ABSTRACT
The invertebrate fauna of five ephemeral forest streams on Hauturu-o-Toi/Little Barrier Island in northern New Zealand was assessed in January 2014. Low summer flows restricted benthic sampling largely to pools that would, during periods of ‘normal’ flow, be main-channel riffle/run habitat. Additionally, adult stages of aquatic insects were sampled by light trapping. Fifty-three aquatic species/morphospecies were recorded during the study, including 25 new records, bringing total island species richness to 65. The fauna was dominated by Ephemeroptera and Trichoptera; species richness of Plecoptera and Diptera was low, and only single species of Mollusca and Crustacea were recorded. Species found were either common with broad New Zealand distributions, or species restricted to the North Island. No species was endemic to the island. Faunal comparisons with adjacent mainland streams indicated the island had similar assemblages of core taxa, but generally lower species richness, likely resulting from physiographic differences found there.
Introduction
Species richness on islands is frequently lower than in mainland regions because the small size of islands increases the likelihood of extinction and their isolation inhibits successful colonisation (Algar and Losos Citation2011). More limited resources and fewer habitats on islands may also reduce species richness relative to mainland areas (MacArthur and Wilson Citation1967). Habitat permanence is an additional factor affecting species richness and is particularly relevant for aquatic species. In general, streams of an ephemeral nature have lower species richness than equivalent perennial streams (Williams Citation1996), being highly variable environments comprising a dynamic mosaic of flowing water, disconnected pools and dry habitat patches (Datry et al. Citation2017). Pools typically expose organisms to higher and more variable water temperatures, higher nutrient concentrations and lower oxygen concentrations than flowing waters (Stubbington et al. Citation2017), making ephemeral streams potentially demanding environments for their inhabitants. Nevertheless, some invertebrate species are tolerant of such conditions (Richardson and Danehy Citation2007) and a number of studies have found that invertebrate communities of ephemeral streams comprise subsets of taxa found at perennially flowing sites (Boulton and Suter Citation1986; Storey and Quinn Citation2008; Stubbington et al. Citation2017 and references within). Most of the invertebrates in ephemeral streams are considered to be generalists with broad environmental preferences (Stubbington et al. Citation2017) although a few species are specialists found only where flow is intermittent (e.g. Towns Citation1985; Dieterich and Anderson Citation2000; Santos and Stevenson Citation2011).
Hauturu-o-Toi/Little Barrier Island (36.20° S; 175.08° E), hereafter called Hauturu, lies on the northern edge of the Hauraki Gulf, 75 km from central Auckland and 22 km from the nearest mainland. It is a roughly circular island of volcanic origin and is thought to have been formed by two separate events 3 and 1.5 million years ago (Lindsay and Moore Citation1995). It has a land area of c. 3000 hectares and is positioned in a warm humid climate with mild winters and an annual rainfall of c. 1400 mm (McEwen Citation1987). Mount Hauturu is the island’s highest point at 722 m above sea level and most of the island’s approximately 35 streams drop down steeply through precipitous ravines and are often ephemeral in nature (Hamilton Citation1961), particularly during summer.
In 1895 Hauturu became the first of New Zealand’s Nature Reserves (Department of Conservation Citation2017). Although much of the island is now in a natural state, about one third of the vegetation had previously been felled or burnt (Veitch Citation2001). Following numerous eradication operations, present-day Hauturu is free of non-native browsing and predatory mammals and as a result is now home to an exceptional assemblage of native species including a high diversity of rare birds, reptiles and both species of New Zealand bat (O'Donnell et al. Citation2013; Hitchmough et al. Citation2016; Department of Conservation Citation2017; Robertson et al. Citation2017).
Although the terrestrial flora and fauna of Hauturu are well documented, few studies have been made on the freshwater fauna. Fish surveys carried out at eight streams on the island have collectively reported the presence of longfin eel (Anguilla dieffenbachii), banded kokupu (Galaxias fasciatus) and redfin bully (Gobiomorphus huttoni) and, although not a fish, the freshwater shrimp Paratya curvirostris (Wade Citation2014; NZFFD Citation2018). Wise (Citation1956) first reported on the aquatic insects from streams in the south-western sector of Hauturu (sampled November 1954), presenting a list of 17 taxa belonging to seven orders. Subsequently, Winterbourn (Citation1964) assessed the benthic invertebrate fauna of four streams in the same sector of the island (sampled August 1963), reporting an additional 22 taxa including the first records of freshwater snails, amphipods and flatworms. However, since that time juvenile and adult stages of many more species have been described from northern New Zealand, new genera have been established in several orders, and the fauna has undergone numerous nomenclatural changes (e.g. McLellan Citation1977, Citation1991, Citation1993; Cowley Citation1978; Towns and Peters Citation1996) resulting in many of the names used in the earlier surveys being no longer appropriate.
Given the close proximity of Hauturu to the mainland, stream invertebrate communities on the island are likely to have affinities with streams on the nearby mainland. However, due to the small size of Hauturu, and the intermittent nature of its streams, we hypothesised that stream invertebrate communities on Hauturu would differ in composition from those of perennially flowing streams on the mainland. The primary objective of the present study was to re-examine the macroinvertebrate fauna of the streams sampled in 1963 by Winterbourn (Citation1964), particularly in the light of substantial advances in the taxonomy of stream invertebrates in the intervening half-century. We also investigated whether the stream invertebrate fauna of Hauturu had a distinct species composition by comparing it with previously documented faunas of forested, stony, perennially flowing streams in three adjacent regions of the North Island mainland: the Coromandel Peninsula, Auckland and the vicinity of Whangarei.
Materials and methods
The streams
Most streams on Hauturu flow through fragmented volcanic rock, particularly on the northern and eastern coasts, and numerous waterfalls occur along their courses. During dry weather, particularly in summer, many of the streams have no surface water, although subsurface flow may be present (Hamilton Citation1961). On the southern and western sides of the island, streams broaden out in their lower courses and have partially alluvial beds. The streams enter the sea, either through boulder beaches, or via waterfalls, and the beds of most streams are dominated by boulders, cobbles and coarse gravels.
Five streams in the south-western sector of Hauturu were included in the present study (, , Supplementary Table S1); four previously investigated by Winterbourn (Citation1964) and Hut Bay Creek. At the sampling sites, four of the streams consisted of boulder–cobble dominated substratum and patches of gravels and/or bedrock. Substrata of one stream (Parihakoakoa Stream) consisted predominantly of bedrock, overlain by a scattering of large cobbles, pebbles and gravels. At the time of sampling, stream channels had minimal surface flow and water was limited in four of the streams to a series of pools connected by subsurface flow or occasionally, shallow (<5 cm deep) sections of surface water. In contrast, Hut Bay Creek had shallow, continuously flowing surface water and may be spring-fed; a number of springs are known in that part of the island (Hamilton Citation1961; 2018 personal communication from Richard Walle, Hauturu Ranger). All streams flowed through native forest with overhead canopy cover (78–95%).
Figure 1. Map of northern New Zealand displaying locations of sampling sites used in the study. Enlargement indicates locations of sampling sites on Hauturu. For symbol details see and Supplementary Table S1.
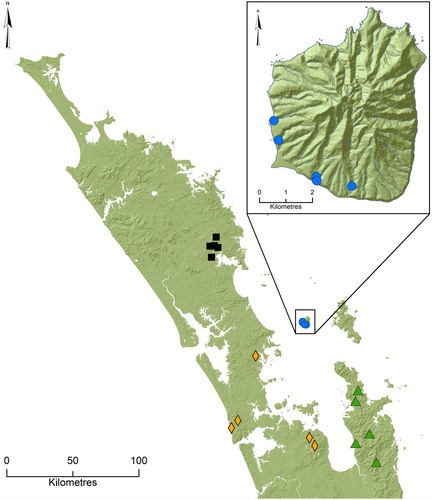
Table 1. Physico-chemical measurements obtained for the five study streams in January 2014. Values given are means of the measurements made (see Methods). Temp. = water temperature, Cond. = conductivity, DO = dissolved oxygen, Width = wetted width of stream channel, Sub. = substrate size, Pfan. = Pfankuch stability index score.
Physico-chemical factors
Several environmental measurements were taken at each stream (). Water temperature (°C), pH, conductivity (µS cm−1) and dissolved oxygen (g m−3) were measured at three positions on each stream using a YSI Pro Plus meter at the time of invertebrate sampling. Wetted width of each stream channel was measured using a laser rangefinder (Bosch PLR-50) and overhead canopy cover was assessed with a convex spherical densiometer (Model-A, Forestry Suppliers Inc.) (Lemmon Citation1956, Citation1957). Hydrologic stability was assessed (by SRP) using the Pfankuch (Citation1975) stability index, which incorporates 15 stability attributes of upper and lower banks, and the stream bed. Composition of the streambed substrate was determined using a modified version of the pebble count method of Wolman (Citation1954) and entailed measurement of the long axes of 50 particles (sizes ranging from fine gravels to boulders) collected at one pace intervals while walking upstream in a zig-zag manner. Air temperature was logged at each site using Hobo® pendant data loggers (Model UA-002-64, Onset Computer Corporation) to confirm that overnight air temperature was likely to have been suitable (>12°C) for aquatic insect flight activity (see Pohe et al. Citation2018 and references within).
Stream invertebrate sampling
Invertebrate sampling was carried out in the lower reaches of the five streams between 15 and 18 January, 2014. Each stream was sampled once. Samples were collected at three points on Parihakoakoa and Tirikakawa streams and at four points on Awaroa and Haowhenua streams and Hut Bay Creek (number of points sampled was based on available habitat but sampling effort was comparable at each stream). Because surface water was mainly confined to pools and occasional small riffles at the time of sampling, it was impractical to take strictly quantitative samples. Therefore, a D-net (width 33 cm; mesh size 0.25 mm) was swept through pools while benthic substrata and organic matter were being disturbed. Any large pieces of organic matter, e.g. tree branches, were washed in the D-net. Kick samples were taken from riffles where present. All samples were preserved in 70% ethanol.
Light trapping
Adult stages of aquatic insects were collected at one point per stream using a light trap comprising four 8-watt ultraviolet fluorescent tubes (total 32-watts; model F8T5 BLB tubes) placed over an 8-litre white plastic tray following Pohe et al. (Citation2018). The tray was half-filled with water containing 30 ml of biodegradable detergent to reduce surface tension and so enhance retention of arriving insects. The trap was placed beside the stream and the lights activated for five hours from sunset. Light traps were operated once at each stream. Traps were emptied the following morning and Ephemeroptera, Plecoptera, Trichoptera and Megaloptera catches were removed from the trays in the field and preserved in 70% ethanol. Only representative selections of micro-caddisfly (Hydroptilidae) were collected from Awaroa and Tirikakawa streams, due to the high numbers of individuals captured and the limited time available for processing them. The marine caddisfly Philanisus plebeius (Chathamiidae) was recorded in low numbers in light traps at three stream sites (Parihakoakoa, Awaroa and Haowhenua) but is not considered further as it does not have stream-dwelling larvae.
Identification of Hauturu invertebrates
Stream invertebrates and light-trapped insects from Hauturu were examined under a dissecting microscope at 10–63x magnification and were identified to the lowest taxonomic level possible using Marshall (Citation1975), Ordish (Citation1984), Neboiss (Citation1986), Ward (Citation1995), Towns and Peters (Citation1996), Winterbourn et al. (Citation2006) and Chapman et al. (Citation2011). In most cases (c. 80%), individuals were identified to species level. For the remainder, there was a high level of confidence for discrete morphospecies designations and therefore, hereafter the term species is used for all Hauturu 2014 sampling results. Identifications of adult caddisflies were checked and corrected where necessary by trichopteran specialist, Brian Smith (Freshwater Biologist, NIWA Hamilton).
Faunal comparison with adjacent mainland regions
Hauturu stream invertebrate faunas were compared with faunas previously reported from streams in three mainland regions (Coromandel Peninsula, Auckland and Whangarei) in close proximity to the island (). The mainland data had been collected by Regional Councils using the standard ‘hard-bottomed’ stream sampling protocol (Stark et al. Citation2001) for State of the Environment (SoE) reporting (MFE Citation2015) and were obtained for two dates for each of five streams within each mainland region (see Supplementary Table S1 for details). The Hauturu dataset comprised the data collected by Winterbourn (Citation1964) from four streams, and the data obtained in the present study (five streams), and therefore provided for a near-balanced comparison with respect to numbers of streams and times sampled. For our comparisons, coded abundance data (0, 1, 5, 20, 100; see Stark Citation1998) were generated for each stream, based on averaged results of each pair of sampling events. This was more complex for the Hauturu stream pairs, due to taxonomic additions and changes introduced since the 1963 sampling was undertaken. Best effort was made to match historical species lists with currently recognised taxa, an approach for which we have a good level of confidence in the outcome. All streams used in the comparison (mainland and Hauturu) were located in native forest with closed canopies, as indicated by Google Earth Pro Version 7 and NZ Topo Map (http://www.topomap.co.nz) software, and had predominantly stony substrata as indicated for the mainland sites by use of the ‘hard-bottomed’ stream sampling protocol. The mainland stream sites were widely distributed within each region. Because mainland data had been identified to the taxonomic level used to calculate macroinvertebrate community indices (MCI) (Stark Citation1985, Citation1998) for SoE reporting, Hauturu data were adjusted to that level for regional comparisons. Thus, most of the 102 taxa included in the Hauturu–mainland comparison were identified to genus or family, rather than species.
Statistical analyses
A cluster analysis based on presence/absence data for all species (benthic and light-trapped individuals combined) was used to compare the faunal composition of the five streams on Hauturu. The Bray–Curtis similarity index and unweighted paired-group average linkage (UPGMA) were used for clustering in the software package Paleontological Statistics (PAST) version 3.20 (Hammer et al. Citation2001). Taxonomic richness in the four regions (n = 20, five streams in each region) was compared with a Kruskal–Wallis H-test, followed by Mann–Whitney U-tests to find where significant differences lay using PAST 3.20. Nonmetric multidimensional scaling (NMS) incorporating the Bray–Curtis distance measure was used to ordinate invertebrate communities in taxonomic space for stream sites on Hauturu and the three mainland regions (n = 20, five streams in each region). Cube-root transformed coded abundance data were calculated for each stream so that an element of relative abundance was included in the ordination. The solution with the lowest stress in two dimensions was selected as only small reductions in stress were obtained when additional axes were included. Monte Carlo simulations (250 runs) were used to test the statistical significance of the ordination. Nonmetric multidimensional scaling in conjunction with the Bray–Curtis distance measure is a robust method for analysing community data sets, which often have a high proportion of zeros, leading to non-normally distributed data (McCune and Grace Citation2002). Spearman rank correlation was used to test whether there was an association between taxonomic richness and ordination scores for axes 1 and 2. The multi-response permutation procedure (MRPP) with the Bray–Curtis distance measure was used to test whether macroinvertebrate stream communities from the four regions were significantly different. Indicator species analysis (ISA) was used to identify which invertebrate taxa were most influential in determining regional differences (Monte Carlo test with 1000 runs). NMS, MRPP and ISA were all run in the statistical software PC-ORD version 6.22 (McCune and Mefford Citation2011).
Results
Physico-chemical factors
Canopy cover of the five streams on Hauturu ranged from 78 to 95%, average wetted widths from 1.8 to 3.0 m and mean substrate size (maximum particle length) from 12 to 38 cm (). Hydrologic stability, as measured by the Pfankuch index, was assessed as ‘good’ or ‘fair’ in all five streams, which also had similar water temperatures (15.0–19.5°C). pH of four streams was slightly acidic (5.8–6.8), whereas Hut Bay Creek was circumneutral (6.9–7.1). Dissolved oxygen (DO) of stream/pool water varied markedly amongst sampling sites with Parihakoakoa (0.7–1.0 g m−3) and Tirikakawa (1.9–2.3 g m−3) water having particularly low oxygen concentrations. In contrast, DO exceeded 4.8 g m−3 in the other three streams (mean 7.1 g m−3) and in Hut Bay Creek, mean saturation of 8.3 g m−3 was recorded.
Hauturu aquatic invertebrate fauna
Fifty-three invertebrate species were identified from the five streams on Hauturu in 2014. They included aquatic stages collected from the stream benthos, and adult stages of insects taken at lights (). Numbers of species found at individual streams ranged from 17 to 32 (). Forty-seven (89%) of the 53 species were insects, of which three mayfly (Ephemeroptera) species and 10 caddisfly (Trichoptera) species were only collected at lights. Gastropoda, Amphipoda, Tricladida and Nematomorpha were represented by single species and only two oligochaete species were found. Trichoptera and Ephemeroptera were the best represented insect orders with 18 and 14 species, respectively. The most abundant benthic species collected in the five streams were the mayflies Zephlebia borealis and Arachnocolus phillipsi, the caddisfly Polyplectropus altera, the snail Potamopyrgus antipodarum and larval Chironominae (Diptera).
Table 2. Macroinvertebrate fauna recorded from five streams on Hauturu, 15–18 January 2014. L = adult insects collected by light-trapping, S = stream dwelling taxa collected by kick/sweep sampling.
Table 3. Numbers of taxa collected from Hauturu during the 2014 survey from benthic samples, numbers of EPTM (Ephemeroptera, Plecoptera, Trichoptera, Megaloptera) species collected in light traps and total numbers of taxa recorded at each stream.
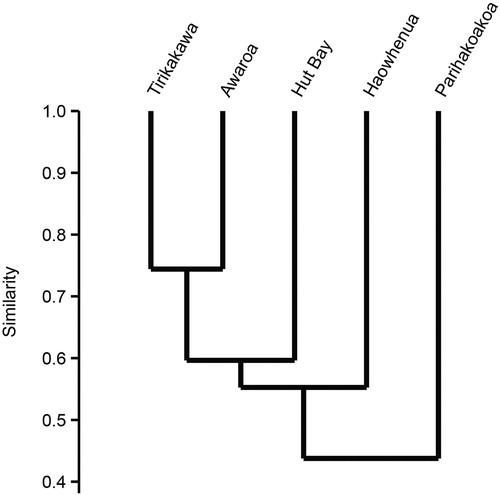
Of the 110 adult and subadult mayflies captured in light traps at the five streams Isothraulus abditus, Z. borealis and Acanthophlebia cruentata were taken in greatest numbers. No species was present in light traps at all five streams but two (A. cruentata and Z. borealis) were trapped at four of them. Stenoperla prasina was the only adult stonefly taken at lights (3 streams). Ten freshwater caddisfly species belonging to eight families were collected by light trapping, but only Polyplectropus altera was taken at all five streams. The dobsonfly Archichauliodes diversus was taken at three streams. Cluster analysis of presence–absence invertebrate data showed that the faunas of Tirikakawa and Awaroa streams were most similar (74%; ), and that the fauna of Parihakoakoa Stream, which had the fewest species, was most divergent.
Faunal comparison with adjacent mainland regions
One hundred and two MCI level invertebrate taxa were recorded in the dataset for the four-region comparison. Of these, 46 (45%) were found on Hauturu, 52 (51%) on the Coromandel Peninsula, 81 (79%) in Auckland and 80 (78%) near Whangarei (). Nine of the 18 mayfly taxa, five of eight stoneflies, 13 of 24 caddisflies and eight of 20 dipterans recorded in the four regions were found on Hauturu. However, only one of the seven freshwater snails (Potamopyrgus antipodarum) and one of eight crustaceans (Paracalliope fluviatilis) were found on Hauturu.
Table 4. Total numbers of taxa, numbers of EPT (Ephemeroptera, Plecoptera, Trichoptera) taxa, Diptera, Mollusca, Crustacea and the top five most abundant taxa, for Hauturu and three adjacent regions of the North Island mainland. All groups are calculated at the MCI level of identification. Data derived from five streams in each region, sampled on two occasions. Top five most abundant taxa are generated from averaged coded abundances.
A significant difference in the number of MCI level taxa was found in streams from the four regions (χ2 = 13.25, P < 0.01). Pairwise comparisons showed that streams on Hauturu and Coromandel Peninsula were significantly less species-rich than streams in Auckland and Whangarei (P < 0.05 for both). The two-dimensional NMS ordination based on coded abundance data () had a final stress value of 0.12 and was significant when tested against Monte Carlo simulations (P < 0.01 for both axes). The ordination clearly separated benthic macroinvertebrate communities on Hauturu from mainland communities, whose envelopes showed a degree of overlap (). Neither axis was significantly correlated with species richness (P > 0.05). The multi-response permutation procedure (MRPP) also indicated significant differences between the Hauturu communities and those in each of the three mainland regions (P < 0.01) and significant differences between stream faunas of the Coromandel and Auckland, and Coromandel and Whangarei regions (). However, no significant difference was found between the Auckland and Whangarei stream communities. Indicator species analysis showed that five species including the caddisfly Plectrocnemia and the poorly known mayflies Arachnocolus phillipsi and Isothraulus abditus were largely responsible for the differences in benthic macroinvertebrate communities between Hauturu and the other three regions (). A further five species were significant indicators for Whangarei and Auckland streams, but no significant indicator species were identified for Coromandel streams.
Figure 3. NMS ordination plot of benthic stream invertebrate communities based on coded abundance data (see Methods) in four regions: Hauturu, Coromandel, Auckland and Whangarei (stress = 0.12). Labels indicate individual sites that correspond to site details in Supplementary Table S1.
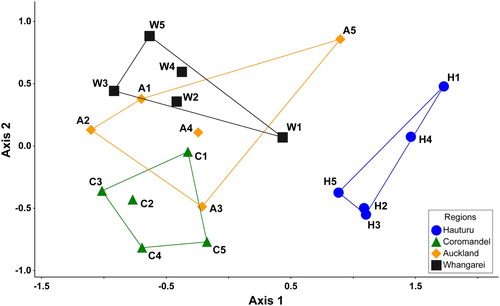
Table 5. Pairwise comparisons of differences in benthic macroinvertebrate community composition between Hauturu, Coromandel, Auckland and Whangarei groups of streams using the multi-response permutation procedure (MRPP). Within-group homogeneity values (A) and their associated P-values are shown.
Table 6. Results of an indicator species analysis (ISA) of benthic macroinvertebrate community composition at Hauturu, Auckland and Whangarei. No indicator taxa were found for Coromandel. Only taxa with significance values <0.05 are shown.
Discussion
Sixty-five aquatic invertebrate species have now been reported from Hauturu/Little Barrier Island. Thirty-nine of these were recorded by Wise (Citation1956) and/or Winterbourn (Citation1964), one (Paratya curvirostris) during fish surveys in May 2000 (NZFFD Citation2018) and the remaining 25 were recorded for the first time in the present study. However, three stoneflies (Acroperla trivacuata, Zelandoperla sp., and Zelandobius sp.), three mayflies (Deleatidium (D.) myzobranchia, Zephlebia versicolor and Nesameletus ornatus) and larvae belonging to the dipteran family Ceratopogonidae, all of which were recorded in 1963, were not found in 2014. The mosquito Aedes antipodeus, reported by Wise (Citation1956), was not recorded in either of the two subsequent surveys. Absence in 2014 of nymphs of at least two of the three stonefly species may be a reflection of their life cycles as Winterbourn (Citation1966) found that adults of A. trivacuata and Z. decorata had completed emergence in January in the Auckland region. Nymphs attributed to Z. versicolor in 1963 may have been those of Z. spectabilis, a species with a broadly similar appearance described by Towns (Citation1983) and found on Hauturu in the present study. Nymphs of D. (D.) myzobranchia are known from fast-flowing water (Towns and Peters Citation1996), conditions not present during the 2014 study; thus they may only be found further upstream during times of low flow. Nymphs of Nesameletus ornatus and Zephlebia versicolor, which have been recorded on Hauturu, as well as others such as Z. nebulosa and Deleatidium (D.) angustum, which have not, are all known from small streams and streamlets amongst organic detritus and trailing vegetation (Towns and Peters Citation1996; Hitchings and Staniczek Citation2003) and therefore could potentially occur higher in the catchments of Hauturu streams. Our sampling was confined to the lower reaches of each stream for logistical reasons. To refine the Hauturu faunal list further, small streams in the upper reaches would be valuable habitats to sample, and seepages and springs would be worth including as they have been known to contribute considerably to stream faunal lists (Collier and Smith Citation2006). Additionally, the inclusion of passive intercept traps (e.g. Malaise traps) could help sample species occupying habitats that are difficult to sample like seeps, and are also not attracted to light traps.
No species unique to Hauturu were identified in the 2014 survey. Rather the stream fauna consisted largely of species that are typically found in the northern half of the North Island, or are common species with widespread distributions within New Zealand. Examples of the former are the mayflies Acanthophlebia cruentata, Arachnocolus phillipsi, Isothraulus abditus, Mauiulus aquilus, Zephlebia borealis and Z. inconspicua, whereas widespread species found included the stoneflies Stenoperla prasina and Austroperla cyrene, the dobsonfly Archichauliodes diversus, two mayflies Deleatidium (D.) fumosum and D. (D.) cf. lillii and the snail Potamopyrgus antipodarum. Our record of the nematomorph Gordionus maori is interesting as it is a recently described species known previously only from the South Island (Yadav et al. Citation2018). Sixteen of the 24 mayflies recorded by Towns (Citation1987) from Great Barrier Island, which lies about 20 km east of Hauturu, were found on Hauturu. However, Atalophlebioides cromwelli and two Austroclima species that have been found on Great Barrier and elsewhere in the northern North Island (Towns Citation1987; Towns and Peters Citation1996) were not recorded on Hauturu.
Although streams on Hauturu are ephemeral, or intermittent, in the sense that flow is periodically disrupted so that surface water is absent or confined to pools, it was apparent that the invertebrate fauna is not characterised by ephemeral stream specialists per se, but rather a subset of species found in perennial and intermittent streams on the adjacent mainland. This finding is consistent with that of Storey et al. (Citation2011) who found that the invertebrate fauna in both pools and flowing waters of ephemeral headwater streams in the Auckland region were similar to those in adjacent perennial streams. As many of the species found in pools on Hauturu inhabit riffles elsewhere, they would be expected to colonise them on Hauturu when flowing water is present. At the time of the 1963 stream survey on Hauturu surface flow was greater than in 2014, enabling both riffles and pools to be sampled in Awaroa and Tirikakawa streams (Winterbourn Citation1964). Thus, of the 16 species identified by Winterbourn (Citation1964) from riffles in those two streams, 10 were also found in pools in 1963, and 11 of them inhabited pools in 2014. These observations provide further support for the notion that numerous members of the riffle fauna move into pools when flow declines.
Species lists and presence-absence clustering indicated that the five Hauturu streams shared a large number of invertebrate species, not surprisingly, given their close proximity within native forest and their physico-chemical similarity. Parihakoakoa, the smallest stream, had the most distinctive fauna, as in 1963 (Winterbourn Citation1964). In 2014 the lowest dissolved oxygen concentration and high conductivity were found in its pools, and its stream channel was the most unstable as indicated by its relatively high Pfankuch index score, together suggesting that Parihakoakoa Stream may provide the most demanding physico-chemical environment for aquatic invertebrates. In particular, the low dissolved oxygen concentrations in pools during periods of low flow may prevent populations of some species from becoming established, while its smaller, less stable substrata may preclude colonisation by some species. Thus, no stoneflies or megalopterans, and only two of the 6–9 mayfly species collected from the other four streams, were found there. Nymphs of Isothraulus abditus, one of the two mayflies found in Parihakoakoa Stream, inhabit pools and backwaters and were found at DO concentrations as low as 1.7 g m−3 in a stream near Whangarei (Pohe et al. Citation2019). Their presence in Parihakoakoa Stream is in line with these findings and supports the suggestion that they can tolerate conditions of low dissolved oxygen. Less is known about the habitat of Arachnocolus phillipsi, the other mayfly found in Parihakoakoa Stream, although Towns and Peters (Citation1979, Citation1996) reported that nymphs were most abundant in slow-flowing reaches of first order streams on wood, leaves and vegetation trailing in the water. Towns (Citation1987) also found nymphs of A. phillipsi with those of I. abditus and Zephlebia borealis on wood and leaves in pools of first order streams on Great Barrier Island as well as on a variety of organic and inorganic substrata in larger streams.
Our comparison of the invertebrate fauna of Hauturu streams with stream faunas in three adjacent parts of the North Island mainland showed that the Hauturu streams were grouped together in ordination space, whereas streams in the Auckland, Whangarei and Coromandel regions of the mainland formed a separate, partially overlapping, cluster. Taxonomic richness of the Hauturu fauna (all streams combined) was also substantially lower than that found in the Auckland and Whangarei groups of streams (46 taxa vs 81 and 80 taxa, respectively), although only six more species were found in the Coromandel group of streams than on Hauturu. These faunal differences may largely reflect the morphology of the streams, rather than an inability of species to colonise them. Thus, Hauturu streams have limited or no low-gradient downstream reaches, in contrast to most mainland streams. Low-gradient reaches provide habitats for a number of species that have not been found on Hauturu but have been reported in streams in the other three regions. Among them are the caddisflies Pycnocentrodes and Hudsonema, the damselfly Austrolestes, molluscs other than Potamopyrgus antipodarum, small Crustacea (Amphipoda other than Paracalliope fluviatilis, Copepoda, Isopoda and Ostracoda), the crayfish Paranephrops planifrons, leeches (Hirudinea) and nemertean worms.
Conclusions
Our survey of five streams on Hauturu resulted in the addition of 25 new species records, bringing the total number of aquatic invertebrates known from the island to 65. Stream faunas on Hauturu are dominated by the immature stages of insects including a notably high diversity of Ephemeroptera. Despite its relatively small size and island status, its isolation does not appear to have been a significant barrier to colonisation. Thus, the stream fauna of Hauturu resembles the faunas of comparable forest streams on the nearby mainland in accordance with our primary hypothesis. However, the notable absence of some species does make the fauna different, and at least in part may be a consequence of a relative absence of low-gradient habitat in the form of downstream reaches. The ephemeral nature of Hauturu streams appears to have little effect on the species composition of the invertebrate fauna, which resembles that of many ephemeral and perennial streams in the Auckland region (Storey et al. Citation2011) and hence is consistent with the results of an increasing number of studies elsewhere (Datry et al. Citation2017).
Supplementary Figure S2
Download PDF (6 MB)Supplementary Table S1
Download PDF (654.2 KB)Acknowledgements
We thank Hauturu rangers, Richard Walle and Leigh Joyce, and their children, Mahina and Liam for welcoming us to the island, and hosting our stay. The Little Barrier Island (Hauturu) Supporters’ Trust are acknowledged for kindly funding transport to and from the island, as is Dave Wade for providing the transport. We thank Fiona McKenzie (Environmental Officer, Ngati Manuhiri) for assistance on Hauturu, and Ngati Manuhiri for permission to conduct the study, and collect samples. The Department of Conservation issued a research and specimen collection permit (permit 45516-RES). Technical assistance at the University of Canterbury was provided by Linda Morris, Nick Etheridge, Alan Woods and Matt Walters. We gratefully acknowledge Brian Smith (NIWA Hamilton) for checking the identification of all light-trapped Trichoptera specimens. We also acknowledge Associate Editor Dr Jonathan Banks and two anonymous reviewers for comments that improved the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Stephen R. Pohe http://orcid.org/0000-0002-7357-5185
M. Lyn Wade http://orcid.org/0000-0002-8700-2468
Michael J. Winterbourn http://orcid.org/0000-0002-3544-2613
Olivier J.-P. Ball http://orcid.org/0000-0003-4734-2107
Additional information
Funding
References
- Algar AC, Losos JB. 2011. Evolutionary assembly of island faunas reverses the classic island–mainland richness difference in Anolis lizards. Journal of Biogeography. 38:1125–1137. doi: 10.1111/j.1365-2699.2010.02466.x
- Boulton AJ, Suter PJ. 1986. Ecology of temporary streams — an Australian perspective. In: De Deckker P, Williams WD, editors. Limnology in Australia. Dordrecht (The Netherlands): Dr W. Junk Publishers; p. 313–327.
- Chapman MA, Lewis MH, Winterbourn MJ. 2011. Guide to the freshwater Crustacea of New Zealand. Christchurch (New Zealand): New Zealand Freshwater Sciences Society. 188 p.
- Collier KJ, Smith BJ. 2006. Distinctive invertebrate assemblages in rockface seepages enhance lotic biodiversity in northern New Zealand. Biodiversity and Conservation. 15:3591–3616. doi: 10.1007/s10531-005-5395-8
- Cowley DR. 1978. Studies on the larvae of New Zealand Trichoptera. New Zealand Journal of Zoology. 5:639–750. doi: 10.1080/03014223.1978.10423816
- Datry T, Bonada N, Boulton AJ. 2017. General introduction. In: Datry T, Bonada N, Boulton AJ, editors. Intermittent rivers and ephemeral streams: ecology and management. London (UK): Academic Press; p. 1–20.
- Department of Conservation. 2017. Te Hauturu-o-Toi Little Barrier Island Nature Reserve. 2017 Management Plan. Wellington, New Zealand: Department of Conservation. 64 p.
- Dieterich M, Anderson NH. 2000. The invertebrate fauna of summer-dry streams in western Oregon. Archiv für Hydrobiologie. 147:273–295. doi: 10.1127/archiv-hydrobiol/147/2000/273
- Hamilton WM. 1961. Little Barrier Island (Hauturu). Bulletin 137. Wellington (New Zealand): New Zealand Department of Scientific and Industrial Research. 180 p.
- Hammer Ø, Harper DAT, Ryan PD. 2001. PAST: paleontological statistics software package for education and data analysis. Palaeontologia Electronica. 4:1–9.
- Hitchings TR, Staniczek AH. 2003. Nesameletidae (Insecta: Ephemeroptera). Fauna of New Zealand 46. Lincoln (New Zealand): Manaaki Whenua Press. 72 p.
- Hitchmough R, Barr B, Lettink M, Monks J, Reardon J, Tocher M, van Winkel D, Rolfe J. 2016. Conservation status of New Zealand reptiles, 2015. New Zealand Threat Classification Series 17. Wellington, New Zealand: Department of Conservation. 14 p.
- Lemmon PE. 1956. A spherical densiometer for estimating forest overstory density. Forest Science. 2:314–320.
- Lemmon PE. 1957. A new instrument for measuring forest overstory density. Journal of Forestry. 55:667–669.
- Lindsay JM, Moore P. 1995. Geological features of Little Barrier Island, Hauraki Gulf. Tane. 35:25–38.
- MacArthur RH, Wilson EO. 1967. The theory of island biogeography. Princeton (NJ): Princeton University Press. 203 p.
- Marshall JW. 1975. A photographic guide to some freshwater Oligochaeta found in Canterbury streams. Mauri Ora. 3:19–25.
- McCune B, Grace JB. 2002. Analysis of ecological communities. Gleneden Beach, Oregon, USA, MjM Software Design. 304 p.
- McCune B, Mefford MJ. 2011. PC-ORD. Multivariate analysis of ecological data. Version 6. Gleneden Beach (OR): MjM Software Design.
- McEwen WM. 1987. Ecological regions and districts of New Zealand. Parts 1–4. New Zealand Biological Resources Centre, publication no. 5. Wellington (New Zealand): Department of Conservation. 326 p.
- McLellan ID. 1977. New alpine and southern Plecoptera from New Zealand, and a new classification of the Gripopterygidae. New Zealand Journal of Zoology. 4:119–147. doi: 10.1080/03014223.1977.9517945
- McLellan ID. 1991. Notonemouridae (Insecta: Plecoptera). Fauna of New Zealand 22. Auckland (New Zealand): Department of Scientific and Industrial Research. 62 p.
- McLellan ID. 1993. Antarctoperlinae (Insecta: Plecoptera). Fauna of New Zealand 27. Lincoln (New Zealand): Manaaki Whenua Press. 70 p.
- MFE. 2015. Ministry for the Environment national indicator data for river condition in New Zealand. Collected by Regional Councils and the National Institute of Water and Atmospheric Research (NIWA), collated and processed by NIWA and protected by copyright owned by the Ministry for the Environment on behalf of the Crown; [accessed 9 July 2015].
- Neboiss A. 1986. Atlas of Trichoptera of the SW Pacific–Australian region. Series Entomologica 37. Dordrecht (The Netherlands): Dr W. Junk Publishers. 286 p.
- NZFFD. 2018. New Zealand Freshwater Fish Database. [accessed 15 June 2018]. https://nzffdms.niwa.co.nz/search.
- O'Donnell CFJ, Christie JE, Lloyd B, Parsons S, Hitchmough RA. 2013. Conservation status of New Zealand bats, 2012. New Zealand Threat Classification Series 6. Wellington, New Zealand, Department of Conservation. 8 p.
- Ordish RG. 1984. Hydraenidae (Insecta: Coleoptera). Fauna of New Zealand 6. Wellington (New Zealand): Department of Scientific and Industrial Research. 56 p.
- Pfankuch DJ. 1975. Stream reach inventory and channel stability evaluation. Montana, USA, United States Department of Agriculture Forest Service. 27 p.
- Pohe SR, Winterbourn MJ, Harding JS. 2018. Comparison of fluorescent lights with differing spectral properties on catches of adult aquatic and terrestrial insects. New Zealand Entomologist. 41:1–11. doi: 10.1080/00779962.2017.1398704
- Pohe SR, Winterbourn MJ, Goldstien SJ, Ball OJ-P, Harding JS. 2019. Distribution, nymphal habitat, genetic structure and conservation of the New Zealand mayfly Isothraulus abditus (Insecta: Ephemeroptera) and a description of its subimago. New Zealand Journal of Zoology. 46:13–30. doi: 10.1080/03014223.2018.1464034
- Richardson JS, Danehy RJ. 2007. A synthesis of the ecology of headwater streams and their riparian zones in temperate forests. Forest Science. 53:131–147.
- Robertson HA, Baird K, Dowding JE, Elliott GP, Hitchmough RA, Miskelly CM, McArthur N, O’Donnell CFJ, Sagar PM, Scofield RP, Taylor GA. 2017. Conservation status of New Zealand birds, 2016. New Zealand Threat Classification Series 19. Wellington (New Zealand): Department of Conservation. 23 p.
- Santos AN, Stevenson RD. 2011. Comparison of macroinvertebrate diversity and community structure among perennial and non-perennial headwater streams. Northeastern Naturalist. 18:7–26. doi: 10.1656/045.018.0102
- Stark JD. 1985. A macroinvertebrate community index of water quality for stony streams. Water and soil miscellaneous publication no. 87. Wellington (New Zealand): Water and Soil Directorate, Ministry of Works and Development. 53 p.
- Stark JD. 1998. SQMCI: a biotic index for freshwater macroinvertebrate coded-abundance data. New Zealand Journal of Marine and Freshwater Research. 32:55–66. doi: 10.1080/00288330.1998.9516805
- Stark JD, Boothroyd IKG, Harding JS, Maxted JR, Scarsbrook MR. 2001. Protocols for sampling macroinvertebrates in wadeable streams. New Zealand Macroinvertebrate Working Group Report No. 1. Prepared for the Ministry for the Environment. Sustainable Management Fund Project No. 5103. 57 p.
- Storey RG, Quinn JM. 2008. Composition and temporal changes in macroinvertebrate communities of intermittent streams in Hawke’s Bay, New Zealand. New Zealand Journal of Marine and Freshwater Research. 42:109–125. doi: 10.1080/00288330809509941
- Storey RG, Parkyn S, Neale MW, Wilding T, Croker G. 2011. Biodiversity values of small headwater streams in contrasting land uses in the Auckland region. New Zealand Journal of Marine and Freshwater Research. 45:231–248. doi: 10.1080/00288330.2011.555410
- Stubbington R, Bogan MT, Bonada N, Boulton AJ, Datry T, Leigh C, Vander Vorste R. 2017. The biota of intermittent rivers and ephemeral streams: aquatic invertebrates. In: Datry T, Bonada N, Boulton AJ, editors. Intermittent rivers and ephemeral streams: ecology and management. London (UK): Academic Press; p. 217–243.
- Towns DR. 1983. A revision of the genus Zephlebia (Ephemeroptera: Leptophlebiidae). New Zealand Journal of Zoology. 10:1–51. doi: 10.1080/03014223.1983.10423888
- Towns DR. 1985. Limnological characteristics of a South Australian intermittent stream, Brown Hill Creek. Australian Journal of Marine and Freshwater Research. 36:821–837. doi: 10.1071/MF9850821
- Towns DR. 1987. The mayflies (Ephemeroptera) of Great Barrier Island, New Zealand: macro- and micro-distributional comparisons. Journal of the Royal Society of New Zealand. 17:349–361. doi: 10.1080/03036758.1987.10426476
- Towns DR, Peters WL. 1979. New genera and species of Leptophlebiidae (Ephemeroptera) from New Zealand. New Zealand Journal of Zoology. 6:439–452. doi: 10.1080/03014223.1979.10428383
- Towns DR, Peters WL. 1996. Leptophlebiidae (Insecta: Ephemeroptera). Fauna of New Zealand 36. Lincoln (New Zealand): Manaaki Whenua Press. 143 p.
- Veitch CR. 2001. The eradication of feral cats (Felis catus) from Little Barrier Island, New Zealand. New Zealand Journal of Zoology. 28:1–12. doi: 10.1080/03014223.2001.9518252
- Wade ML. 2014. A survey of aquatic invertebrates and fish in a selection of intermittent streams on Hauturu/Little Barrier Island [Unpublished BAppSci thesis]. Whangarei, New Zealand: NorthTec. 50 p.
- Ward JB. 1995. New Zealand Trichoptera: keys to the adults. March 1995. Unpublished identification guide. Christchurch, New Zealand: Canterbury Museum. 17 p.
- Williams DD. 1996. Environmental constraints in temporary fresh waters and their consequences for the insect fauna. Journal of the North American Benthological Society. 15:634–650. doi: 10.2307/1467813
- Winterbourn MJ. 1964. A survey of the stream fauna of Little Barrier Island. Tane. 10:59–69.
- Winterbourn MJ. 1966. Studies on New Zealand stoneflies. 2. The ecology and life history of Zelandoperla maculata (Hare) and Aucklandobius trivacuatus (Tillyard) – (Gripopterygidae). New Zealand Journal of Science. 9:312–323.
- Winterbourn MJ, Gregson KLD, Dolphin CH. 2006. Guide to the aquatic insects of New Zealand. 4th ed. Bulletin of the Entomological Society of New Zealand 14. 108 p.
- Wise KAJ. 1956. Aquatic insects of Little Barrier Island. Records of the Auckland Institute and Museum. 4:321–327.
- Wolman MG. 1954. A method of sampling coarse river-bed material. Transactions of the American Geophysical Union. 35:951–956. doi: 10.1029/TR035i006p00951
- Yadav AK, Tobias ZJC, Schmidt-Rhaesa A. 2018. Gordionus maori (Nematomorpha: Gordiida), a new species of horsehair worm from New Zealand. New Zealand Journal of Zoology. 45:29–42. doi: 10.1080/03014223.2017.1329155