wide as long; lateral carinae bulging. Median carina vestigial, its (presumed) former position only recognisable by a small spot. Tegulae () present. Hind tibiae with numerous bristles and four lateral teeth (). Tegmina (forewings) (): length 3.37 mm, translucent pale orange yellow (73), with some darker spots near the veins, the latter are strong yellowish brown (74).
Figures 7–9. (7) Right tegmen (female); (8) hindlegs, ventral view; (9) second antennal segment (scape) and flagellum/arista.
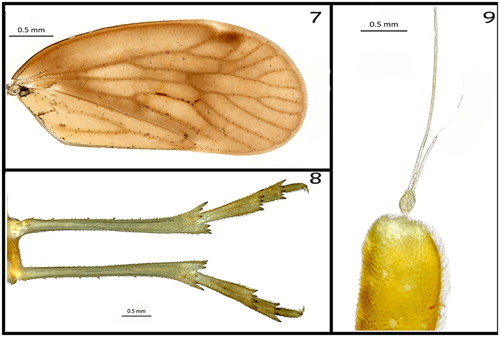
Intraspecific variation of tegmina (): there is considerable variation in the vein configuration as shown in . Variation is expected since one of the most common troglomorphisms in Auchenorrhyncha is the reduction of tegmina and wings. Such reductions may lead to polymorphisms, as observed in the specimens of this study. The adult specimen we observed in the cave did not appear to be flighted; however, it displayed jumping behaviour.
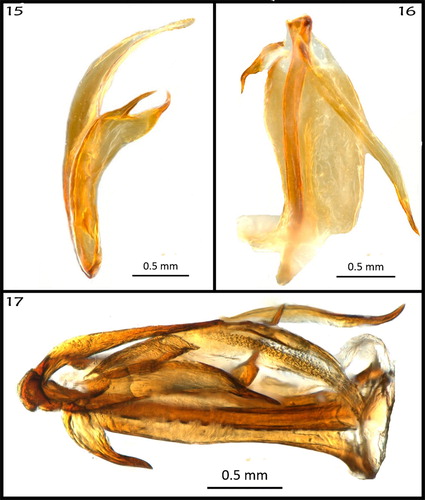
Male genitalia (): as described and figured by Fennah (Citation1975). Our photographs reveal additional details of anal segment (, ), pygofer (), genital styles () and especially of the aedeagus (, –): length 0.649 mm, with four spinose processes: two on phallobase (one arising right laterally near apex, short, stout, in repose curved laterobasally; one arising left laterally near apex, long, slender, directed ventrally), and two on flagellum, arising from a common base on right side, distinctly shorter than membranous portion of flagellum.
Figures 10–14. Male genitalia. (10) Genital capsule, in situ, right lateral view; (11) aedeagus, left lateral view; (12) genital segment (pygofer), right lateral view; (13) anal segment, right lateral view; (14) genital styles, left lateral view.

Etymology
In the original description Fennah (Citation1975) did not provide any information on the origin of the genus or species name. Therefore, we present it here. In Latin Confuga, means ‘refugee, one who takes refuge’. The name persephone refers to a daughter of Zeus and Demeter. According to Greek mythology, Persephone was kidnapped by her uncle Hades and taken to the Underworld, from where she was rescued by her half-brother Hermes. From then on, Persephone spent half of the year on Olympus (during summer and spring) with the other gods and the other half of the year in the underworld (during winter and autumn), where she became known as Koré, the queen of the underground world.
Discussion
Habitat
Council Cave, sometimes referred to in the past as Motupipi Cave, lies in a small limestone block immediately south of Dry River, near the settlement of Motupipi in Golden Bay (). The limestone has been pulled up by uplift on the nearby Pikikiruna Fault, leaving it dipping at an angle of approximately 55° to the west. The block is approximately 1 km long and mostly less than 100 m wide. At its highest point, it reaches about 60 m above the alluvial plain lying to the west. Similar limestone ‘hogsbacks’ extend discontinuously to the northeast for about 9 km, before ending in the limestone block at Tarakohe. The Motupipi limestone is presumed to be continuous beneath the bed of Dry River with a larger block immediately north of Dry River. This larger karst area extends from Dry River to The Grove Scenic Reserve, which it underlies. The Motupipi limestone also continues beneath the land surface to the west, probably for a considerable distance, but well below the local water table.
Figures 22–27. (22) Location of Council Cave on the South Island of New Zealand; (23) Council Cave entrance; (24) general aspect of the karst area around Council Cave; (25) cave interior, showing speleothems on which adult specimens of C. persephone can be found; (26) carcass of a male found on a speleothem; (27) Council Cave map; the yellow area represents the sites where specimens are more commonly found; the red star marks the site where the female described in this study was found.
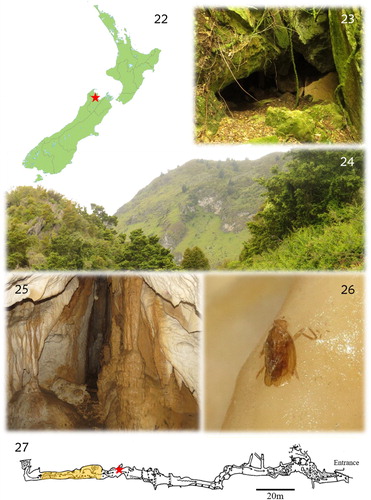
Council Cave is the largest known cave in this limestone block. The cave’s main passage is 170 m in length and runs parallel with the edge of the limestone (, ). The main entrance () lies at about the level of the pasture, which extends from the western side of the limestone. Just below and west of this passage lies a second passage of similar length (as far as it has been explored to date), which at most times holds a small stream that floods long sections of it. There are several entrances into this passage along the western edge of the limestone.
The northernmost section of the Motupipi limestone block, containing Council Cave, lies in Council Cave Scenic Reserve. The reserve still supports a reasonable amount of native vegetation (), but it is heavily covered by adventives such as hawthorn, barberry, banana passionfruit and blackberry. Rough pasture occurs on gentler slopes towards the southern end, where it is grazed by stock.
The initial interest in Council Cave was in its being the only known habitat of the cave spider Spelungula cavernicola in Golden Bay, with all other locations being known in the Oparara and lower catchments. Subsequently, this cave was considered by Johns (Citation1991) as one of the most important caves in New Zealand for its fauna, which presents troglobitic beetles, harvestmen, diplurans, centipedes and spiders.
Since the species description there have been no further sightings of C. persephone, with the exception of a small group of nymphs observed in July 1994 by Ian Millar. In 1996 a temperature and humidity assessment was also undertaken (I. Millar, Department of Conservation, Nelson, New Zealand) using miniature dataloggers to measure those parameters in an area in which specimens were observed (a region close to the deeper portion of the cave, located around 130 m from the entrance; ). The dataloggers were placed in the site for 6 days before being retrieved. The results showed an unvarying temperature of 11.86 °C and humidity of 100% throughout this period. During the last survey to the cave (21 November 2015) we did not observe any nymphs, although we did a fairly detailed search throughout the cave, especially in areas with roots where cave planthoppers are usually most often observed.
Conservation issues and threat status
Confuga persephone is the only known New Zealand Auchenorryncha species to be adapted to the deep cave zone, and is only known with certainty from Council Cave to date, where it is rarely encountered. Given the low densities in which the population has been observed since the species description, it is plausible to assume that the species habitat extends to mesocaverns.
Unfortunately we do not have any information on potential host species or even host specialisation. The surface vegetation above and around the cave is diverse and does not allow clear association of roots to one or more plant species. It is, however, safe to assume that C. persephone feeds on one or more host species native to New Zealand. As the surface vegetation is strongly being altered by adventive species (see above), it appears likely that the native species providing the crucial root resources for C. persephone are under threat from competition with these species, which consequently may lead to the species extinction. Additional threats may be posed by current karst surface management, logging and fire.
In 1999 a workshop was held in New Zealand where the strategies of three programmes regarding threatened species recovery were discussed: the IUCN Red List system (IUCN Citation1994); the Lange and Norton proposal for the classification of New Zealand’s Threatened and Uncommon plants (de Lange & Norton Citation1998; de Lange et al. Citation1999); and the species priority system used by the New Zealand Department of Conservation (DOC) (Molloy & Davis Citation1994). It was concluded that none of the three systems was fully effective. A new classification system based on elements of these three systems was proposed, which would also address marine, freshwater and terrestrial taxa (Molloy Citation2002). This system was subsequently revised and refined, resulting in production of the New Zealand Threat Classification System Manual (Townsend et al. Citation2008). This new system aims to indicate the threat level of extinction of the species for decision-making (Stephens Citation1997; Stephens et al. Citation2002).
At present, C. persephone is ranked within this system as Naturally Uncommon, with a qualifier of Range Restricted (Stringer et al. Citation2012). The Naturally Uncommon ranking is applied to taxa ‘whose distribution is naturally confined to specific substrates … , habitats … , or geographic areas … , or taxa that occur within naturally small and widely scattered populations’ (Townsend et al. Citation2008, p. 24). This distribution is not considered to be a result of human disturbance and, more importantly, populations ‘may be stable or increasing’ (Townsend et al. Citation2008, p. 24). This category has tended to become a repository for those cave-dwelling species which have been ranked to date. However, although the population has not been monitored since its description (which would allow access to information regarding population size and fluctuations), it is plausible to assume a reduction in population size in the cave during the last 4 decades. The 19 individuals mentioned in the original description (collected on four distinct occasions, between 1973 and 1974) indicate that the population was probably larger than was observed in subsequent years. In 1994 only a small group of nymphs was observed in the cave. Furthermore, considering our last visit to the cave (where a single living specimen was found), it is plausible to assume that the population may be in decline (or at least migrating to other subterranean habitats inaccessible to humans). Thus, even without access to reliable information on population fluctuations (an important information for an accurate classification of the taxon), it can be considered that any new threat could quickly affect the entire population, leading to the extinction of this species. However, even considering the scarce information regarding this species, it can be considered At Risk, given the apparent restriction to a single locality, associated with an apparently decreasing population density.
Given C. persophone’s potential restriction to a single locality, associated with an apparently decreasing population density, we suggest that this species current ranking may not reflect its true conservation status. The low numbers in the only known habitat for this species, combined with apparent decline in those numbers, suggests that a ranking as Nationally Vulnerable or Nationally Endangered would be more appropriate.
Acknowledgements
We would like to express our sincere thanks to Marconi Souza Silva (UFLA), Dave Smith, Chris Stephenson and Andy MacDonald (from the Nelson Speleological Group) for their assistance in the field trip. We are especially grateful to Andy MacDonald for taking the photographs of the living specimen.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Rodrigo Lopes Ferreira http://orcid.org/0000-0003-3288-4405
Additional information
Funding
References
- Cavichioli RR, Takiya DM. 2012. Description of a new species of Wolfniana and new records of Rotigonalia (Hemiptera: Cicadellidae: Cicadellinae) from the state of Amazonas, Brazil. Zoologia (Curitiba). 29(1):85–88. doi: 10.1590/S1984-46702012000100011
- Culver DC. 1982. Cave life: evolution and ecology. Cambridge (Mass): Harvard University Press.
- de Lange PJ, Heenan PB, Given DR, Norton DA, Ogle CC, Johnson PN, Cameron EK. 1999. Threatened and uncommon plants of New Zealand. N.Z.J. Bot. 37:603–628. doi: 10.1080/0028825X.1999.9512657
- de Lange PJ, Norton DA. 1998. Revisiting rarity: a botanical perspective on the meanings of rarity and the classification of New Zealand’s uncommon plants. R.S.N.Z. Miscellaneous Series. 48:145–160.
- D’Urso V, Grasso R. 2009. First record of troglobitic Fulgoromorpha from Italy (Hemiptera, Auchenorrhyncha, Cixiidae). Dtsch Entomol Z. 56(1):7–14. doi: 10.1002/mmnd.200900002
- Eberhard S. 2001. Cave fauna monitoring and management at Ida Bay, Tasmania. Rec Aust Mus. 64:97–104. doi: 10.18195/issn.0313-122x.64.2001.097-104
- Fennah RG. 1975. New cavernicolous cixiid from New Zealand (Homoptera: Fulgoroidea). N.Z.J. Zool. 2(3):377–380. doi: 10.1080/03014223.1975.9517880
- Gibert J, Danielpol DL, Stanford JA. 1994. Groundwater ecology. New York (NY): Academic Press, 571 pp.
- Gines A, Gines J. 1992. Karst phenomena and biospeleological environments. In: Mus. Nac. Cienc. Natur, editors. The natural history of biospeleology, monografias. Madrid (Spain); 677p.
- Hoch H. 1991. Cave-dwelling Cixiidae (Homoptera Fulgoroidea) from the Azores. Bocagiana. 149:1–9.
- Hoch H. 1994. Homoptera (Auchenorrhyncha Fulgoroidea) 313–325. In: Jubertie C, Decu V, editors. Encyclopaedia biospeologica. Tome I. Moulis (France): Société de Biospéologie; 834 pp.
- Hoch H. 2013. Trirhacus helenae sp. n., a new cave-dwelling planthopper from Croatia (Hemiptera: Fulgoromorpha: Cixiidae). Dtsch Entomol Z. 60(2):155–161. doi: 10.1002/mmnd.200700015
- Hoch H, Asche M. 1993. Evolution and speciation of cave-dwelling Fulgoroidea in the Canary Islands (Homoptera: Cixiidae and Meenoplidae). Zool J Linn Soc. 109(1):53–101. doi: 10.1111/j.1096-3642.1993.tb01259.x
- Hoch H, Ferreira RL. 2012. Ferricixius davidi gen. n., sp. n. the first cavernicolous planthopper from Brazil (Hemiptera, Fulgoromorpha, Cixiidae). Dtsch Entomol Z. 59(2):201–206.
- Hoch H, Ferreira RL. 2013. Potiguara troglobia gen. n., sp. n. the first record of a troglobitic Kinnaridae from Brazil (Hemiptera: Fulgoromorpha). Dtsch Entomol Z. 60(1):33–40.
- Hoch H, Howarth F. 1989. Six new cavernicolous cixiid planthoppers in the genus Solonaima from Australia (Homoptera: Fulgoroidea). Syst Entomol. 14:377–402. doi: 10.1111/j.1365-3113.1989.tb00291.x
- Hoch H, Naranjo M, Oromí P. 2012. Witness of a lost world: Meenoplus roddenberryi sp.n., a new cavernicolous planthopper species (Hemiptera, Fulgormorpha, Meenoplidae) from Gran Canaria. Dtsch Entomol Z. 59(2):207–215.
- IUCN. 1994. IUCN red list categories. Prepared by the IUCN Species Survival Commission. Gland (Switzerland): IUCN.
- Johns PM. 1991. Distribution of cave species of northwest Nelson, Westland and Canterbury. The Weta. 14:11–21.
- Molloy J. 2002. Classifying species according to threat of extinction: a system for New Zealand. No. 22. Wellington (NZ): Biodiversity Recovery Unit, Dept. of Conservation.
- Molloy J, Davis A. 1994. Setting priorities for the conservation of New Zealand’s threatened plants and animals. Wellington (NZ): Department of Conservation.
- Mundie DA. 1995. The NBS/ISCC Color System /David A. Mundie Pittsburgh (PA): Polymath Systems 535.6 dc20. [accessed 2006 February]. http://www.anthus.com/Colors/Cent.html.
- Oliarinony R, Elouard JM, Sartori M. 2016. Complementary description of the genus Manohyphella Allen, 1973 (Insecta: Ephemeroptera: Teloganodidae), with some comments on its ecology in the Andasibe area (East Coast, Madagascar). Afr Invertebr. 57(1):1–14. doi: 10.3897/afrinvertebr.57.8449
- Sartori M. 2014. Complementary description of Dudgeodes ulmeri Sartori, 2008 and Teloganopsis media Ulmer, 1939 (Ephemeroptera: Teloganodidae, Ephemerellidae). Ent Mitt Zool Mus Hamburg. 17(192):161–166.
- Saturnino R, Rodrigues BVB, Bonaldo AB. 2015. Alpaida (Araneae: Araneidae) from the Amazon Basin and Ecuador: new species, new records and complementary descriptions. Zoologia (Curitiba). 32(3):241–256. doi: 10.1590/S1984-46702015000300008
- Simon KS, Pipan T, Culver DC. 2007. A conceptual model of the flow and distribution of organic carbon in caves. J Caves Karst Stud. 69(2):279–284.
- Souza SM, Martins RP, Ferreira RL. 2011. Trophic dynamics in a neotropical limestone cave. Sub Biol. 9:127–138.
- Stephens RTT, Brown DJ, Thornley NJ. 2002. Measuring conservation achievement: concepts and their application over the Twizel area. Science for Conservation. 200:114 p.
- Stephens T. 1997. Measuring conservation achievement. In: Blaschke PM, Green K, editors. Biodiversity now! Joint Societies Conference, Wellington, 1997. Selected papers. Wellington (NZ): Department of Conservation; p. 13–39.
- Stone FD, Howarth FG, Hoch H, Asche M. 2005. Root communities in lava tubes 477–484. In: Culver DC, White WB, editors. Encyclopedia of caves. London: Elsevier Academic Press; 654 pp.
- Stringer IAN, Hitchmough RA, Larivire M-C, Eyles AC, Teulon DAJ, Dale PJ, Henderson, RC. 2012. The conservation status of New Zealand Hemiptera. New Zealand Entomologist. 35(2):110–115. (Supplementary material online.) doi: 10.1080/00779962.2012.686314
- Townsend AJ, de Lange PJ, Duffy CAJ, Miskelly CM, Molloy J, Norton DA. 2008. New Zealand threat classification system manual. Wellington (NZ): New Zealand Department of Conservation; 35 p.
- White WB. 2005. Volcanic caves, 599–602. In: Culver DC, White WB, editors. Encyclopedia of caves. London: Elsevier Academic Press; 654 pp.