ABSTRACT
We evaluate evidence that reducing mammalian predators benefits threatened and declining grasshoppers in the Mackenzie Basin, New Zealand. Long-term population trends of Sigaus minutus are investigated under three control regimes: high intensity, indirect control through prey reduction and no control. We then test whether predator control benefits conservation management of Brachaspis robustus by translocating wild-caught individuals to areas of moderate versus no predator control. A significant positive trend in S. minutus counts occurred under high intensity and indirect control, suggesting that mammalian predator control is beneficial. Differences in the decline of translocated B. robustus were observed between moderate and no predator control release sites but could not be unequivocally attributed to predator densities. We recommend replicated predator control studies be undertaken to develop a predator management strategy which will enable grasshopper recovery, and investigate the potential for meso-predator release and prey-switching under regimes that target specific mammals.
Introduction
A notable characteristic of New Zealand’s historic biota is the absence of certain functional groups, including predatory land mammals (Holdaway Citation1989; McGlone Citation2006). Predatory land mammals were first introduced when Polynesians arrived to New Zealand c. AD 1280 and brought with them the kiore (Rattus exulans) and the domestic dog (Canis lupus familiaris) (Holdaway Citation1989; Wilmshurst et al. Citation2008). When European’s arrived in the late 1700s, there was a second much larger wave of introductions which included the domestic cat (Felis catus), the hedgehog (Erinaceus europaeus), and several species of Mustelidae and Rodentia (King Citation1990). Because the historic fauna of New Zealand did not evolve with predatory land mammals, many native and endemic species lacked defense mechanisms appropriate for avoiding predation by introduced mammals (Daugherty et al. Citation1993). Consequently, many native and endemic species went extinct after the arrival of predatory land mammals, and many extant species are currently threatened or in decline as a result of their continued presence in native ecosystems (Holdaway Citation1989; Department of Conservation Citation2017).
There are several traits seen in New Zealand invertebrates which are associated with vulnerability to predation by introduced mammals, and like other endemic fauna, a number of these traits have been attributed to lack of co-evolution with mammals (Gibbs Citation2010). For example, many New Zealand invertebrate species exhibit a freeze response and rely on visual crypsis when threatened (White Citation1994; Gibbs Citation1998; Lester et al. Citation2014). This response provides an effective defense against predators that hunt by sight, such as the native birds and lizards with which many New Zealand invertebrate species co-evolved (Daugherty et al. Citation1993). However, introduced predatory mammals are often olfactory hunters; therefore, a freeze response when threatened does not prevent detection (Gibbs Citation1998; Jones et al. Citation2005; Lester et al. Citation2014). Other traits that make many New Zealand invertebrate species vulnerable to predation by introduced mammals include flightlessness, living on the ground, and gigantism (Daugherty et al. Citation1993; Gibbs Citation1998; Stringer and Hitchmonger Citation2012). Large invertebrates are particularly vulnerable because they are often preferentially targeted by predatory mammals as a higher value food resource (St Clair Citation2011; Barker Citation2016).
Although there has been a recent and major shift in the focus of conservation science in New Zealand to eradicate all introduced mammalian predators by 2050 (Predator Free 2050 (Bell Citation2016; Owens Citation2017)), there are currently no tools available to achieve such large-scale eradication (Department of Conservation Citation2017; Linklater and Steer Citation2018). In the interim, significantly reducing the pressure of predatory mammals on threatened or declining species remains a priority of conservation management in New Zealand (Parkes et al. Citation2017). In some instances, low or moderate intensity predator control is adequate to achieve a conservation benefit (Basse et al. Citation2003; Whitehead et al. Citation2008), and is preferred to high intensity predator control because it is more time, cost, and resource efficient. However, mammalian predator control research in New Zealand is routinely focused on the benefit it provides for endemic bird species. For threatened invertebrates, much less is known about the conservation benefit mammalian predator control can provide, let alone the appropriate level of intensity for it to be implemented cost-effectively (Lester et al. Citation2014).
The distribution of Sigaus minutus Bigelow (Orthoptera: Acrididae), an At Risk – Declining grasshopper found throughout the Mackenzie Basin, includes several populations present in areas where mammalian predator control is implemented. Endemic to the South Island of New Zealand, S. minutus is a small (males up to 10 mm, females up to 17 mm in body length), rugose grasshopper which is diversely coloured and visually cryptic (Jamieson Citation1996; Morris Citation2002; Trewick et al. Citation2014). Currently, two populations of S. minutus exist in locations that receive predator control. The first population (Upper Ohau River) receives direct and high intensity mammalian predator control set up for the protection of black-fronted terns (Chlidonias albostriatus) on a braided river island (Woolmore et al. Citation2010). The second population (Tekapo River) receives indirect predator control as a result of prey reduction in that the continuous suppression of rabbits (Oryctolagus cuniculus) to very low levels (<1 rabbit per kilometre) for more than a decade (DOC, unpublished data) has likely resulted in significant reductions to populations of feral cats and ferrets at the site (Pierce Citation1987; Norbury et al. Citation2009). As part of annual grasshopper surveys in the Mackenzie Basin, the Department of Conservation has monitored these two populations of S. minutus since 2007, along with a third (Lower Ohau River) in an area of no predator control.
Another Threatened – Nationally Endangered braided river grasshopper, Brachaspis robustus Bigelow (Orthoptera: Acrididae), has many of the traits described above that make it susceptible to predation by introduced mammals (Trewick et al. Citation2014). It is a large (males up to 17 mm and females up to 38 mm in body length), flightless and ground-dwelling insect which relies on visual crypsis when threatened (Bigelow Citation1967; Morris Citation2002; Trewick et al. Citation2014). Body colour varies from shades of pale to dark grey through to reddish-brown allowing it to blend in with its habitat among riverbed gravels and silts. Endemic to the Mackenzie Basin, its range is severely restricted, and the few remaining populations are patchy in distribution and show trends of decline (Department of Conservation, unpublished data). Because diets of predatory mammals in the Mackenzie Basin have been found to contain high proportions of invertebrates (Murphy et al. Citation2004; Jones et al. Citation2005; Dowding et al. Citation2015) it is thought that introduced predatory mammals pose a significant threat to the persistence of B. robustus. Currently none of the few remaining natural populations of B. robustus receive any mammalian predator control.
Here we evaluate whether there is evidence that reducing mammalian predators is beneficial for threatened and declining grasshoppers in the Mackenzie Basin using two datasets. First, we investigate the long-term population trends of S. minutus under the three different predator control regimes; high intensity, indirect through prey reduction, and none. Second, we test whether a moderate intensity mammalian predator control regime is adequate for conservation management for B. robustus by translocating wild-caught B. robustus to two adjacent locations which differ in their predator management; (1) an area of moderate predator control and (2) an adjacent area of no predator control. The aim of this analysis is to inform conservation managers about appropriate predator management options for protecting threatened and declining grasshoppers.
Materials and methods
Monitoring of S. minutus
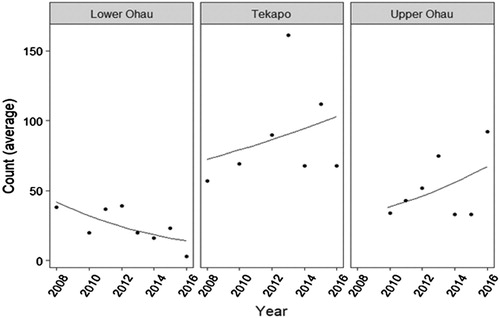
Wild populations of the At Risk – Declining braided river grasshopper S. minutus have been monitored by the Department of Conservation at sites in the Upper Ohau River, Lower Ohau River and Tekapo River in the Mackenzie Basin (), as part of annual grasshopper surveys since 2007. All three sites were located on braided river bed and primarily had a rocky substrate. Ground cover at all sites was comprised of vegetative litter (∼ 4%), vascular and non-vascular plants, and bare soil and gravel. Non-vascular plants and bare ground made up ∼ 44% and ∼ 43% of the ground cover at the Lower Ohau River site respectively. The Tekapo River site had ∼ 46% bare ground and vascular plants made up ∼ 32% of the ground cover. Over half (∼ 57%) of the ground cover at the Lower Ohau River site was vascular plants, and ∼20% was bare ground (Department of Conservation Citation2011, unpublished data). The Upper Ohau River site receives high intensity predator control targeting seven mammalian species for the protection of black-fronted terns, while the Lower Ohau River site receives no predator control. The Tekapo River site is situated within the Tekapo Scientific Reserve which receives high intensity rabbit control. Because rabbits are the main prey item for several predatory mammals in the Basin, removal of rabbits can result in reduced presence of predatory mammals (Pierce Citation1987; Norbury et al. Citation2002) (). Plot search monitoring at these sites was conducted annually by two to three observers. Observers used a slow walk to systematically search the entire plot on three days within a two-week period each February. The total number of all orthoptera observed during each visit were recorded including Phaulicridium spp., Sigaus ‘species A’, S. minutus, katydids and crickets. The number of each species was recorded along with weather conditions, temperature, time of day and search duration. No monitoring took place at any site in 2009, the Tekapo River site was not monitored in 2011, and only two visits were made to each site in 2015.
Figure 1. The locations of the Upper Ohau, Lower Ohau, and Tekapo sites, and the kakī aviary complex in the Mackenzie Basin, New Zealand.
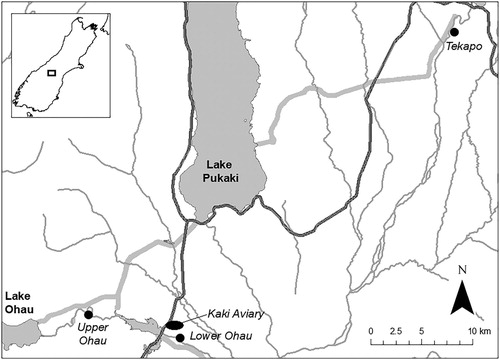
Table 1. Descriptions of the predator control implemented at (1) each of the three sites where S. minutus is monitored annually by the Department of Conservation and (2) the two areas at the kakī aviary complex which received translocated B. robustus.
Impacts of moderate levels of predator control on translocated B. robustus
In February 2015, a group of 186 B. robustus of mixed age, source site, and gender were released into receiving habitats in, and adjacent to, the kakī/black stilt aviary complex near Twizel (). Half were released into an area of moderate mammalian predator control and half into an immediately adjacent area absent of predator control (). The moderate control site had a 1.2 m high wire mesh fence that had top and bottom electric fence wires on outriggers, and continuous set kill trapping (DOC250, DOC150 traps) inside the fence boundaries (see ). Control was considered ‘moderate’ because the fence excluded hedgehogs, but only deterred cats and mustelids (this study), and was somewhat ‘leaky’ for the duration of the study as a result of damage that was not immediately detected.
The presence of mammalian predators was determined for two seasons using tracking tunnels. In March 2015, ten Black Trakka™ tracking tunnels (100 mm × 100 mm × 500 mm) baited with fresh rabbit were run for 25 days in each of the two areas (moderate and no mammalian predator control) to estimate the presence of predatory mammals (excluding cats due to small tunnel size) and lizards. From December 2015 to March 2016, ten larger tracking tunnels (approx. 200 mm × 200 mm × 1100 mm, constructed from black corflute with a wooden base) were run in each of the two areas to estimate the presence of predatory mammals (including cats due to larger tunnel size) and lizards. The tunnels were active for three weeks per month and were baited with peanut butter for the first night (to target mice) and fresh rabbit for the remaining twenty nights (to target larger predatory mammals). Visual assessment of inked footprints was used to identify which predators (cats, hedgehogs, rodents, mustelids, lizards) had passed through a tunnel. The percentage of tunnels tracked by a predator type in each area was calculated for each month the tunnels were active.
Released grasshoppers were monitored fortnightly for a year using mark-recapture until individuals could no longer be detected in February 2016. This coincided with the expected end of the natural lifecycle of the translocated grasshoppers. Release sites consisted of six identical 15 × 15 m gravel plots created on site at the beginning of the study, three inside the predator fence area, and three outside. Gravel plots in the two areas were separated by an average of 150 m. Monitoring was not conducted during the winter of 2015 (May to August) due to cold temperatures and snow fall, conditions under which the grasshoppers were not expected to be active. The number and identity of individuals sighted during each monitoring occasion was recorded and mark-recapture data was used to manually generate estimates of the minimum number of individuals present, taking into account higher counts and sightings of missing individuals on subsequent monitoring occasions.
Data analyses
All analyses were carried out using R version 3.2.1 (R Core Team Citation2016). Annual mean counts of S. minutus were modelled using a Poisson mixed effects model in the lme4 package (Bates et al. Citation2014). In 2007, monitoring was conducted by three first-time observers, rather than two observers including at least one with experience (as per 2008 onwards), and was therefore excluded from analysis. Counts from the Upper Ohau river were limited to 2010 onwards to coincide with when predator control was implemented. Model fit was assessed using visual assessment for normally distributed residuals. The final model was selected for based on a comparatively low AIC score. Welch’s T-tests (to account for unequal variances) were used to compare the percentage of tunnels tracked by each predator type (cats, hedgehogs, rodents, mustelids and lizards) from December 2015 to March 2016 in the moderate and no mammalian predator control areas which received translocated B. robustus.
Results
Trends in S. minutus populations
Under high intensity predator control implemented at the Upper Ohau River site, S. minutus counts showed a significant positive trend over time. On average, counts increased by 10% per year (p < .001). A similar trend was seen under the indirect predator control implemented at the Tekapo River site where counts increased significantly by an average of 4.5% each year (p < .001). In contrast, at the Lower Ohau River site where no predator control was implemented, counts significantly decreased by 13% on average each year (p < .001) ().
Experimental translocation of B. robustus
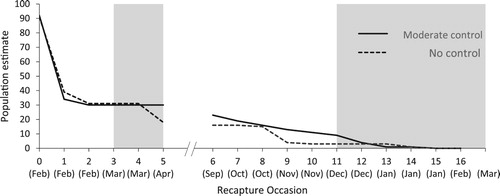
Translocated populations of B. robustus in both the moderate control area and no control area showed a steep decline during the first two weeks post-release, with the number of individuals sighted falling to around a third of those released. The number of individuals in the moderate control area then remained stable until the onset of winter, after which there was a gradual decline. In the area with no control, there were two additional distinct periods of decline, once in late autumn and another in late spring (). Thirteen and eleven individuals were lost over each two-week period respectively.
Figure 3. The minimum possible number of B. robustus individuals present during fortnightly mark-recapture monitoring of translocated grasshoppers in the moderate mammalian predator control area and the adjacent no control area. The break indicates where monitoring stopped for the winter. The grey shading indicates the periods when tracking tunnels were run.
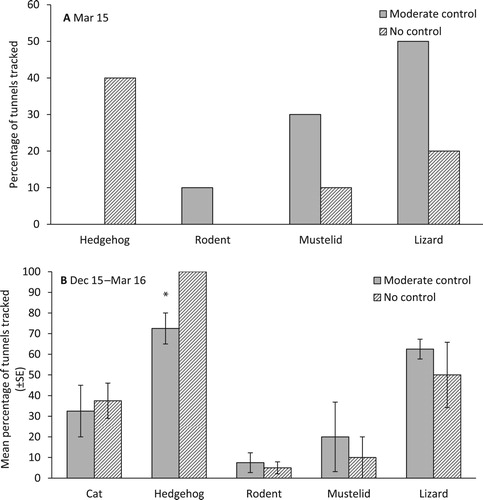
In March 2015, more tunnels were tracked by hedgehogs in the no control area than the moderate control area. In contrast, more tunnels were tracked by rodents, mustelids and lizards in the moderate control area than the no control area (A). From November 2015 to March 2016, the mean percentage of tunnels tracked by cats, rodents, mustelids and lizards in the moderate and no mammalian predator control areas did not differ significantly (cat, p = .75, d.f. = 5.3; rodent, p = .67, d.f. = 4.9; mustelid, p = .63, d.f.= 4.9; lizard, p = .50, d.f. = 3.5). The mean percentage of tunnels tracked by hedgehogs was significantly higher in the no control area than the moderate control area (p = .04, d.f. = 3) (B).
Figure 4. Percentage of tunnels tracked by lizards and predatory mammals during A, 25 days in March 2015, and B, three weeks per month from December 2015 to March 2016, within the moderate and no mammalian predator control areas which received translocated B. robustus grasshoppers. *significant at p < 0.05.
Discussion
Long term trends of S. minutus
The significant increase of S. minutus counts under predator control regimes at the Upper Ohau River and Tekapo River sites suggests that removing predatory mammals from the environment is beneficial for this At Risk grasshopper, and this is in contrast to the Lower Ohau River site, where predators were not controlled, and where counts significantly declined over time. However, we did not study the complexity of interactions following the removal of rabbits at the Tekapo Scientific Reserve. The lack of rabbits at this site would not have resulted in a reduced presence of all mammalian predators, only those for which rabbits are the main prey item (i.e. feral cats, stoats and ferrets) (Pierce Citation1987; Norbury et al. Citation2002, Citation2009). In contrast hedgehog, skink and mice numbers may have been expected to increase following the removal of rabbits due to release from predation by larger predatory mammals, and improved habitat quality and food availability as vegetation recovers. One possible reason for why S. minutus numbers may have increased despite predicted increases in hedgehog, skink and mice predators in the reserve, is that the plot where the grasshoppers were monitored is situated on the lower terraces near the Tekapo River. The lower terraces have poorer soils and relatively little vegetation resulting in lower quality habitat relative to other parts of the reserve, which may not support larger populations of hedgehogs, skinks and mice than were present prior to rabbit removal. Future work which explores the potential benefit that mammalian predator control through primary prey reduction could provide should incorporate monitoring of meso-predator populations to better understand responses at various trophic levels.
While the correlation between increasing S. minutus populations and low predator densities are compelling, there are several caveats which need to be addressed. The single population monitored under each of the predator control methods (high intensity, indirect, and none) makes it difficult to distinguish the predator control treatment from the location effect. To fully tease apart the predator control effect from the location effect would require long term predator control regimes to be implemented at multiple locations throughout the Mackenzie Basin where S. minutus are present. Furthermore, the grasshopper surveys were conducted in February at which time the populations are composed mostly of juveniles. The emergence of S. minutus nymphs is likely to be associated with environmental variables such as temperature (Fischer Citation1994). Variation in the timing of emergence compared to the timing of monitoring may account for some of the year-to-year variability seen in counts of S. minutus. Because the life history of S. minutus is not well-studied, it is difficult to evaluate exactly how environmental variables may have influenced S. minutus counts in this study. A suggested improvement would be to conduct grasshopper counts in December when the population consists mostly of adults. This would result in more accurate and comparable counts year-to-year as adults are larger and therefore easier to count and identify correctly to species. Furthermore, as high mortality rates are often associated with the juvenile stage of insects, counts of adults would be more biologically meaningful.
Experimental translocation of B. robustus
The moderate predator control implemented during the translocation of B. robustus was not adequate to achieve an obvious conservation advantage for the species. The steep population decline seen in both the moderate control area and the no control area in the first fortnight following release is assumed to be a normal response to translocation. Many factors that can cause population decline are present during the initial stage of a translocation, such as unfamiliar surroundings leading to dispersal (Heidinger et al. Citation2009), starvation (if individuals are not able to find adequate food resources in unfamiliar surroundings) and increased vulnerability to predation (when unfamiliar surroundings result in an inability to find appropriate refuges (Jacquot and Solomon Citation1997; Yoder et al. Citation2004; Marable et al. Citation2012)). Stress-induced mortality may also occur as a result of the translocation procedure (Letty et al. Citation2007, Teixeira et al. Citation2007, Dickens et al. Citation2010). Without undertaking the technically difficult task of fine-scale tracking to determine the fate of individual grasshoppers, it was not possible to tease out the relative importance of these factors in the initial decline observed in this particular translocation.
After the initial decline, grasshoppers in the no control area had two episodes where the minimum estimated number of individuals present dropped by 13 and 11 individuals in a fortnight (between March – April and October – November respectively). No such steep declines were seen in the moderate control area where the maximum number of individuals lost over any single fortnight was estimated at no more than five. Given that the initial population decline appears to have stabilised before these steep decline events in the no control area, one explanation is that they were a result of hedgehog predation. A higher tracking rate of hedgehogs was detected in the no control area and hedgehogs are known to exploit food patches, meaning they will stay in a resource-rich area and remove large numbers of prey in a short amount of time (Jones et al. Citation2005). We note, however, that if predation was a key factor explaining declines, it did not result in all grasshoppers being completely extirpated from non-predator managed sites.
Several challenges remain in understanding the population trends observed following the B. robustus translocation. In particular, two key challenges are to better determine the fate of released individuals, and to better understand the roles of different species of mammalian predators. Post-release fate tracking is both a technically challenging and time-consuming endeavour for grasshoppers because they moult several times over the course of a spring-autumn period. The mark-recapture tracking used during these translocations was only effective at determining the fate of individuals that remained alive and stayed within or immediately adjacent to the plots. The small body size and highly cryptic nature of B. robustus makes them difficult to detect visually, and dead bodies, even if present, were never recovered to determine cause of death. Tracking using telemetric devices attached to the pronotum could have been used to partially overcome this problem (Kissling et al. Citation2014). However, most individuals were mid- to late-instar nymphs rather than adults at the time of translocation and subsequent moults would have meant any telemetric devices would have been shed along with the exoskeleton, if the device itself did not prevent successful moulting in the first instance.
The differential roles of key mammalian predator species
It is unlikely that all mammals pose an equal threat to grasshoppers. Because the predator control programme in the high intensity site (Upper Ohau River) used a range of tools to control seven species of mammalian predators (all species except mice) (Woolmore et al. Citation2013), it remains unclear whether any one or a subset of these predators are key in suppressing S. minutus populations. However, the significantly reduced presence of hedgehogs within the moderate control area suggest they are one of the key predators of B. robustus.
Hedgehogs are present in higher abundance than any other predatory mammal throughout the Mackenzie Basin (Keedwell and Brown Citation2001), and exhibit a diet comprised mostly of invertebrates (Moss and Sanders Citation2001). Scat analysis has shown grasshoppers (White Citation1994), and other orthoptera (Jones et al. Citation2005) form a substantial component of the hedgehog diet in the Mackenzie Basin. Of concern is their ability to consume numerous invertebrates in a short space of time. Jones et al. (Citation2005) found hedgehogs could exploit rich food patches by consuming large numbers of individuals in a single night. For Mackenzie Basin grasshoppers, which tend to have a patchy distribution and loose congregations, this behaviour is particularly threatening.
While invertebrates are not the primary prey of cats, they do make up a substantial proportion of their diet and are a common alternative prey item. In the Mackenzie Basin, invertebrates have been found in 10-30% of cat guts and scats (Pierce Citation1987; Murphy et al. Citation2004). Grasshopper remains have also been found in cat scats both in the Mackenzie Basin (Pierce Citation1987; White Citation1994) and other parts of New Zealand (O'Donnell et al. Citation2017). In years when cats’ main prey, rabbits, have experienced population crashes due to poisoning, the percentage of cat guts containing invertebrates increased to around 50%, suggesting a switch to smaller available prey (Murphy et al. Citation2004). White (Citation1994) suggested this prey switching caused the loss of marked individuals in his study of B. robustus in 1991–1992. Even when rabbit prey are available, the percentage of cat guts containing invertebrates is still higher in the Mackenzie Basin than for other ecosystems in New Zealand; in other grassland regions between 5% and 10% of cat guts contain invertebrates (Langham Citation1990) while in forests it is <1% (Harper Citation2005). It is likely that kittens are a bigger threat to grasshoppers than adult cats as they are often too small to catch rabbits so must rely on smaller prey (Gillies Citation2001).
Although stoats have been found to consume significantly fewer invertebrates than cats, their diets still contain a substantial proportion of invertebrates (Murphy et al. Citation2004). Like cats, there is evidence that stoats will prey-switch and consume more invertebrates when rabbit densities are low (Murphy et al. Citation2004). The same occurs with ferrets, however ferrets have been found to consume fewer invertebrates than both stoats and cats (Murphy et al. Citation2004). For both stoats and ferrets, it is the smaller females which consume the most invertebrates and therefore pose the biggest threat to the grasshoppers (Pierce Citation1987; Dowding et al. Citation2015). The low abundance of stoats in this ecosystem (Keedwell and Brown Citation2001) and the low consumption of invertebrates expected from ferrets suggests the threat of mustelids to Mackenzie Basin grasshoppers is likely to be small.
Little is known about the diet of rodents in the Mackenzie Basin, however studies on off-shore islands have revealed that most often their presence has negative impacts (decline or suppression) on invertebrate populations (St Clair Citation2011). On the mainland, kiore and ship rats (Rattus rattus) have negatively impacted populations of New Zealand weta species (Hemideina), and appear to threaten the largest individuals most strongly (Rufaut and Gibbs Citation2003; Ruscoe et al. Citation2013). All rats identified in the Mackenzie Basin area have been Norway rats (Department of Conservation, unpublished data). Keedwell and Brown (Citation2001) found the catch trapping rate for rats in the Ohau, Tekapo and Pukaki Rivers to be the second lowest (to stoats) of the key predatory mammals. The low abundance of rats in this ecosystem means their threat to grasshopper populations is likely to be small. Less is known about the abundance of mice in the Mackenzie Basin, but there are no reported observations of large scale eruptions in the last three decades, and only three mice were detected in over 2700 days of video monitoring at bird nests in several Mackenzie Basin braided rivers (Sanders and Maloney Citation2002). In snow tussock ecosystems in Fiordland, grasshoppers can make up 13% of the alpine mouse diet (Wilson and Lee Citation2010). If mice were abundant in braided river ecosystems they could be threatening to the persistence of Mackenzie Basin grasshoppers.
In braided river ecosystems in the Mackenzie Basin, introduced predatory mammals are top terrestrial predators (Murphy et al. Citation2004). Control activities, or eradication initiatives such as Predator Free 2050, which aim to remove some, or all, introduced mammalian predators from the ecosystem could result in a meso-predator release (Soulé et al. Citation1988; Linklater and Steer Citation2018). The consequence being an increase in the abundance of grasshopper predators at lower tropic levels; particularly birds and lizards, and possibly predatory invertebrates such as spiders (Norbury et al. Citation2013, Watts et al. Citation2014). At the translocation sites for B. robustus, the tracking rate of skinks in the moderate control area was found to be greater than in the no control area, suggesting that a meso-predator release may have occurred at that site. Because many of the bird and lizard species in this ecosystem are also threatened or in decline (Department of Conservation Citation2017), it is not expected that removal of predatory mammals will result in stronger predation pressure from non-mammalian predators than was historically present. However, it is not well understood how populations of mammalian predators at lower trophic levels, such as mice or stoats, might respond to the removal of apex predatory mammals in these ecosystems (Pierce Citation1987; Keedwell and Brown Citation2001). A better understanding of these types of interactions are needed to ensure control or eradication of a sub-set of mammals does not have unintended negative consequences for populations of threatened invertebrates.
Conclusions
We provide evidence here that the high intensity predator control undertaken for black-fronted tern protection was correlated with a significant increase in S. minutus counts over time, as did indirect predator control through prey reduction. This suggests that predatory mammals do limit S. minutus population growth. Differences in the decline of translocated B. robustus individuals were observed between moderate and no predator control release sites but could not be unequivocally attributed to predator densities due to challenges in determining the fate of released individuals. Although further replicates would likely generate stronger support for our findings, we suggest that a more pressing priority is to determine which control strategy is most beneficial. Even though S. minutus appeared to benefit from indirect predator control, we caution that it may not be an effective conservation strategy for all threatened or declining grasshopper species because prey-switching is known to occur in some of the key predatory mammals (Keedwell and Brown Citation2001; Norbury Citation2001). In particular, large insect species like B. robustus may be more strongly targeted by predatory mammals as an alternative prey item because they provide a higher energetic benefit than small insects (Pyke et al. Citation1977). If prey reduction is implemented as a management technique, we recommended that predatory mammals be controlled simultaneously to reduce the chance of prey-switching to invertebrates (Keedwell and Brown Citation2001).
Because predator control implemented both in the Upper Ohau River and the moderate control area of the translocation were designed to control for introduced mammals in general, it remains unclear whether any one or several of these predators are key in suppressing grasshopper populations. It is also unclear whether B. robustus is more vulnerable to predatory mammals than the much smaller S. minutus. To determine exactly how to protect threatened and declining grasshoppers from introduced mammalian predators, further study is needed to identify the intensity of control required, the predators for which management is needed, and any unintended increases in predation pressure caused by meso-predator release or prey-switching (Soulé et al. Citation1988, Norbury et al. Citation2013, Watts et al. Citation2014). Given the threat status of these two Mackenzie Basin grasshoppers, however, we suggest that their future conservation management should prioritise high intensity mammalian predator control for the full suite of predators.
Acknowledgements
We are grateful to the Department of Conservation and Project River Recovery staff at the Te Manahuna/Twizel Office for providing annual monitoring data on Sigaus minutus, and for their support throughout this project. A special thanks to Warren Chinn, Carol Burke and all those involved in the translocation of B. robustus, and to those who were involved in the annual grasshopper surveys. We thank two anonymous reviewers for their helpful comments on this manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Jennifer C. Schori http://orcid.org/0000-0002-7119-3538
Tammy E. Steeves http://orcid.org/0000-0003-2112-5761
Tara J. Murray http://orcid.org/0000-0001-8332-5406
Additional information
Funding
References
- Barker GM. 2016. Land snail communities respond to control of invasive rats in New Zealand forests. New Zealand Journal of Ecology. 40(3):310–320.
- Basse B, Flux I, Innes J. 2003. Recovery and maintenance of North Island kokako (Callaeas cinerea wilsoni) populations through pulsed pest control. Biological Conservation. 109(2):259–270.
- Bates D, Mächler M, Bolker B, Walker S. 2014. Fitting linear mixed-effects models using lme4. Journal of Statistical Software. 67(1):1–48.
- Bell M. 2016. Accelerating predator free New Zealand. Wellington: New Zealand Government. https://fyi.org.nz/request/4307/response/14723/attach/3/Spnog0116090814360.pdf.
- Bigelow RS. 1967. The grasshoppers of New Zealand, their taxonomy and distribution, Lewis JD, editor. Christchurch: University of Canterbury.
- Daugherty CH, Gibbs GW, Hitchmough R. 1993. Mega-island or micro-continent? New Zealand and its fauna. Trends in Ecology and Evolution. 8(12):437–442.
- Department of Conservation. 2017. New Zealand's threatened species strategy. Wellington: Department of Conservation.
- Dickens MJ, Delehanty DJ, Romero LM. 2010. Stress: an inevitable component of animal translocation. Biological Conservation. 143(6):1329–1341.
- Dowding J, Elliott M, Murphy E. 2015. Scats and den contents as indicators of the diet of stoats (Mustela erminea) in the Tasman Valley, South Canterbury, New Zealand. New Zealand Journal of Zoology. 42(4):270–282.
- Fischer JR. 1994. Temperature effect on postdiapause development and survival of embryos of three species of Melanoplus (Orthoptera: Acrididae). Annals of the Entomological Society of America. 87(5):604–608.
- Gibbs G. 2010. Do New Zealand invertebrates reflect the dominance of birds in their evolutionary history? New Zealand Journal of Ecology. 34(1):152.
- Gibbs GW. 1998. Why are some weta (Orthoptera: Stenopelmatidae) vulnerable yet others are common? Journal of Insect Conservation. 2(3):161–166.
- Gillies C. 2001. Advances in New Zealand mammalogy 1990–2000: house cat. Journal of the Royal Society of New Zealand. 31(1):205–218.
- Harper GA. 2005. Numerical and functional response of feral cats (Felis catus) to variations in abundance of primary prey on Stewart Island (Rakiura), New Zealand. Wildlife Research. 35:597–604.
- Heidinger IMM, Poethke H-J, Bonte D, Hein S. 2009. The effect of translocation on movement behaviour – a test of the assumptions of behavioural studies. Behavioural Processes. 82(1):12–17.
- Holdaway RN. 1989. New Zealand's pre-human avifauna and its vulnerability. New Zealand Journal of Ecology. 12:11–25.
- Jacquot JJ, Solomon NG. 1997. Effects of site familiarity on movement patterns of male prairie voles Microtus ochrogaster. American Midland Naturalist. 138(2):414–417.
- Jamieson CD. 1996. The grasshopper Sigaus minutus in Central Otago: a pilot study. Science for Conservation: 42. Wellington: Department of Conservation.
- Jones C, Moss K, Sanders M. 2005. Diet of hedgehogs (Erinaceus europaeus) in the upper Waitaki Basin, New Zealand: Implications for conservation. New Zealand Journal of Ecology. 29(1):29–35.
- Keedwell RJ, Brown KP. 2001. Relative abundance of mammalian predators in the upper Waitaki Basin, South Island, New Zealand. New Zealand Journal of Zoology. 28(1):31–38.
- King CM. 1990. The handbook of New Zealand mammals. Oxford: Oxford University Press.
- Kissling DW, Pattemore DE, Hagen M. 2014. Challenges and prospects in the telemetry of insects. Biological Reviews. 89(3):511–530.
- Langham N. 1990. The diet of feral cats (Felis catus L.) on Hawke’s Bay farmland, New Zealand. New Zealand Journal of Zoology. 17(2):243–255.
- Lester PJ, Brown SDJ, Edwards ED, Holwell GI, Pawson SM, Ward DF, Watts CH. 2014. Critical issues facing New Zealand entomology. New Zealand Entomologist. 37(1):1–13.
- Letty J, Marchandeau S, Aubineau J. 2007. Problems encountered by individuals in animal translocations: lessons from field studies. Écoscience. 14(4):420–431.
- Linklater W, Steer J. 2018. Predator Free 2050: a flawed conservation policy displaces higher priorities and better, evidence-based alternatives. Conservation Letters. e12593.
- Marable MK, Belant JL, Godwin D, Wang GM. 2012. Effects of resource dispersion and site familiarity on movements of translocated wild turkeys on fragmented landscapes. Behavioural Processes. 91(1):119–124.
- McGlone MS. 2006. Becoming New Zealanders: immigration and the formation of the biota. In: Allen RB, Lee WG, editors. Biological invasions in New Zealand. Berlin: Springer; p. 17–32.
- Morris SJ. 2002. Identification guide to grasshoppers (Orthoptera: Acrididae) in Central Otago and Mackenzie country. DOC Science Internal Series no. 26. Wellington: Department of Conservation.
- Moss K, Sanders M. 2001. Advances in New Zealand mammalogy 1990–2000: hedgehog. Journal of the Royal Society of New Zealand. 31(1):31–42.
- Murphy EC, Keedwell RJ, Brown KP, Westbrooke I. 2004. Diet of mammalian predators in braided river beds in the central South Island, New Zealand. Wildlife Research. 31(6):631–638.
- Norbury G. 2001. Conserving dryland lizards by reducing predator-mediated apparent competition and direct competition with introduced rabbits. Journal of Applied Ecology. 38:1350–1361.
- Norbury G, Byrom A, Pech R, Smith J, Clarke D, Anderson D, Forrester G. 2013. Invasive mammals and habitat modification interact to generate unforeseen outcomes for indigenous fauna. Ecological Applications. 23(7):1707–1721.
- Norbury G, Heyward R, Parkes J. 2002. Short-term ecological effects of rabbit haemorrhagic disease in the short-tussock grasslands of the South Island, New Zealand. Wildlife Research. 29(6):599–604.
- Norbury G, Heyward R, Parkes J. 2009. Skink and invertebrate abundance in relation to vegetation, rabbits and predators in a New Zealand dryland ecosystem. New Zealand Journal of Ecology. 33(1):24–31.
- Owens B. 2017. The big cull: Can New Zealand pull off an audacious plan to get rid of invasive predators by 2050? Nature. 541:148–150.
- O'Donnell C, Weston K, Monks J. 2017. Impacts of introduced mammalian predators on New Zealand’s alpine fauna. New Zealand Journal of Ecology. 41(1):1–22.
- Parkes JP, Nugent G, Forsyth DM, Byrom AE, Pech RP, Warburton B, Choquenot D. 2017. Past, present and two potential futures for managing New Zealand's mammalian pests. New Zealand Journal of Ecology. 41(1):151–161.
- Pierce RJ. 1987. Predators in the Mackenzie Basin: their diet, population dynamics, and impact on birds in relation to the abundance and availability of their main prey (rabbits). Wellington: Wildlife Service, Department of Internal Affairs.
- Pyke GH, Pulliam HR, Charnov EL. 1977. Optimal forgaing: a selective review of theory and tests. The Quarterly Review of Biology. 52(2):137–154.
- R Core Team. 2016. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
- Rufaut CG, Gibbs GW. 2003. Response of a tree weta population (Hemideina crassidens) after eradication of the Polynesian rat from a New Zealand island. Restoration Ecology. 11(1):13–19.
- Ruscoe WA, Sweetapple PJ, Perry M, Duncan RP. 2013. Effects of spatially extensive control of invasive rats on abundance of native invertebrates in mainland New Zealand forests. Conservation Biology. 27(1):74–82.
- Sanders MD, Maloney RF. 2002. Causes of mortality at nests of ground-nesting birds in the Upper Waitaki Basin, South Island, New Zealand: a 5-year video study. Biological Conservation. 106(2):225–236.
- Soulé ME, Bolger DT, Alberts AC, Wrights J, Sorice M, Hill S. 1988. Reconstructed dynamics of rapid extinctions of chaparral-requiring birds in urban habitat islands. Conservation Biology. 2(1):75–92.
- St Clair JJH. 2011. The impacts of invasive rodents on island invertebrates. Biological Conservation. 144(1):68–81.
- Stringer IAN, Hitchmough RA. 2012. Assessing the conservation status of New Zealand's native terrestrial invertebrates. New Zealand Entomologist. 35(2):77–84.
- Teixeira CP, De Azevedo CS, Mendl M, Cipreste CF, Young RJ. 2007. Revisiting translocation and reintroduction programmes: the importance of considering stress. Animal Behaviour. 73(1):1–13.
- Trewick SA, Johns PM, Hitchmough RA, Rolfe J, Stringer IAN. 2014. Conservation status of New Zealand Orthoptera, 2014. New Zealand Threat Classification Series 16. Wellington: Department of Conservation.
- Watts C, Thornburrow D, Cave V, Innes J. 2014. Beetle community changes following pest mammal control at two biodiversity sanctuaries in Wellington, New Zealand. Journal of the Royal Society of New Zealand. 44(2–3):61–87.
- White EG. 1994. Ecological research and monitoring of the protected grasshopper Brachaspis robustus in the Mackenzie Basin. Wellington: Department of Conservation.
- Whitehead AL, Edge K-A, Smart AF, Hill GS, Willans MJ. 2008. Large scale predator control improves the productivity of a rare New Zealand riverine duck. Biological Conservation. 141(11):2784–2794.
- Wilmshurst JM, Anderson AJ, Higham TFG, Worthy TH. 2008. Dating the late prehistoric dispersal of Polynesians to New Zealand using the commensal Pacific rat. Proceedings of the National Academy of Sciences. 105(22):7676–7680.
- Wilson DJ, Lee WG. 2010. Primary and secondary resource pulses in an alpine ecosystem: snow tussock grass (Chionochloa spp.) flowering and house mouse (Mus musculus) populations in New Zealand. Wildlife Research. 37(2):89–103.
- Woolmore C, Anderson S, Garside R. 2010. Project river recovery annual report, 1 July 2009–30 June 2010. Twizel: Department of Conservation.
- Woolmore C, Anderson S, Garside R. 2013. Project river recovery annual report, 1 July 2012–30 June 2013. Twizel: Department of Conservation.
- Yoder JM, Marschall EA, Swanson DA. 2004. The cost of dispersal: predation as a function of movement and site familiarity in ruffed grouse. Behavioral Ecology. 15(3):469–476.