ABSTRACT
The new species Quinquelaophonte aurantius sp.nov. is described, based on specimens collected in Portobello Bay, New Zealand. The species is distinguishable for having long fine setules in the anal operculum distal edge, a breadth ratio of caudal rami length above 3.5, and a rudimentary antenna abexopodal spine. This new species differentiates from Q. parasigmoides and Q. wellsi on the following autapomorphs; short spine-like outer seta in segment 2 of females P3 endopod and the partial reduction of setae in spines from male P3 and P4, longer V-shaped caudal rami, an almost non-existent terminal portion of the antenna exopod with short lateral setae. Phylogenetic analyses demonstrate the position of Quinquelaophonte within the family Laophontidae.
Publication LSID: urn:lsid:zoobank.org:pub:D5E5EB23-8348-446D-9606-EA8B27EC0EDC
LSID for Q. aurantius: urn:lsid:zoobank.org:act:834C4218-209B-4B70-8AD5-35512B5FCB29
Introduction
The genus Quinquelaophonte (Wells et al. Citation1982) has 12 described species: Q. quinquespinosa (Sewell Citation1924), Q. bunakenensis (Mielke Citation1997), Q. koreana (Lee Citation2003), Q. wellsi (Hamond Citation1973), Q. prolixasetae (Walker-Smith Citation2004), Q. candelabrum (Wells et al. Citation1982), Q. capillata (Wilson Citation1932), Q. longifurcata (Lang Citation1965), Q. parasigmoides (Bozic Citation1969), Q. varians (Bjornberg Citation2010) and Q. aestuarii (Sciberras et al. Citation2014). To date, Q. candelabrum is the only species of Quinquelaophonte described in New Zealand (Wells et al. Citation1982), and is one of the most common harpacticoids found in intertidal mud and fine sand sediments in estuaries and harbours.
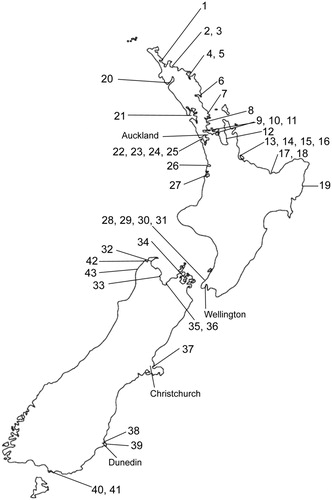
As part of a nationwide sampling, the presence of an undescribed second species of Quinquelaophonte inhabiting the same substrata was identified. This species presents a smaller distribution than Q. candelabrum (, ). Quinquelaophonte aurantius sp. nov. is easily cultured in the laboratory and their sensitivity to pollutants has been validated for estuarine sediment toxicity testing (Stringer et al. Citation2012a; Stringer et al. Citation2014). In this study, we describe this new harpacticoid copepod species of the genus Quinquelaophonte, obtained from field samples collected at Portobello Bay, in Dunedin, New Zealand.
Table 1. Known locations of Quinquelaophonte candelabrum and Q. aurantius sp. nov. in New Zealand. Numbers correspond to locations shown in .
Materials and methods
Specimens collection and treatment
Specimens were collected from sediment samples in Portobello Bay, Dunedin (45°50’14.3’'S170°39’25.9E’’). Sediment samples were gently sieved out with a 100-μm plastic mesh, and fixed in 4% formalin before being transferred to 70% ethanol for storage. Specimens were cleared in lactic acid, dissected in water, mounted in Reyne's Medium and ringed with clear nail varnish for identification. Drawings were made with a camera lucida tube on a Zeiss Universal microscope equipped with Nomarski differential interference contrast microscopy. Terminology of tagma and appendages follows Huys & Boxshall (Citation1991). In this paper, ‘medial’ signifies ‘towards the middle or centre of the animal or of the structure being described’. P1–P4 refers to the ‘swimming’ legs and P5–P6 to the legs modified for reproductive purposes. Individual ‘segments’ of rami are numbered from proximal to distal (i.e. exopod–1, exopod–2, exopod–3). Setal formula of P1–P4 is given in the simple Langian form (Lang Citation1934). The total length of individuals was measured from the base of the rostrum to the apex of the caudal rami. Mean length is expressed ±1SD. The wide variation in length represents the difference between extremely contracted and fully relaxed individuals. This mean value probably is considerably smaller than the true mean of a collection of living adults.
Material examined
Type specimens were deposited in the Auckland Museum, New Zealand. Holotype: adult female preserved in ethanol (reg. no. MA73574, Auckland Museum). Allotype: adult male preserved in ethanol (reg. no. MA73575, Auckland Museum). Paratypes: 4 females – 1 preserved in ethanol and 3 dissected and mounted on slides (reg. no. MA73576, Auckland Museum); 5 males – 2 preserved in ethanol and 3 dissected and mounted on slides (reg. no. MA73577, Auckland Museum).
DNA extraction, sequencing, and phylogenetic analyses
Total genomic DNA was extracted from 100 pooled adult copepods using a PowerSoil DNA isolation kit (Qiagen, Hilden, Germany) following the manufacturer's protocol. DNA extractions were quantified using a NanoPhotometer (Implen, Munich, Germany) to check for DNA quantity and quality (260/280 ratio), and stored at −20°C until further analysis. An approximately 700 bp section of the cytochrome c oxidase subunit I gene (COI) was amplified using the primers LCO1490 and HCO2198 (Vrijenhoek Citation1994). The polymerase chain reaction (PCR) amplifications were carried out in 50 µl reaction volumes containing; 25 µL of MyTaqTM 2x PCR master mix (Bioline, MA, USA), both forward and reverse primers 0.4 µM and template DNA (ca. 50–150 ng). Thermocycling conditions consisted of an initial denaturing step of 95°C, 4 min, followed by 40 cycles of 94°C, 1 min; 50°C, 1 min; 72°C, 90 s; with a final extension step of 72°C, 10 min. An approximately 1800 bp section of the 18S ribosomal DNA gene region (rDNA) was amplified using the primers EukA and EukB (Medlin et al. Citation1988). PCRs were made up as described above with thermocycling conditions of initial denaturing step of 94°C, 2 min, followed by 30 cycles of 94°C, 30 s; 56°C, 1 min; 72°C, 90 s; with a final extension step of 72°C, 10 min. Amplification products were purified using AxyPrep PCR cleanup kits (Axygen, California, USA) and sequenced in both directions, using the PCR primers, by an external contractor (Genetic Analysis Services, University of Otago, Dunedin). Forward and reverse sequences were aligned using Geneious v8.1.5 (Kearse et al. Citation2012) and conflicts resolved by manual inspection.
The 18S rDNA sequence from Q. aurantius was aligned with publicly available Harpacticoida 18S rDNA sequences (Yeom et al. Citation2018) from GenBank using the ClustalW algorithm (Thompson et al. Citation1994) in Geneious. For subsequent analyses, sequence data matrices were truncated to 1695 bp. Bayesian analyses were carried out in Geneious using MrBayes 3.1.2 (Ronquist et al. Citation2012) using the evolutionary model (general time reversible with gamma-distributed rate variation across sites and a proportion of invariable sites, GTR + G + I). Analyses of alignments were carried out in two simultaneous runs with four chains each 2.1 × 106 generations, sampling every 1000 trees. A 50% majority-rule consensus tree was drawn from the last 1000 trees. All final split frequencies were <0.01.
Results
Taxonomy
Quinquelaophonte aurantius sp. nov. ().
Figure 2. Quinquelaophonte aurantius sp. nov. female: A–C, urosomites 2–6 dorsal, ventral and lateral; D, P6 and genital field; E, anal operculum; F, labrum; G, paragnaths.
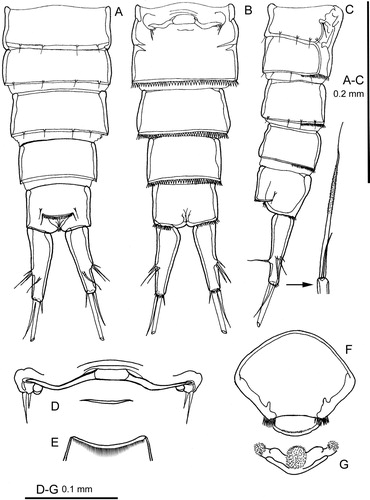
Figure 3. Quinquelaophonte aurantius sp. nov. female: A–C, right caudal ramus dorsal, ventral and outer lateral; D, antennule; E, antennule segment 6 in posterior view; F, mandible; G, mandible gnathobase in another orientation; H, maxillule. [int = internal; ext = external].
![Figure 3. Quinquelaophonte aurantius sp. nov. female: A–C, right caudal ramus dorsal, ventral and outer lateral; D, antennule; E, antennule segment 6 in posterior view; F, mandible; G, mandible gnathobase in another orientation; H, maxillule. [int = internal; ext = external].](/cms/asset/90e05b36-e818-4177-abdc-563f7c80fe82/tnzz_a_1548496_f0003_ob.jpg)
Figure 4. Quinquelaophonte aurantius sp. nov. A–F, female: A, antenna; B, maxilla; C, maxilla, distal endites; D, maxilliped; E, P1 and intercoxal sclerite; F, P.5; G, male P5.
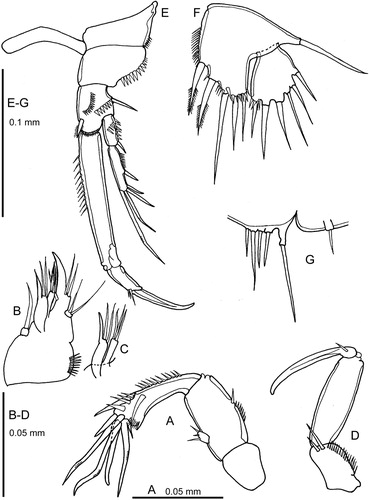
Figure 6. Quinquelaophonte aurantius sp. nov. A–B, P3 and intercoxal sclerite, female and male (note comment in text on setation of male exopod-3).
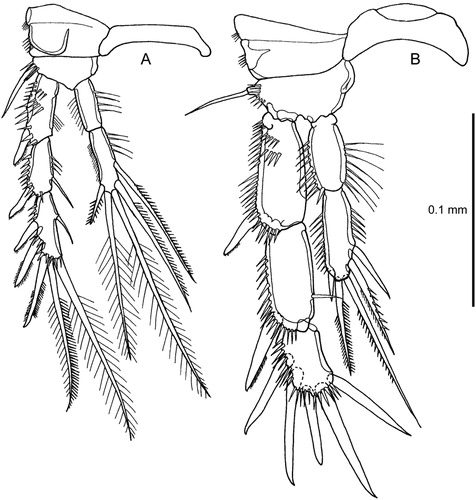
Figure 7. Quinquelaophonte aurantius sp. nov. A–B, P4 and intercoxal sclerite, female and male (note comment in text on setation of male exopod-2 and -3); C, male, pair of P6.
Etymology
The specific name alludes to the latin word for orange (aurantius), in reference to the species body's colour when alive.
Type location
Portobello Bay, Otago Harbour, New Zealand (, location 38).
Diagnosis
Body surface almost naked, without a comprehensive cover of minute setules, denticles or pustules; anal operculum with long fine setules; caudal ramus almost four times as long as maximum breadth; female antennule 6–segmented; antenna abexopodal seta spine-like and small; P1 exopod–2 with two setae and three spines; P1 endopod–2 with minute accessory seta; exopod–3 of female P3 and P4 with six setae and spines; female P5 exopod with six setae; male P5 with a total of five setae. Adult length: Females: 608–842 µm; mean 737 ± 55 µm (n = 34). Males: 632–808 µm, mean 715 ± 49 µm (n = 23).
Female description
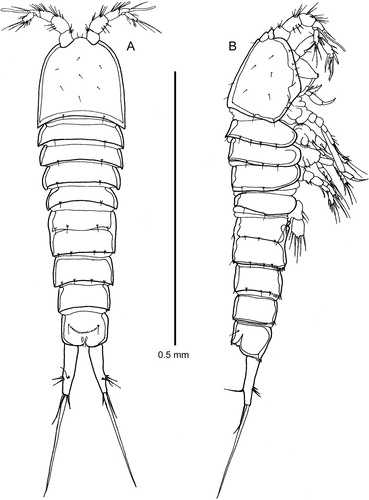
Habitus
(A–B): almost cylindrical. Urosome slightly tapering posteriorly. Somites well demarcated from each other. Hyaline frill broad on cephalic shield but rudimentary elsewhere. Rostrum small, fused to cephalic shield, with two sensilla. Urosomites 2–3 fused to form a genital double somite (A–C). Outline of urosomite-2 obvious in dorsal view. Junction between the two somites marked by a prominent dorsal and lateral chitin ridge bearing sensilla that ventrolaterally extends anteriorly and posteriorly as though marking the ventral margin of the pleura. Anal operculum (E) well developed, semilunar, margin heavily chitinised, clothed with fine setules and flanked each side by a sensillum. Posterior margin of all somites (including urosomite–2) except urosomites 5–6 with several sensilla on small pedestals. Ventral and ventrolateral posterior margins of urosomites 3–6 a row of small spinules. Dorsal posterior margin of all urosomites without spinules. Almost all body surfaces with a thin layer (about 2μm) of mucus in which a large number of minute particles are entrapped. Generally, the body surface without apparent surface ornamentation except for some sensilla on the cephalic shield.
Genital field
(D): The pair of P6 widely separated; each with two short setae. Adjacent and medial to the P6 is a small lobe that possibly conceals the gonopore. Copulatory pore not displaced posteriorly and possibly hidden beneath a central chitin ridge. This central region is linked on each side to the P6 region by a tubular structure whose medial portion is somewhat enlarged and may be the receptaculum seminis.
Labrum
(F) and paragnaths (G): Labrum very large and very prominent in lateral view of whole animal (B). Paragnaths complex, with two minutely setulose lateral knobs and a large similarly setulose central knob.
Caudal ramus
(A–C): cylindrical, slightly conical, with the inner margin convex proximally; maximum length 3.5–3.8 times as long as maximum width; seven setae. Origins of setae I–III closely adjacent about two-thirds along the inner margin. Seta I rudimentary. Setae IV and V with a common base. Seta IV short and very slender. Seta V 2–3 times as long as the ramus, without a cleavage plane; middle portion set all round with minute setules only just visible under high magnification (x1825) Nomarski optics (C). Seta VI at inner distal corner, very short. Seta VII well developed, biarticulate at its base, borne on a pedestal just distal to the origin of setae I–III.
Antennule
(D–E): short, six-segmented. Segment four has a terminal aesthetasc fused basally with a long seta and accompanied by another long seta in a trithek. Segment six with a terminal trithek similar to that on segment four, although the aesthetasc is very fine and delicate. Setal formula: 1–[1], 2–[8], 3–[7], 4–[2 + aesthetasc], 5–[1], 6–[11 + aesthetasc].
Mandible
(F–G,): with a strongly chitinised, but relatively simple gnathobase consisting of five short blunt teeth, three of which appear to be on a distinct plate. Palp a single element, exopod and endopod not recognisable but represented by one and three setae respectively; basis with one terminal seta.
Maxillule
(H): arthrite with five terminal teeth and a subterminal lateral spinule; posterior surface with a row of spinules, but without setae. Coxa distinct, with two long terminal setae. Basis endite with three setae. Rami absorbed into basis. Endopod with three setae. Exopod with two setae.
Antenna
(A): large and robust. Allobasis with a small abexopodal spine like seta that is not much longer than the adjacent spinules. Endopod–2 with two stout spines on inner margin, one of which is displaced medially and some extremely large, stout spinules distally; terminally with two claw-like spines and three weakly geniculate spines, the outer sharing a common base with a very small spine. Exopod reduced to one very small segment with three-minute terminal setae.
Maxilla
(B–C): syncoxa compact, with three endites; proximal endite with 1 seta, distal endites each with three elements. Basis with a fused terminal pinnate claw with two setae at its base on the inner side and one on the outer side. Endopod recognisable (in some specimens it appears to be distinct from the basis) with two setae.
Maxilliped
(D): long and slender, prehensile. Syncoxa with two setae. Basis unornamented. Endopod a long claw with a minute seta at its base.
P1
(E): The pair of P1 widely separated by a long slender intercoxal sclerite. Praecoxa large and prominent; with small spinules at the outer distal corner. Coxa much broader than long, outer side rounded and ornamented with strong spinules. Basis narrow, much longer than broad, with origin of exopod considerably proximal to that of endopod. Outer margin with a stout spine. Inner spine spinulose, subterminal and originating slightly medial to inner margin. Anterior surface of basis with outer and medial antero-posterior rows of stout spinules. Distal margin above origin of endopod ornamented with small spinules. Exopod two-segmented and extends to about halfway along endopod–1. Exopod–2 almost twice as long as exopod–1. Exopod–1 with a distal outer spine and ornamented with stout spinules along outer edge and in a proximal transverse row on anterior surface. Exopod–2 with two weakly geniculate setae terminally and three outer spines. Endopod elongate, prehensile, two-segmented. Endopod–1 about four times as long as endopod–2. Endopod–1 with long fine setules on proximal part of inner margin. Endopod–2 terminally with a long claw, a minute seta and two small spinules.
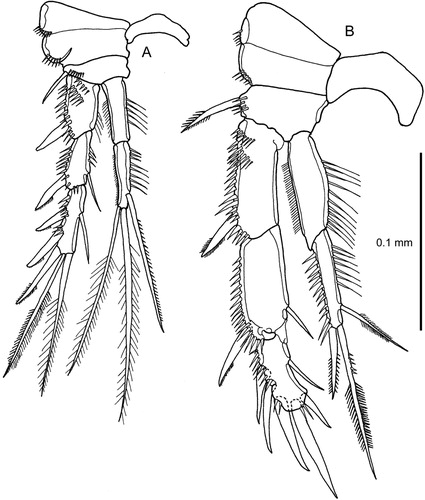
P2
(A): the pair of P2 separated by a slender intercoxal sclerite that is shorter than that of the other legs. Praecoxa large and prominent but suture with coxa less well defined than in P1. Praecoxa with spinules at outer distal corner. Coxa with spinules at outer distal corner; sclerotisation of outer margin continues medially in an arc. Basis narrow, especially on outer side, with a short row of stout spinules around the origin of the stout outer spine. Exopod with three, endopod with two segments; endopod extends beyond the end of exopod–2. Exopod segments approximately equal in length but progressively more slender; outer distal corner of exopod–1 and –2 produced as a rounded projection; outer margin of segments densely ornamented with long stout spinules; proximal anterior surface of exopod–1 with two diagonal rows of stout spinules. Endopod segments approximately equal in length, elongate, slender; inner margin ornamented with very long fine setules; outer margin of endopod–1 with extremely fine setules, that of endopod–2 with minute spinules.
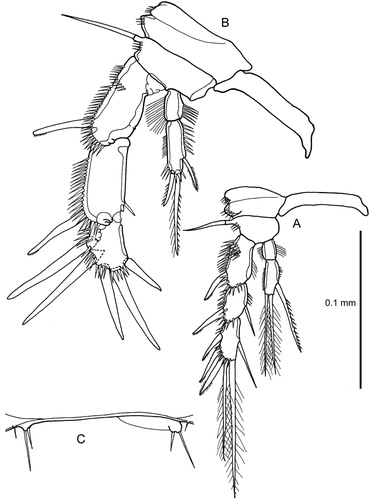
P3
(A): differs from P2 only in the longer intercoxal sclerite, a narrower and less well-defined praecoxa, slightly different proportions of exopod and endopod segments and the presence of five setae and spines on endopod–2.
P4
(A): intercoxal sclerite of similar proportions to that of P1 and P3. Praecoxa similar to P3. Coxa as P2–P3. Basis as P2–P3 but with outer side projecting as a long pedestal. Exopod slightly shorter and endopod much shorter than P2–P3. Endopod extends only halfway along exopod–2. Exopod–1 considerably longer than exopod 2 or 3. Endopod–2 almost twice as long as endopod–1. Ornamentation of rami as P2-P3.
P5
(F): the pair of P5 are separate. Basis and endopod fused as a baseoendopod. Outer side of basis expressed as a narrow pedestal with a slender seta. Endopod lobe well developed; ornamented with fine setules on inner margin and spinules on distal margin. Exopod not fused to basis, only slightly longer than endopod lobe; broadly triangular in shape, making it difficult to be certain which setae are on the inner, outer and distal margins; inner distal corner with spinules. Baseoendopod with two bipinnate spines and three setae, exopod with six short plain setae.
Variability
was noted in body length. Caudal ramus seta V varied relative to urosome length; in females of similar body length this seta varied between being almost as long as the entire urosome (excluding caudal ramus) to being equal only to urosomites 3–6.
Male Description
The male differs from the female in the following aspects:
Habitus
(A–B): urosomites 2–3 not fused and without trace of ventral pleura margins. Venter of urosomite–3 with a proximal row of fine setules situated in a shallow groove. Urosomite–4 with a similar groove but without ornamentation.
Antennule
(C–G): chirocerate; nine segments, with segments 2–3 and 5–6 fused together. The major articulation is between segments 6 and 7. Segments 5–6 form a very large and bulbous unit, with small traces of its amalgamation from two precursors visible on the outer margin. The portion distal to segments 5–6 comprises three segments that are poorly demarcated from each other. The visible line of demarcation between segments 7 and 8 is very weak and that between segments 8 and 9 is variably present or incomplete. Aesthetasc on segment four shares a common base with a long seta in an acrothek; another long seta originates on an adjacent but distinctly separate pedestal. Inner margin of segment four has a large minutely spinose digitate pad. Immediately distal to this pad is a bifid lobe; proximal to the pad is a large stoutly spinulose structure, a small seta and a pedestal with a small acutely pointed hyaline structure. A similar structure appears on segment five. While it is possible these structures have been derived from setae they are excluded from the setal formula. Setal formula: 1-[1], 2-3–[9], 4–[7], 5–[10 + spiniform process + aesthetasc + hyaline structure], 6–[0], 7–[1], 8-9–[10].
P2
(B): much longer and more stoutly built than female. Intercoxal sclerite broader in proximal-distal axis than in the female. Protopod similar to female except that the coxa lacks a medial sclerotised arc and inner margin of basis appears to be more heavily chitinised. Ornamentation of rami as female except that the setules and spinules on the outer margin of the endopod are much stronger. Exopod–3 very strongly chitinised, especially around the origin of the outer and terminal spines, where the segment margin is extended so that the spines appear to be set into deep sockets. Terminal setae represented by very stout spines in the male. Exopod–3 linked to exopod–2 by a flexible membrane at inner proximal corner that enables exopod–3 to be swivelled inwards through approximately 90˚. Endopod less modified. Endopod–1 less cylindrical than in female, inner margin convex; outer distal corner a unguiform projection. Inner seta of endopod–2 much shorter than in female and outer seta replaced by a much shorter plumose spine. Outer margin of endopod–2 with strong spinules.
P3
(B): similar to P2 in general build; junction between exopod–2 and –3 similarly modified. Endopod not extending to the end of exopod–2; relatively shorter and much more robust than in female. Endopod–2 setae much shorter than in female; outer seta replaced by a very short stout spine. Outer distal corner of endopod–1 not unguiform. Inner seta much reduced in size on exopod–2 and –3, and usually absent from exopod–3 (in10 males examined this seta was only present on the right leg in one and on the left in two; never present on both legs).
P4
(B): similar to P2 in general build; junction between exopod–2 and –3 similarly modified. The two proximal outer spines of exopod–3 very long. Endopod–2 more cylindrical than in female but with a rounded base and apex; all setae much shorter and outer seta replaced by a spinulose spine. Outer distal corner of endopod–1 not unguiform. Inner seta of exopod–2 rudimentary or absent (it was present on the left leg of all 10 males examined, but on the right leg in only one). Nine of the 10 males examined lack an inner seta on exopod–3; in one male a rudimentary seta was present on the right leg only (this is the same male that has a seta on the right P3 exopod–3). Setal formula for male P1-P4 swimming legs (E–7A) ().
Table 2 . Summary setal formula for male and female Q. aurantius sp. nov. P1-P4 swimming legs.
P5
(G): reduced to a long outer pedestal (the remnant of the basis) bearing a long seta and four small inner pedestals each bearing a seta (there is no evidence to indicate whether these are rudiments of the exopod or endopod or a mixture of both).
P6
(C): left P6 represented by a broad but shallow articulated genital lappet bearing two setae at its outer margin. Right P6 reduced to two small pedestals fused to somite margin, each bearing a seta.
Variability
was noted in body length, relative length of caudal ramus seta V (of a similar order to that in the female), the level of reduction of setation in P3 and P4 and the degree of segmental fusion in the antennule.
Habitat distribution
Quinquelaophonte aurantius is widespread in the Otago Harbour, and at Taipa in Doubtless Bay (; sites 2 and 38). Habitat preferences has been correlated to finer sediments (mud to medium sand particles <500 µm), and upper tidal reaches with pH between 8 and 8.8 (Stringer et al. Citation2012b).
Remarks
Reference DNA sequences obtained from adult copepods of Q. aurantius have been deposited in GenBank as follows: COI (MH444814) and 18S rDNA (MH444815).
Phylogenetic analyses
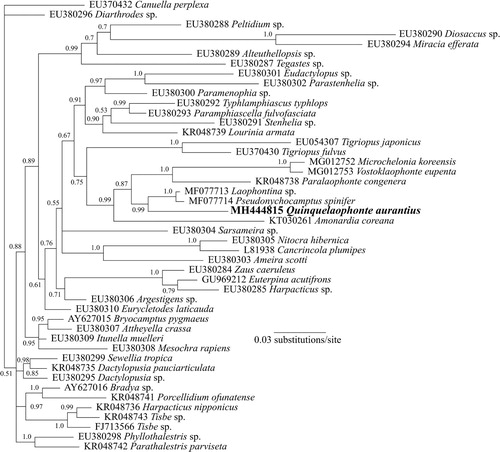
The phylogenetic tree based on the nuclear 18S rDNA gene () shows that currently available sequences from genera of the family Laophontidae (Paralaophonte, Pseudonychocamptus, Laophontina, Microchelonia, Vostoklaophonte) group together with the sequence from Q. aurantius with very high support (87% Bayesian posterior probability) (Yeom et al. Citation2018). In particular, the analysis suggests a close relationship between Quinquelaophonte, Pseudonychocamptus and Laophontina.
Discussion
Analysis of all Quinquelaophonte species shows that Q. aurantius is most similar to Q. parasigmoides and Q. wellsi in major structures. Regarding the geographic distribution, species Q. parasigmoides is known only from marine beach sand on Réunion Isle (Indian Ocean, east of Madagascar) and Q. wellsi from saline lakes in South Australia. Ten females and ten males of Q. aurantius were examined for variability of structural characters. Based on the descriptions provided for Q. parasigmoides and Q. wellsi, there appear to be four autapomorphs for male Q. aurantius. Three relate to the partial reduction of setae and spines in the male P3 and P4. However, a number of other structural differences between these three species are shown on . Phylogenetic analyses demonstrate the position of Quinquelaophonte within the family Laophontidae.
Table 3. Characters of Q. aurantius sp. nov. compared with Q. parasigmoides and Q. wellsi. * =Autapomorph. (a) 5 setae on both legs in 7 of the 10 individuals examined; 5 on left and 6 on right in 1 of 10; 6 on left, 5 on right in 2 of 10; (b)1 female (of 2 examined) without seta on second segment on both legs; (c) 1 female (of 2 examined) with two inner setae on left leg only; (d) Exp. 2 inner seta: present on left leg of all 10 individuals; only on 1 of 10 on right leg; (e) Exp. 3 inner seta: 9/10 seta absent; 1/10 seta rudimentary on right leg only.
Despite the distribution similarity between Q. aurantius and Q. candelabrum in New Zealand, different morphological characteristics are evident on the urosomite, the antennule and the shape of the seta V. Unlike Q. aurantius males, the two rows of fine setules in the proximal part of the venter are not situated in a groove in the somite surface of Q. candelabrum (Figure S1). Further, the urosomite 4 does not have a ventral grove. Another main difference lies on the male antennule, where segments 5–6 and 7–9 are completely fused, and the distal digitate element on the digitate spine is extremely long (Figure S2). Last, seta V in Q. candelabrum has a pipette like shape, while Q. aurantius sp. nov. has a pronounced V shape in both sexes.
Supplementary material
Download MS Word (367.1 KB)Acknowledgements
We would like to thank the colleagues mentioned in who made their material available to us, and Janet Adamson (Cawthron Institute) for the extraction of DNA material.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Louis A. Tremblay http://orcid.org/0000-0002-3586-3995
Additional information
Funding
References
- Bjornberg T. 2010. Quinquelaophonte varians n. sp.(Copepoda, Harpacticoida, Crustacea) and notes on its developmental stages. Pan-American J Aquat Sci. 62(1):62–77.
- Bozic B. 1969. Copépodes harpacticoïdes de la Réunion. Bulletin du Muséum National d’Histoire Naturelle. 41(4):867–882.
- Hamond R. 1973. The Australian species of Robertsonia (Crustacea, Harpacticoida), with a revised key to the genus. Vol. 28 Records of the Australian Museum. 18).
- Huys R, Boxshall G. 1991. Evolutionary trends within the Copepoda. Copepod Evol Ray Soc. (ed), 169, London, 315–370.
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C. 2012. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 28(12):1647–1649. doi: 10.1093/bioinformatics/bts199
- Lang K. 1934. Marine Harpacticiden von der Campbell-Insel und einigen anderen südlichen Inseln, von Karl Lang.(Arbeit aus dem Zoologischen Institut Lund.). H. Ohlssons Buchdruckerei.
- Lang K. 1965. Copepoda Harpacticoidea from the Californian Pacific Coast. Kungliga Svenska Vetenskapsakademiens Handlingar, 4th series. Stockholm, Sweden: Almqvist & Wiksell.
- Lee W. 2003. A marine harpacticoid, Quinquelaophonte koreana sp. nov. from a sandy beach in Korea (Crustacea: Copepoda). Zool Sci. 20(5):657–668. doi: 10.2108/zsj.20.657
- Medlin L, Elwood HJ, Stickel S, Sogin ML. 1988. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene. 71(2):491–499. doi: 10.1016/0378-1119(88)90066-2
- Mielke W. 1997. On a small collection of Laophontidae (Copepoda) from Sulawesi, Indonesia. Microfauna Mar. 11:223–250.
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. 2012. Mrbayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 61(3):539–542. doi: 10.1093/sysbio/sys029
- Sciberras M, Bulnes VN, Cazzaniga NJ. 2014. A new species of Quinquelaophonte (Copepoda: Harpacticoida) from Argentina. Zoologia (Curitiba). 31(5):496–502. doi: 10.1590/S1984-46702014000500010
- Sewell RS. 1924. Fauna of the Chilka Lake: Crustacea Copepoda.
- Stringer TJ, Glover CN, Keesing V, Northcott GL, Gaw S, Tremblay LA. 2014. Development of acute and chronic sediment bioassays with the harpacticoid copepod Quinquelaophonte sp. Ecotoxicol Environ Saf. 99:82–91. doi: 10.1016/j.ecoenv.2013.10.002
- Stringer TJ, Glover CN, Keesing V, Northcott GL, Tremblay LA. 2012a. Development of a harpacticoid copepod bioassay: selection of species and relative sensitivity to zinc, atrazine and phenanthrene. Ecotoxicol Environ Saf. 80:363–371. doi: 10.1016/j.ecoenv.2012.04.008
- Stringer TJ, Korsman JC, Peralta G, Keesing V, Tremblay LA, Glover CN. 2012b. Effects of environmental gradients on the distribution of harpacticoid copepods in an intertidal flat, Portobello Bay, Otago Harbour. N Z J Mar Freshwat Res. 46(3):385–397. doi: 10.1080/00288330.2012.697069
- Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL w: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22(22):4673–4680. doi: 10.1093/nar/22.22.4673
- Vrijenhoek R. 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol. 3(5):294–299.
- Walker-Smith GK. 2004. A new species of Quinquelaophonte (Crustacea: Copepoda: Harpacticoida: Laophontidae) from Port Phillip Bay, Victoria, Australia. Mem Mus Vic. 61(2):217–227. doi: 10.24199/j.mmv.2004.61.14
- Wells J, Hicks G, Coull B. 1982. Common harpacticoid copepods from New Zealand harbours and estuaries. N Z J Zool. 9(2):151–184. doi: 10.1080/03014223.1982.10423847
- Wilson CB. 1932. The copepods of the woods hole region, Massachusetts. Bull US National Mus. 158:1–635.
- Yeom J, Nikitin MA, Ivanenko VN, Lee W. 2018. A new minute ectosymbiotic harpacticoid copepod living on the sea cucumber Eupentacta fraudatrix in the East/Japan Sea. PeerJ. 6:e4979. doi: 10.7717/peerj.4979

![Figure 8. Quinquelaophonte aurantius sp. nov. male: A–B, urosome ventral and lateral; C1, antennule (setation shown only for segments 1–2) [C3, segment 3; C4, segment 4; C5, segment 5; C6, segments 6-7]. (Note that C3–C6 are figured in a slightly different orientation from their equivalents in C1).](/cms/asset/f3428543-14d3-4e90-a2b3-c73f8a6908ed/tnzz_a_1548496_f0008_ob.jpg)