ABSTRACT
One new genus and ten new species of antipatharian corals are described from the New Zealand region. Differential diagnoses are given and comparisons are made to related nominal species. Described as new is the genus Acanthosaropathes and the following species: Antipathes coronata, A. dicrocrada, A. polyhedra, Acanthosaropathes uniseriata, Phanopathes ctenocrada, Cupressopathes simplex, Stylopathes stenotes, Stauropathes stellata, Telopathes tasmaniensis, and Heteropathes intricata. This research highlights the diversity of deepsea corals for the region, improves knowledge of the distributional limits of species, which in turn can inform assessments of species prevalence for conservation initiatives and management decisions.
Introduction
This is the second in a series of papers describing new taxa of antipatharian corals from New Zealand and adjacent areas in the Southwest Pacific. In the first paper (Opresko Citation2015) the following seven species were described: Antipathes leptocrada, A. craticulata, Asteriopathes octocrada, Phanopathes zealandica, Tetrapathes latispina, Parantipathes dodecasticha, and Parantipathes robusta. Here an additional ten species are described and a new genus is established in the family Aphanipathidae. The specimens on which this study is based are deposited in the Invertebrate Collection at the National Institute of Water and Atmospheric Research (NIWA), Wellington, New Zealand. The samples were collected primarily during a series of exploratory research cruises conducted by NIWA and its predecessor, the New Zealand Oceanographic Institute, over the past fifty-plus years. Additional samples came from Ministry for Primary Industries (MPI) Government Observed fishing trips. Provisional identifications of the black corals from these cruises were published in the New Zealand Inventory of Biodiversity (Cairns et al. Citation2009). The NIWA collection forms the basis for an on-going monographic revision of the New Zealand black coral fauna. An accompanying field guide describing the commonly occurring genera of black corals from the New Zealand region was published by Opresko et al. (Citation2014).
Material and methods
The material reported upon in this paper was collected during research cruises both in and outside the New Zealand EEZ including: Challenger Centenary Cruise (1974), Norfolk Rise Biology (1975), Norfolk Ridge II (1977), Southern Benthomass (1981), Cook Island Expedition (1981), Bay of Plenty Geology (1989), Kermadec Volcanics NZAPLUME-1 (1999), Bay of Plenty and Southern Kermadec Arc Seamounts (2000), Kermadec Ridge Volcanics (2002), Kermadec Volcanics NZAPLUME-III (2004), Bay of Plenty and Hikurangi Plateau Seamounts (2004). Some specimens were obtained as fishery bycatch from commercial fishing vessels and retained by MPI government-observers. All samples are held in stewardship in the NIWA Invertebrate Collection, Greta Point, Wellington, NZ.
Specimens were studied at both NIWA and at the US National Museum of Natural History (USNMNH), Smithsonian Institution, Washington, DC. Subsamples of some specimens have been deposited at the USNMNH (Museum Catalog numbers are designated with the prefix ‘USNM’). Specimens from the collections of the Natural History Museum, London, are indicated with the prefix ‘NHMUK’ before the catalog number.
The microscopic skeletal elements of the specimens were examined using a Zeiss EVO MA 15 scanning electron microscope (SEM) housed at the USNMNH. The specimens were coated with a 30–40 nm thick layer of 60% gold: 40% palladium prior to scanning. Measurements of the microscopic skeletal features were made directly using a dissecting microscope equipped with an ocular micrometer or from the photographs taken with the SEM. SEM stub numbers are from a series established by the author at the USNMNH. All SEM stubs are deposited at the USNMNH. Copies of the SEM photos are kept at NIWA.
The holotypes and paratypes of all new species are deposited in the NIWA Invertebrate Collection (NIC), with selected schizoholotypes and schizoparatypes also deposited in the collections of the USNMNH.
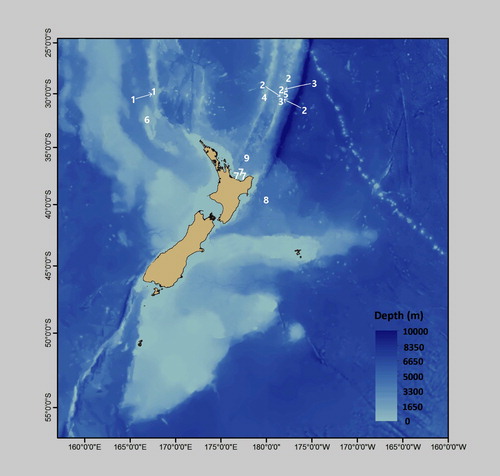
The approximate locations where the specimens described in this report were collected are indicated in .
Figure 1. Distribution of described species (positions shown are approximate). #1 = Antipathes coronata; #2 = Antipathes dicrocrada; #3 = Antipathes polyhedra; #4 = Acanthosaropathes uniseriata; #5 = Phanopathes ctenocrada; #6 = Cupressopathes simplex; #7 = Stylopathes stenotes; #8 = Stauropathes stellata; #9 = Heteropathes intricata. Not shown, Telopathes tasmaniensis (44.33°S, 147.17°E).
Taxonomic account of new species
Phylum Cnidaria
Class Anthozoa
Order Antipatharia
Family Antipathidae Ehrenberg, Citation1834
Genus Antipathes Pallas, 1766
Type species
Antipathes dichotoma Pallas, Citation1766: 126 (see Opresko Citation2003a).
Diagnosis
Corallum branched. Branching bushy, bramble-like, broom-like or fan-shaped; spines triangular or cone-shaped in lateral view; smooth or covered, to varying degrees, with small papillae; apex of spines simple or with one or more lobes or bifurcations. Polyps from less than 1 to 3 mm in transverse diameter.
Remarks
The genus currently contains about 70 nominal species. Species are usually differentiated by colony morphology, as well as size, shape, and surface ornamentation of the skeletal spines.
Molecular phylogenetic reconstructions using mitochondrial and nuclear markers (Brugler et al. Citation2013; Bo et al. Citation2018) indicate that A. dichotoma, the type species of the genus, is more closely related to species of the family Aphanipathidae than it is to any other species traditionally placed in the genus Antipathes. If these results are supported by more comprehensive genetic DNA sequencing studies, such as those using ‘ultra-conserved elements’ (see Quattrini et al. Citation2017), it will necessitate either a major revision of the families Antipathidae and Aphanipathidae or the involvement of the International Commission of Zoological Nomenclature to resolve the resulting nomenclatural issues.
Distribution
Species of Antipathes are known primarily from tropical and subtropical waters; usually at depths less than 200 m; however, some species may occur much deeper.
Antipathes coronata n. sp.
(–)
Material examined
Holotype. NIWA 15099, NZOI Stn P26, 29.92°S, 167.75°E, Norfolk Ridge, 130–301 m, 27 January 1977 (schizoholotype USNM 1202901/SEM stub 318).
Paratype. NIWA 19777, NZOI Stn P26, 29.92°S, 167.75°E, Norfolk Ridge, 130–301 m, 27 January 1977 (schizoparatype USNM 1202914).
Type locality
Norfolk Ridge, off south side of Norfolk Island, 29.92°S, 167.75°E, 130–301 m.
Description
Corallum branched, bushy, with stiff, straight to slightly curved, vertically directed branches; with narrow branch angles, often 30° or less. Branching usually unilateral with successive orders of branches often arising on the same side of the lower order branches; however, in places loosely and irregularly bilateral. Terminal branchlets up to 4 cm in length. Polypar spines smooth, conical, slightly curved distally, up to 0.15 mm tall, and with knob-like protuberances mainly near apex. Polyps up to 0.8 mm in transverse diameter, with 8–9 polyps per cm.
The holotype (NIWA 15099, A) is only a part of a colony and is about 13 cm tall. The lower part of the stem and the holdfast were not collected. Branching is to the third and fourth order. Branches and branchlets are very strongly directed distally such that the distal branch angles are generally less than 45° and most are closer to 30°. Branchlets 1 cm long are about 0.35 mm in diameter at the base (including spines); those 2 cm long are 0.4 mm in basal diameter; and a small branch 3.5 cm long and with three branchlets is 0.5 mm in basal diameter. The smallest terminal branchlets tend to occur at the distal end of the next lower order branches and are usually spaced 1 cm or more apart; most are 2–3 cm in length.
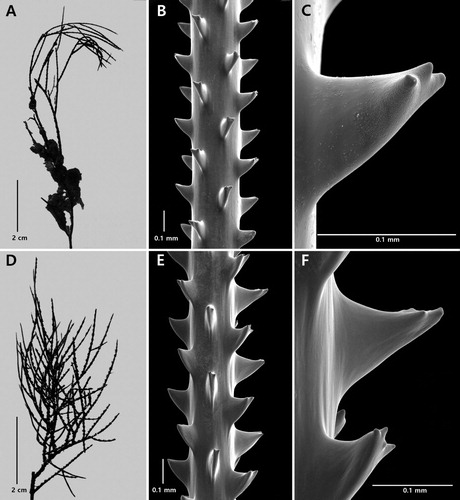
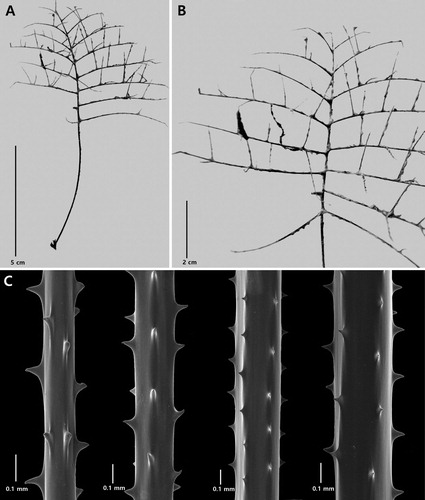
Figure 2. Antipathes coronata n. sp., holotype NIWA 15099: A, corallum; B, polyps; C, single spine; D, sections of branchlets (C and D from schizoholotype, USNM 1202901/SEM stub 318).
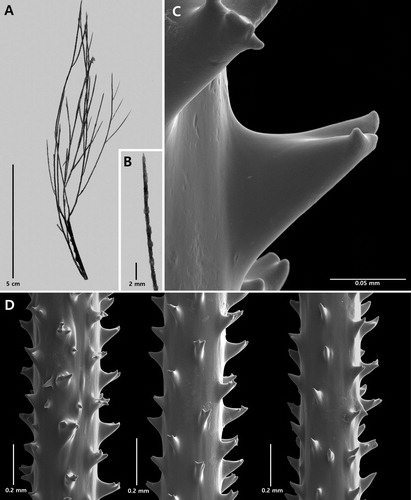
On branchlets 0.3 mm in diameter, the polypar spines are 0.12–0.15 mm tall and about 0.1 mm wide at the base (in an axial direction). The spines (C,D) have a smooth surface and a variable number of knobby or finger-like processes, primarily at the apex, although some knobs occur lower down, almost at the base. As many as five knobs can be seen on a single spine (in lateral view). The knobs are not regular in size or shape: their maximum height is about 0.017 mm. They are more common on the distal side of the spines compared to the proximal side which is often smooth. The abpolypar spines on the branchlets are 0.08–0.12 mm tall and have fewer apical knobs than the polypar spines. Small simple spines, about one-half the size of the polypar spines, are occasionally found interspersed between the larger ones. The spines are arranged in axial rows, five of which are visible in lateral view, and within each row they are spaced 0.22–0.3 mm apart resulting in five spines per mm. On the thicker branchlets, the rows become much less regular in arrangement.
The polyps (B) are 0.64–0.77 mm in transverse diameter and arranged uniserially on the branchlets, with eight polyps per cm.
The paratype (NIWA19777) is a large, almost complete colony from the same station as the holotype. It is about 51 cm tall with ten or more orders of branching. The branching is loosely bilateral or irregularly uniserial, and, as in the holotype, on some parts of the corallum up to five successive orders of branches originate on the same side of the lower order branches. The spines are similar to those of the holotype in being smooth and apically knobbed. The polypar spines are up to 0.15 mm tall, and the abpolypar spines up to 0.12 mm. Five rows of spines are visible in lateral view and within the rows the spines are 0.23–0.28 mm apart. The polyps are up to 0.8 mm in transverse diameter and there are about 4.5 polyps per 5 mm (equivalent to 9 per cm).
Discussion
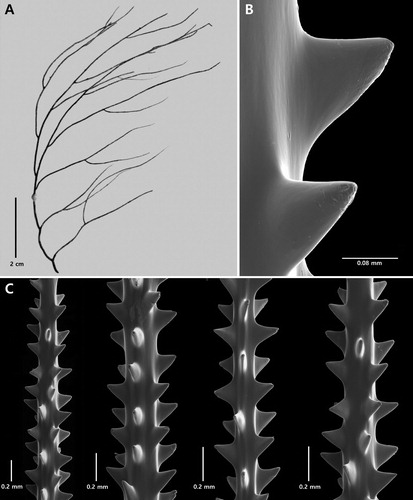
Antipathes coronata n. sp. is morphologically very similar to A. elegans Thomson & Simpson, Citation1905, and A. gallensis Thomson & Simpson, Citation1905, both of which also form bushy colonies with straight, relatively stiff, vertically directed branches (). The type of A. elegans was described by Thomson and Simpson as a small, pseudodichotomously branched colony with short, compressed, triangular spines whose upper margin is sub-horizontal and lower margin convex. Re-examination of the type specimen revealed, however, that the polypar spines (B,C) often have two or three apical knobs (sometimes more), similar to those found in A. coronata. The knobs tend to occur very close to the apex of the spine, are usually no more than 0.013 mm tall, and tend to be arranged in a row at right angles to the direction of the axis. Abpolypar spines generally do not have as many knobs as the polypar spines and some are without knobs. The upper part of the polypar spines is also covered with numerous minute papillae, giving the surface a granulated appearance when examined under high magnification (C). Similar spines are found in A. gallensis (E,F). Antipathes coronata differs from these two species in that the spines have a smooth surface and the apical knobs are less regular in size, number and arrangement. Also, in A. coronata the spines on thicker branchlets (i.e. diameter of 0.35 mm) tend to have more apical knobs, and small secondary spines are interspersed between the primary spines. Branchlets of a similar thickness were not available for study for A. elegans and A. gallensis.
Figure 3. Type specimens of Antipathes elegans Thomson & Simpson, Citation1905 (A–C) and Antipathes gallensis Thomson & Simpson, Citation1905 (D–F): A, corallum (NHMUK 1908.2.18.2); B, section of branchlet; C, single spine; D, corallum (NHMUK 1908.2.18.21); E, section of branchlet; F, single spine (B and C from schizoholotype, USNM 100390/SEM stub 165; E and F from schizoholotype, USNM 100385/SEM stub 166).
DNA sequencing data are not available for A. coronata or A. gallensis; however, ITS1 and ITS2 data (Lapian et al. Citation2007) for specimens identified as A. elegans were evaluated by Wagner et al. (Citation2010) who found that the species is closely related (98.795% and 97.653% similarity estimates based on the Kimura 2-parameter distance) to specimens identified as Antipathes griggi Opresko, Citation2009. Using the same markers, Bo et al. (Citation2018) found the same specimen to be related to Antipathes fruticosa Gray, Citation1857. The latter two species have skeletal spines similar to those of A. coronata; therefore, it would be expected that A. coronata would fall into this same clade.
Two other species that superficially resemble A. coronata are Antipathes pseudodichotoma Silberfeld, Citation1909a, and Antipathes sarothrum Pax, Citation1932. In both species, the branches are relatively long and ascending. Silberfeld (Citation1909a, Citation1909b) described the spines of A. pseudodichotoma as flat (compressed laterally), 0.128 mm tall on an axis 0.355 mm in diameter; the distal edge forming a right angle with the axis and with the tip curved upward. No mention was made of the spines having papillae or apical knobs. The type of A. pseudodichtoma is assumed to be lost as it is not among the other Silberfeld types in the Zoologische Staatssammlung München. Regarding Antipathes sarothrum, Pax (Citation1932) reported that the spines are 0.12–0.14 mm tall, and that they are unbranched and non-papillose; however, a re-examination of a sample of the type with the SEM revealed that the polypar spines are slightly forked at the apex, but do not have the multiple apical knobs as is typical for A. coronata.
Etymology
From the Latin, corona (crown) in reference to the sometimes crown-like appearance of the apex of the spines.
Distribution
Known only from the type locality, Norfolk Ridge (, #1).
Antipathes dicrocrada n. sp.
(Figures 1, 4–5)
Material examined
Holotype. NIWA 19806, NIWA Stn TAN0205/63, 30.179°S, 178.570°W, Kermadec Ridge, 157–88 m, 22 April 2002 (schizoholotype USNM 1494016/SEM stub 462).
Paratypes. NIWA 15091, NZOI Stn K826, 28.800°S, 177.800°W, Kermadec Ridge, off Raoul Island, 142–160 m, 25 July 1974; NIWA 19808, NIWA Stn TAN0205/76, 30.017°S, 178.638°W, Kermadec Ridge, 942–735 m, 23 April 2002; NIWA 41692, NIWA Stn TAN0205/63, 30.179°S, 178.570°W, Kermadec Ridge, 157–88 m, 22 April 2002 (schizoparatype USNM 1494033/SEM stub 475).
Type locality
Kermadec Ridge, 30.179°S, 178.570°W, 157–88 m.
Description
Corallum (Figures 4A and 5) relatively small (usually 10 cm or less in height) and branched mostly in one plane. Branching sympodial to pseudodichotomus; branches irregularly bilateral or uniserial; in places with successive orders of branches originating on convex side of curved lower order branches. Polypar spines on branchlets up to 0.17 mm tall. Polyps unknown.
The holotype (NIWA 19806, A) is approximately 9 cm tall and 6 cm wide, with a basal stem diameter of about 0.08 mm. The holdfast was not collected. Five orders of branches are present. Branches and branchlets are mostly 1–2 cm apart, resulting in no more than two branches per cm or two or three branches/branchlets per 2 cm. Longest terminal branchlets are generally 2‒3 cm long and 0.02–0.04 mm in diameter at their midpoint (including spines). Distal angle of insertion of branches and branchlets very variable, 40–80°, with many of the ramifications curved back towards the branch from which they arose.
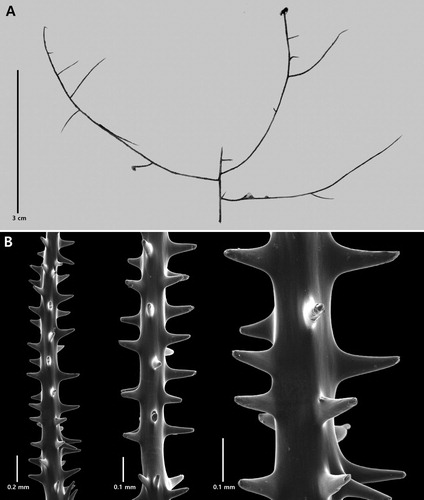
Figure 4. Antipathes dicrocrada n. sp., holotype NIWA 19806: A, corallum; B, single spines; C, sections of branchlets (B and C from schizoholotype, USNM 1494016/SEM stub 462).
Spines (B,C) strong, triangular in lateral view, slightly laterally compressed, smooth, with acute or rounded apex and wide base; without apical lobes or bifurcations. Distal edge of polypar spines at right angles to the axis or slightly inclined distally. Spines unequal in size around circumference of axis. On branchlets 0.15–0.20 mm in diameter polypar spines up to 0.15 mm tall and 0.14–0.16 mm in basal width (in axial direction), abpolypar spines 0.09 mm tall. On branchlets 0.20‒0.25 mm in diameter, polypar spines 0.15‒0.17 mm tall, abpolypar spines 0.10‒0.13 mm tall. Spines on branchlets arranged in axial rows, four or five visible in single lateral view. Within each row spines 0.23‒0.32 mm apart, resulting in a density of 4‒5 per mm. Polyps not present on holotype.
The paratypes () are all small colonies less than 10 cm in height. The branching pattern seen in these specimens varies from being very similar to that in the holotype to more regularly bilateral. The spines are similar to those of the holotype in terms of height (0.13–0.16 mm), but are not as wide at the base (0.08–0.1 mm).
Discussion
Antipathes dicrocrada n. sp. is somewhat similar in branching pattern to Antipathes craticulata Opresko, Citation2015; however, it differs in having less dense branching (1–2 per cm vs. 3–5 per cm), longer terminal branchlets (2–3 cm vs. 0.5–1.3 cm), and shorter spines (<0.18 mm vs. 0.22–0.27 mm).
Although DNA data are not available for A. dicrocrada, the morphology of the spines, suggests that this species would group with Antipathes atlantica Gray, Citation1857, and related species, as in phylogenetic reconstructions based on cox3-cox1 (Brugler et al. Citation2013) and ITS1 and ITS2 (Bo et al. Citation2018).
Etymology
The species name dicrocrada is from the Greek dikros (forked) and krade (branch) in reference to the typical forking of the branches.
Distribution
Known only from the type locality, Kermadec Ridge ( #2).
Antipathes polyhedra n. sp.
(Figures 1, 6)
Material examined
Holotype. NIWA 41689, TAN0205/63, 30.179°S, 178.570°W, Kermadec Ridge, 157–88 m, 22 April 2002 (schizoholotype USNM 1527063/SEM stub 459).
Paratype. NIWA 85952, TAN0205/63, 30.179°S, 178.570°W, Kermadec Ridge, 157–88 m, 22 April 2002 (schizoparatype USNM 1527062).
Type locality
Kermadec Ridge, 30.179°S, 178.570°W.
Description
Corallum small, sparsely branched, bramble-like, with holdfasts present at the tips of some branches. Sub-branches develop on all sides of the lower order branches, although in places the branching tends to be planar. Wide branch angles close to 90°. Spines up to 0.15 mm tall. Polyps about 0.7 mm in transverse diameter.
The holotype (NIWA 41689, A) is a small incomplete colony about 4.5 cm tall by 7.5 cm wide. The apparent stem is narrow, about 0.5 mm in diameter. The branching is very sparse, to the third or fourth order with the lowest order branches up to 5 cm long and the highest order branchlets up to 2 cm long and 0.25 mm in diameter at their base. Distal branch angles are mostly near 90°. Terminal branchlets can be very close together, almost opposite, to as much as 2.0 cm apart. Branch density is also very variable, ranging from one branch in 2 cm to three or four branches in one cm.
Figure 6. Antipathes polyhedra n. sp., holotype NIWA 41689: A, corallum; B, sections of branchlets (B from schizoholotype, USNM 1527063/SEM stub 459).
Spines (B) are relatively tall, narrow, smooth, and unequally developed around the circumference of the axis. On a branchlet about 0.1 mm in diameter, the polypar spines are 0.13 mm tall and about 0.03 mm in diameter at their midpoint; the abpolypar spines 0.07 mm tall. On branchlets 0.12–0.13 mm in diameter, the polypar spines are 0.14–0.16 mm tall, 0.02–0.04 in midpoint diameter, and the abpolypar spines are 0.07–0.10 mm tall. Four or five rows of spines are visible in one aspect. The distance between adjacent spines in one row is quite variable, 0.1–0.3 mm; however, it averages 0.2–0.25 mm, resulting in 4–5 spines per mm (range 3–6 per mm). In places two or three spines occur quite close together.
Soft tissue is present on holotype, but the polyps are degraded; therefore, their size and density could not be determined.
The paratype is similar to the type in having multiple holdfasts, sparse branching, and relatively tall polypar spines 0.1–0.13 mm tall. Soft tissue is also very poorly preserved; consequently the size of the polyps was difficult to discern; however, in one location it appeared that the polyps were about 0.7 mm in transverse diameter, with about six polyps per 5 mm. But this estimate needs to be verified with newer and better preserved specimens.
Discussion
Antipathes polyhedra n. sp. resembles a West Indian species, Antipathes rubusiformis Warner & Opresko, Citation2004. Both species form small irregularly branched colonies with thin branchlets, wide distal angles, and multiple holdfasts. In A. rubusiformis the polypar spines are 0.05–0.1 mm tall, and have a distinctly papillose surface, whereas in A. polyhedra the spines are 0.13–0.16 mm tall and have a smooth surface. Neither species has been sequenced; therefore, the affinities of these two species with others in the genus has yet to be elucidated.
Etymology
The species name polyhedra is from the Greek polys (many) and hedra (base) in reference to the multiple attachment plates.
Distribution
Only known from the Kermadec Ridge, northeast of New Zealand (, #3).
Family Aphanipathidae Opresko, 2004
Genus Acanthosaropathes n. gen.
Type species
Acanthosaropathes uniseriata n. sp.
Diagnosis
Corallum broom-like; branches and branchlets with very wide distal angle, but curved sharply upward very close to base and afterwards mostly straight; arranged primarily uniserially; spines anisomorphic; circumpolypar spines distinctly larger than interpolypar spines; hypostomal spines very reduced. Polyps less than1.0 mm in transverse diameter.
Remarks
Although the genus is morphologically similar to Acanthopathes, and in particular to species that tend to have uniserial branching such as A. humilis (Pourtalès, Citation1867), DNA sequencing studies have not been conducted to determine the relationship of this genus to others in the family. In molecular phylogenetic reconstructions, species of the genus Acanthopathes do not always group with other members of the family Aphanipathidae and this has been attributed, in part, to ‘long branch attraction, a phylogenetic artifact that is a consequence of mutational saturation and/or lineage heterogeneity in the substitution process, whereby rapidly evolving taxa are “attracted” to other long branches or extremely divergent outgroups’ (Brugler et al. Citation2013, p. 20). Including A. uniseriata in future phylogenetic analyses may help resolve these ambiguities.
Etymology
The genus name Acanthosaropathes is derived from the Greek, acantha (thorn), saron (broom) and the standard suffix pathes in reference to the large spines and broom-like shape of the corallum.
Distribution
Known only from New Zealand.
Acanthosaropathes uniseriata n. sp.
(Figures 1, 7–8)
Material examined
Holotype. NIWA 86099, NIWA Stn TAN1213/18, 30.186°S, 179.722°E, Colville Ridge Volcano, 380–440 m, 18 October 2012 (schizoholotype USNM 1491417/SEM stub 460).
Type locality
Colville Ridge, 30.186°S, 179.722°E, 380–440 m.
Description
Large branched, broom-like colony with elongated and very distinctly uniserial branches and branchlets. Spines typically acanthopathid-like with very tall circumpolypar spines up to 0.64 mm and reduced hypostomal spines. Polyps less than 1 mm in transverse diameter.
The holotype () is a large colony about 50 cm tall. The holdfast was not collected. The basal diameter of the stem is approximately 6 mm. The colony is densely branched to the seventh order or more. Except for the stem and the lower portion of some of the larger branches, all the ramifications have numerous orders of very crowded, uniserially arranged higher order branches. The density of the branches is 3 or four per cm. A large branch from the holotype is shown in A. It is 11 cm tall with a lower branch diameter of about 1 mm. There are at least four orders of branching. The branches and branchlets are arranged unilaterally with 3–4 per cm. The highest order end-branchlets vary considerably in length, many are greater than 2 cm, and some as long as 5 cm. The distal angles that the branches and branchlets form with the lower order branches are 90° at their proximal end, but the axis quickly curves distally so that the branches and branchlets, for the most part, extend vertically and run parallel to each other. The density of the highest order branchlets is 1–2 per cm. There are no fusions between the branches or branchlets.
Figure 8. Acanthosaropathes uniseriata n. sp., holotype NIWA 86099: A, branch from holotype; B, close-up view showing polyps; C, sections of branchlets showing spines; D, close up view of forked circumpolypar spine (C and D from schizoholotype, USNM 1491417/SEM stub 460).
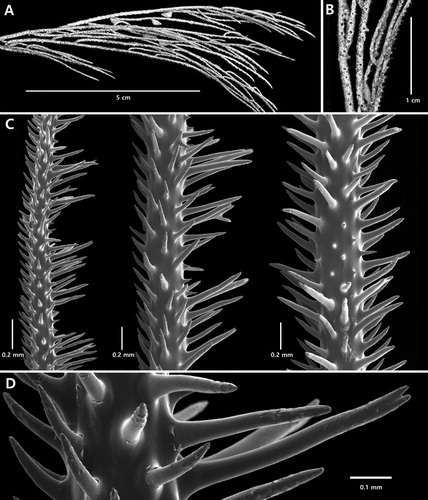
On branchlets about 0.27 mm in diameter, the circumpolypar spines are up to 0.64 mm tall and are about 0.06 mm in diameter near their midpoint; the interpolypar spines are 0.35 mm tall, and the abpolypar spines are 0.21 mm tall (C–D). As many as eight hypostomal spines per polyp are reduced in size; most of these are 0.06–0.07 mm tall although some on the periphery can be up to 0.14 mm tall. A few of the circumpolypar and interpolypar spines are forked (D); the forks in some cases being very pronounced. The spines are spaced on average about 0.2 mm apart in each axial row, resulting in about 5 per mm. Five axial rows are visible in lateral view. All the spines except the hypostomal spines have small papillae scattered near the distal end. The hypostomal spines are smooth, conical and very acute.
The polyps (B) lie in a single row on one side of the branches and branchlets. They are 0.8‒0.9 mm in transverse diameter, the interpolypar space is 0.2‒0.3 mm, and 4 polyps occur along 5 mm of axis (equivalent to 8 per cm).
Discussion
Acanthosaropathes uniseriata n. sp. has a distinct growth form that resembles some species of Aphanipathes such as A. pedata Gray, Citation1857 and A. sarothamnoides Brook, Citation1889; however, it can be distinguished from those species by its anisomorphic spines.
Etymology
The species name uniseriata is derived from the Latin unio (one) and series (row) in reference to the typical branching pattern in which most of the branches and branchlets arise on one side of the lower order branches.
Distribution
Known only from the type locality, Coleville Ridge (, #4).
Genus Phanopathes Opresko
Phanopathes Opresko Citation2004: 214–216, fig. 2.
Type species
Antipathes expansa Opresko and Cairns Citation1992 (by original designation).
Diagnosis
Corallum fan-shaped; highest order branchlets short; unilateral to bilateral, not uniform in size or arrangement. Spines tall, narrow, conical; covered, to varying degrees, with small conical tubercles; apex of spines acute to rounded. Polyps 0.55 mm to about 1.3 mm in transverse diameter.
Remarks
The genus currently contains four species: Phanopathes cancellata (Brook, Citation1889), P. expansa (Opresko and Cairns, Citation1992), P. rigida (Pourtalés, Citation1880), and P. zealandica Opresko, Citation2015. Aphanipathes erinaceus Roule, Citation1905, from the northeast Atlantic likely also belongs to the genus (T. Molodtsova, pers. comm.).
Molecular phylogenetic reconstructions have shown that Phanopathes is closely related to Aphanipathes, and in studies using mitochondrial markers some specimens assigned to Phanopathes have grouped with species of Aphanipathes (see Brugler et al. Citation2013); however, in studies using ITS1 and ITS2 (Bo et al. 018), the two genera were clearly separable.
Distribution
Atlantic, IndoPacific, and New Zealand.
Phanopathes ctenocrada n. sp.
(Figures 1, 9)
Material examined
Holotype. NIWA 19886, NIWA Stn TAN0205/75, 30.023°S, 178.716°W, Volcano L, Kermadec Ridge, 154–240 m, 23 Apr 2002 (schizoholotype USNM 1202936/SEM stubs 325, 373).
Type locality
Kermadec Ridge, 30.023°S, 178.716°W, 154–240 m.
Description
Small colonies sparsely branched mainly to the third and fourth order. Corallum tending to be planar, but not strictly so; some branches and branchlets overlapping. Pattern of branching of highest order ramifications uniserial to irregularly bilateral. Terminal branchlets relatively straight, of varying length, and with wide distal branch angles. Polypar spines up to 0.18 mm tall with a few tubercles near apex. Polyps estimated to be about 1 mm in transverse diameter.
The holotype (NIWA 19886, A) is a small complete colony with a holdfast. It is approximately 9 cm wide by 5 cm tall. The stem and lower order branches are 4–5 cm long and 0.24–0.34 mm in basal diameter. Terminal branches are mostly straight and stiff; very variable in length and spacing; up to about 2 cm long and mostly 2–8 mm apart with up to six branchlets per 2 cm. Distal branch angles are close to 90°. The branching pattern is uniserial to irregularly bilateral with up to 4 branchlets in a row on one side of a lower order branch.
Figure 9. Phanopathes ctenocrada n. sp., holotype NIWA 19886: A, corallum; B, sections of branchlets; C, single spine with tubercles (B and C from schizoholotype, USNM 1202936/SEM stubs 325, 373).
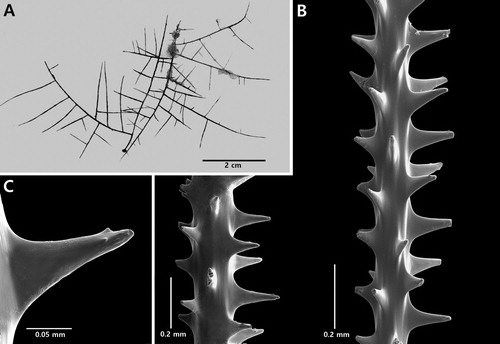
The skeletal spines on the branchlets are cone-shaped, smooth or with up to four small conical tubercles visible in lateral view (including those seen in profile on the edge of the spine) and concentrated primarily near the apex. The spines are dimorphic with the polypar spines larger than abpolypar spines. Polypar spines are 0.13–0.15 mm tall on a branchlet 0.11 mm in diameter; and up to 0.18 mm tall on branchlets 0.17 mm in diameter; the abpolypar spines up to about 0.09 mm tall (on the sample examined many of the spines were damaged). The spines generally project out at right angles to axis and tend to be flared out along the base in proximal and distal directions. They are arranged in axial rows, five of which are visible in lateral view. Within each row the spines are spaced 0.27–0.32 mm apart, resulting in about 4 spines per mm.
Soft tissue is poorly preserved and few of the polyps are recognisable; however, it was estimated that the polyps are about 1 mm in transverse diameter.
Discussion
Phanopathes ctenocrada n. sp. differs from the other four species in the genus in having straighter branches and branchlets with wider distal branch angles. In addition, the colony is not strictly planar. In this regard, it is closest morphologically to P. rigida (Pourtales), but in the latter species, the branchlets are more inclined distally. In terms of branching density, P. ctenocrada is similar to the types of P. rigida and P. zealandica in that all three have branching densities up to about 6–7 per 2 cm whereas in P. expansa and in P. cancellata there are 11–12 branchlets per 2 cm (the latter an estimate from an illustration of the type). Phanopathes ctenocrada has relatively small polypar spines compared to the other species; 0.18 mm tall vs. 0.24 mm in the type of P. expansa, 0.30 mm in the type of P. rigida, 0.34 mm in the type of P. zealandica, and 0.21 mm in the type of P. cancellata (estimated from illustration of the type). In addition, P. ctenocrada has the smallest number of tubercles per spine (those visible in lateral view) of the species in the genus; four vs. eight in P. zealandica, 12 or more in P. rigida and P. cancellata, and 25 or more in P. expansa. The polyps of P. ctenocrada are estimated to be about 1 mm in transverse diameter, slightly greater than those in P. expansa, P. zealandica, and P. cancellata (0.7 mm or less), but smaller than those P. rigida (up to 1.3 mm).
In terms of branching pattern P. ctenocrada is similar to Antipathes sibogae (van Pesch, Citation1914), the type of which was collected on the Borneo Bank in 50–40 m. Antipathes sibogae was originally placed in the genus Aphanipathes by van Pesch; however, based on the shape of the spines, and their lack of tubercles, the species most likely belongs in the genus Antipathes.
Etymology
The species name is derived from the Greek cteno (comb) and crada (branch), in references to the comb-like appearance of some of the branches that bear unilateral higher order branchlets.
Distribution
Known only from Volcano L, Kermadec Ridge ( #5).
Family Myriopathidae Opresko
Genus Cupressopathes Opresko
Cupressopathes Opresko, Citation2001: 352.
Type species
Gorgonia abies Linnaeus, Citation1758 (by original designation).
Diagnosis (emended)
Corallum monopodial or sparsely branched; pinnulate and subpinnulate to varying degrees. Pinnulation pattern not always uniform. Primary pinnules arranged in four irregular rows around circumference of stem and branches (sometimes loosely biserial or subspiral); extending out at nearly right angles to stem or slightly inclined distally. Secondary pinnules ranging from simple and sparse to uniserial to biserial and alternating. Higher order subpinnules uniserial or biserial, usually with very narrow interior angle.
Remarks
Besides the type species, the genus currently contains three other nominal species: Cupressopathes pumila (Brook, Citation1889); C. paniculata (Esper, Citation1796); and C. gracilis (Thomson & Simpson, Citation1905).
Distribution
New Zealand and throughout the IndoPacific.
Cupressopathes simplex n. sp.
(Figures 1, 10)
Material examined
Holotype. NIWA 19863, NZOI Stn P1, 32.590°S, 167.533°E, West Norfolk Ridge, 122 m, 24 Jan 1977 (schizoholotype USNM 1202931/SEM stub 322).
Type locality
West Norfolk Ridge. 32.590°S, 167.533°E, 122 m.
Description
Colony monopodial, very sparsely branched, and pinnulate in a bottlebrush fashion. Primary pinnules in four irregular rows around the circumference of the stem, and extending out subhorizontally. Pinnules mostly simple, some with a single simple subpinnule. Spines thick, cylindrical, smooth, with rounded apex; polypar spines up to 0.14 mm tall. Polyps 0.6–0.8 mm in transverse diameter.
The holotype (NIWA 19863, A) has a stem 22 cm long that extends to top of corallum where it shows signs that it had been broken off and is undergoing regeneration. The stem diameter near the base is 2.5 mm. The corallum is not more than about 3 cm wide. The primary pinnules (B) are up to 1.8 cm long with a basal diameter of 0.5 mm. The pinnules extend out sub-horizontally from all sides of stem and are arranged irregularly, although in places there appear to be four rows. Distances between the primary pinnules are very variable, but where the pinnules are in rows, the distance between two members of same row is about 4 mm, with 14–19 primary pinnules per cm. Some primary pinnules have one simple subpinnule arising 8–10 mm from the point of insertion on the stem. The subpinnules are small, generally less than 5 mm. One primary pinnule near the distal end of stem has five short subpinnules (each 7 mm long or less) coming off in different directions, suggesting the early formation of a branch.
Figure 10. Cupressopathes simplex n. sp., holotype NIWA 19863: A, corallum; B, close-up view of corallum showing pinnulation pattern and polyps; C, sections of pinnules (C from schizoholotype, USNM 1202931/SEM stub 322).
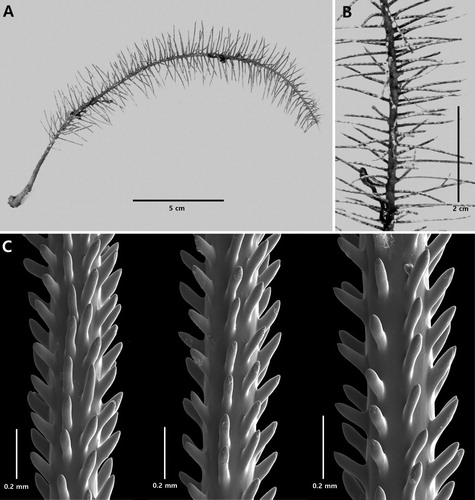
The spines on the pinnules are thick, smooth, cylindrical, blunt with a rounded apex, without apical knobs or bifurcations, and directed distally (C). On pinnules 0.2 mm in diameter, the polypar spines are 0.12–0.14 mm tall and 0.03–0.04 mm wide near their midpoint; the abpolypar spines are the same size or even slightly larger (up to 0.16 mm), and directed more distally. The spines are in axial rows, 5–6 of which are visible in lateral view. In each row the spines are 0.17–0.22 mm apart, resulting in about eight per mm. The polyps occur in a single row on one side of the pinnules, such that they face out in the same direction. The transverse diameter of polyps is 0.6–0.8 mm, and there are 10–12 polyps per cm.
Discussion
Cupressopathes simplex n. sp. superficially resembles Cupressopathes gracilis (Thomson & Simpson, Citation1905). The type specimen of C. gracilis is a small 6 cm tall colony (A), with horizontally directed primary pinnules and simple secondary pinnules often directed downward. The lower unpinnulated section of the stem is 13 mm in length, the next 2 cm has pinnules extending around three sides of the stem, and the upper 3 cm has pinnules in four irregular rows and extending out on all sides. The primary pinnules are up to 8 mm long and the secondary pinnules are 3 mm or less. A re-examination of the type revealed that there are 12 primary pinnules per cm along the stem.
Figure 11. Cupressopathes gracilis (Thomson & Simpson, Citation1905), holotype NHMUK 1908.2.18.22: A, corallum; B, close-up view of spines; C, sections of pinnules showing spines (B and C from schizoholotype: USNM 100388/SEM stub 498).
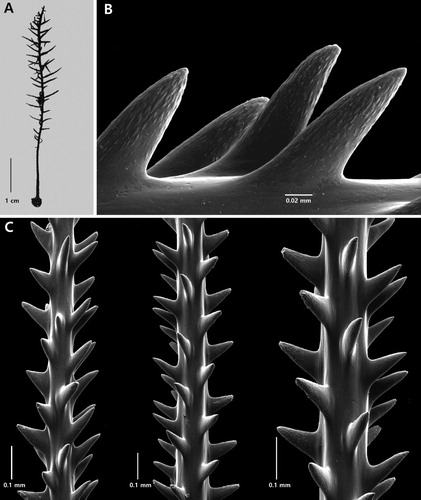
The size of the spines was not reported by Thomson and Simpson (Citation1905); however, examination of a sample of the type revealed that the polypar spines on the pinnules are conical, acute, directed distally, up to about 0.11 mm tall, and covered over half their surface or more with numerous, small elongated papillae (B,C). The polyps were found to be about 1 mm in transverse diameter.
Cupressopathes simplex differs from C. gracilis in having longer primary pinnules, fewer secondary pinnules, a higher pinnular density (14–19 per cm vs. 12 per cm), thicker, blunter spines without papillae, and smaller polyps. The other three nominal species in the genus (C. abies, C. pumila, and C. paniculata) form much larger colonies with many more orders of subpinnules.
Etymology
The specific name simplex is derived from the Latin simplex (simple) referring to the fact that most of the pinnules are simple, with only occasional subpinnulation.
Distribution
Known only from the type locality, West Norfolk Ridge (, #6).
Family Stylopathidae Opresko
Genus Stylopathes Opresko
Stylopathes Opresko, Citation2006: 111–112.
Type species
Arachnopathes columnaris Duchassaing, Citation1870 (by original designation).
Diagnosis (emended)
Corallum monopodial or very rarely branched. Stem (and branches when present) pinnulate. Primary pinnules simple or subpinnulate; arranged, in varying degrees of regularity, in three or four axial rows, and sometimes in quasi-verticillate groupings of three or four. Subpinnules, when present, arranged singly, suboppositely, or in verticils of three or four (rarely five). Spines on pinnules and subpinnules in most species usually not more than 0.1 mm tall, but up to 0.16 mm (see below); smooth, conical, and inclined distally to varying degrees, especially near tips of subpinnules. Polyps 0.6–1.3 mm in transverse diameter.
Remarks
The genus contains the species Stylopathes columnaris (Duchassaing, Citation1870), S. americana (Duchassaing & Michelotti, Citation1860), S. tenuispina (Silberfeld, 1909), S. adinocrada Opresko, 2006, and S. litocrada Opresko, 2006. Species are differentiated primarily by differences in the extent of subpinnulation, the length of the primary pinnules, and size of the spines.
Molecular phylogenetic reconstructions using mitochondrial markers such as cox3-cox1 and intergenic regions (Brugler et al. Citation2013) have shown that species of Stylopathes fall in the same large clade as species of Tanacetipathes, Antipathella and Myriopathes in the family Myriopathidae. Further study is needed to determine whether Stylopathidae should be recognised as only a subfamily of Myriopathidae.
Distribution
Known from the western Atlantic, IndoPacific, New Zealand and Japan.
Stylopathes stenotes n. sp.
(Figures 1, 12)
Material examined
Holotype. NIWA 16047, NIWA Stn TAN0413/23, 36.958°S, 177.363°E, Bay of Plenty, 1174 m, 9 November 2004 (schizoholotype USNM 1174712/SEM stub 294). Other Material: NIWA 16046, NIWA Stn TAN0413/139, 37.321°S, 177.113°E, Bay of Plenty, 763‒529 m, 14 November 2004; NIWA 16048, NIWA Stn TAN0413/70, 37.469°S, 177.207°E, Bay of Plenty, 330‒218 m, 11 November 2004.
Type locality
Bay of Plenty, 36.958°S, 177.363°E, 1174 m.
Description
Corallum monopodial and pinnulated, mostly to the second order (but occasionally to the third order and rarely to the fourth order). Corallum very narrow, generally not more than 2 cm wide. Spines up to 0.16 mm tall. Polyps unknown.
The holotype (NIWA 16047, A,B) is 24 cm tall, 1.5‒2 cm wide, and has a basal stem diameter of 0.9 mm. Primary pinnules on the stem are arranged in four (sometimes only three) axial rows; two lateral and two anterolateral, and in alternating pairs of one lateral and one anterolateral pinnule. Lateral primary pinnules are longer and more extensively subpinnulated than the anterior primaries. The lateral primary pinnules are mostly 5‒8 mm long (maximum about 1 cm), 0.5 mm in basal diameter, and 2.5‒3 mm apart, resulting in 5 per cm on each side of the axis. Anterolateral pinnules are 5‒6 mm long, and about the same distance apart as the laterals. The density of the primary pinnules (total for laterals and anterolaterals) is 16‒20 per cm. The secondary pinnules on the lateral primary pinnules are arranged singly, suboppositely, or in groups of three, and spaced about 2 mm apart (C). There are up to four groups of secondary pinnules on a lateral primary, with a maximum of three secondary pinnules per group. The secondary pinnules closest to the proximal end of the lateral primaries are often as long as the primary. Tertiary pinnules are present on some secondaries and quartenary pinnules are present on some tertiaries. Subpinnulation is usually more extensively developed on the distal sections of lateral primary pinnules. The anterolateral primary pinnules have 1‒7 secondary pinnules which are usually 3 mm or less in length, spaced 2 mm or less apart, and arranged singly, suboppositely, or in groups of three. Tertiary pinnules are only rarely present on the secondaries on the anterolateral primaries. Fusions occur between adjacent subpinnules throughout the corallum.
Figure 12. Stylopathes stenotes n. sp., holotype NIWA 16047: A, corallum; B, close-up lateral view of middle of corallum; C, arrangement of subpinnules; D, sections of pinnules (C–D from schizoholotype, USNM 1174712/SEM stub 294).
A cylindrical, reticulated worm run 3‒3.5 mm in diameter is present along the posterior side of stem. It is formed by the anastomosing of the basal-most sections of the lateral pinnules and subpinnules. In places, small subpinnules extend directly out from surface of worm run.
The pinnular spines (D) are conical, smooth, and inclined distally (especially near distal end of the pinnules and subpinnules, but becoming less distally inclined proximally). On pinnules 0.1‒0.13 mm in diameter, the polypar spines are 0.14‒0.16 mm tall, and the abpolypar spines 0.08‒0.12 mm tall. On pinnules 0.19 mm in diameter, the polypar spines are 0.12 mm, and the abpolypar spines 0.1 mm. The spines are arranged in rows, and spaced 0.14‒0.28 mm apart in each row resulting in 6‒7 spines per mm. Polyps are not present on the holotype.
NIWA 16046 is similar to the holotype, but has fewer subpinnules, and NIWA 16048 has slightly longer pinnules at the basal end of the stem.
Discussion
Stylopathes stenotes n.sp. differs from the other IndoPacific species, S. tenuispina (Silberfeld, 1909) in having much shorter primary pinnules (less than 1 cm vs. up to 4 cm) and more orders of subpinnules (up to three in S. stenotes and one, rarely two, in S. tenuispina). The spines in S. stenotes are slightly taller than those in S. tenuispina (0.16 mm vs. 0.11 mm or less).
Etymology
The species name stenotes is derived from the Greek stenotes (narrowness) in reference to the small diameter of the corallum.
Distribution
Known only from the Bay of Plenty, 218‒1174 m (, #7).
Family Schizopathidae Brook, 1889
Genus Stauropathes Opresko
Stauropathes Opresko, Citation2002: 417.
Type species
Stauropathes staurocrada Opresko, 2002 (by original designation).
Diagnosis
Corallum sparsely to densely branched and pinnulate. Stem and major branches tending to be in one plane. Pinnules simple, in two lateral or anterolateral rows and also arranged in subopposite pairs. Spines smooth, triangular to conical and compressed. Polyps 3–9 mm in transverse diameter.
Remarks
The genus currently contains three species: S. arctica (Lütken, Citation1872), S. punctata (Roule, Citation1905), and S. staurocrada Opresko, 2002.
Molecular studies (Brugler et al. Citation2013) have shown that Stauropathes is very closely related to some species of Bathypathes, and in particular to those species in which the pinnules are arranged suboppositely as in Stauropathes. The mitochondrial markers used to date have not been able to clearly separate the two genera, suggesting that perhaps Stauropathes might merit only subgeneric recognition within Bathypathes.
Distribution
Stauropathes arctica and S. punctata occur in the North Atlantic, S. staurocrada in the Pacific.
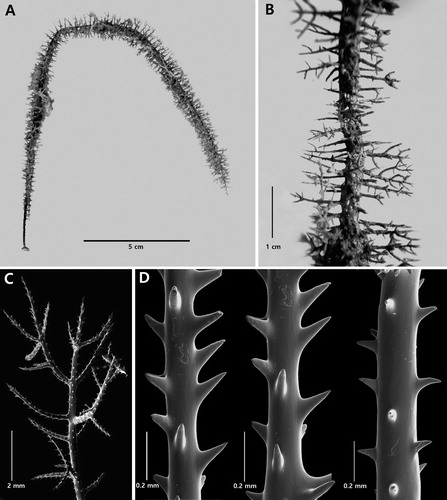
Stauropathes stellata n. sp.
(Figures 1, 13)
Material examined
Holotype. NIWA 16059, NIWA Stn TAN0413/193, 39.453°S, 179.962°E, Hikurangi Plateau, Young Nick Seamount, 2308–2207 m, 19 November 2004 (schizoholotype USNM 1573770/SEM stub 283).
Type locality
Hikurangi Plateau, Young Nick Seamount, 39.453°S, 179.962°E, 2308–2207 m.
Description
Monopodial and planar corallum with the stem extending to the top of the corallum and with two anterolateral rows of stem pinnules arranged in subopposite pairs. Most of the stem pinnules developed into pinnulated branches whose pinnules are also arranged biserially and in subopposite pairs. Proximal pinnules on a few of the longer branches also showing signs of developing into pinnulated branches. Polypar spines up to 0.07 mm tall, and polyps up to 6 mm in transverse diameter.
The holotype (NIWA 16059, A) is about 12 cm tall and 6.5 cm wide. The lower, unpinnulated section of stem is 6 cm in length, and the diameter just above the base is 0.8 mm. The stem possesses a striatum which starts about 2 cm above the base and extends for about 4 cm. The pinnulated part of the stem consists of eight pairs of bilateral, subopposite pinnules which cover a distance of about 5 cm. The pairs are 6‒8 mm apart, and the pinnular density ranges from about 4 per 2 cm on the lower part of the stem to 3 per 2 cm on the upper part (B). In each pair, the pinnule on the left side (viewing the front of the corallum) is set lower (proximal) to the pinnule on the right side. These stem pinnules are up to 4 cm long and about 0.5 mm in diameter near their base; they are longest near the middle of the stem and then decrease in length gradually toward the top of the corallum. The interior angle formed by the two rows is near 75°. All but the two lowermost stem pinnules are developed into pinnulated branches. The pinnules on these branches occur mostly in pairs whose members may be subopposite or offset by up to 2 mm. In the middle of the stem there are four pairs of pinnules on each branch; higher and lower there are fewer. On the lowermost stem pinnule there is only a single pinnule which is about 2.5 cm from the base. The lowermost (proximal) pair of pinnules on successively more distal stem pinnules occur closer to the base such that by fifth highest branch, the first pinnule is only 7 mm from the base. The pairs of pinnules on the branches are varying distances apart, mostly from 5 to 10 mm, such that about three pairs occur along 2 cm of axis. In each pair, one member generally projects distally and the other projects out at nearly a right angle to the plane containing the stem and stem pinnules. The lowermost pinnules on the fifth and sixth stem pinnules from the bottom (on the colony’s right side), have a single short ramification, 1.5–2 cm long, developing near the proximal end, suggesting that this pinnule is developing into a branch. A single simple short ramification about 7 mm is also found on the second pinnule from the base of the fifth stem pinnule from the bottom.
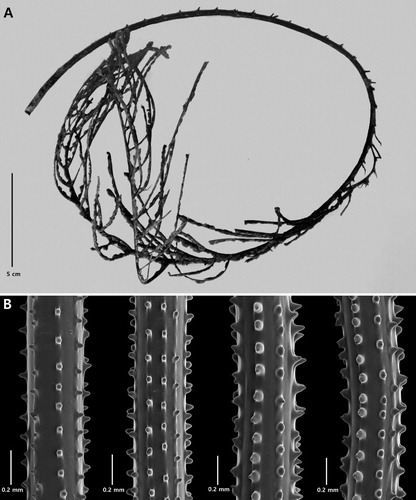
Figure 13. Stauropathes stellata n. sp., holotype NIWA 16059: A, corallum; B, close-up view of corallum; C, sections of pinnules (C from schizoholotype, USNM 1573770/SEM Stub 283).
The spines (C) are conical, smooth, with an acute apex. They are nearly equal on all sides of the axis, or only slightly longer on the polyp side, and they project out almost at right angles to the axis. The polypar spines are 0.06‒0.07 mm tall on pinnules 0.11‒0.28 mm in diameter; the abpolypar spines are 0.05‒0.06 mm. On the pinnules the spines are arranged in axial rows, three or four of which can be seen in lateral view, and there are 4‒6 spines per mm in each row.
Polyps are in poor condition but appear to be 5‒6 mm in transverse diameter with three polyps along 2 cm of axis.
Discussion
Stauropathes stellata n. sp. differs from the other three species in the genus in its more distinct monopodial growth form and limited development of pinnulated branches. Over most of the corallum there is only first order branching (from the stem), and with just some slight indications that second order branching might develop as the colonies increase in size.
The monopodial growth form and the relatively long unpinnulated lower section of the stem are two characters that S. stellata has in common with species in the genus Bathypathes whereas the multiple orders of branching is a characteristic of the genus Stauropathes.
Etymology
The species name stellata is derived from the Latin stellatus (starred), an allusion to general appearance of the corallum.
Distribution
Known only from the type locality; 2308–2207 m (, #8).
Genus Telopathes MacIsaac & Best
Telopathes MacIsaac & Best, in MacIsaac et al., Citation2013: 243.
not Telopathes, Brugler et al., Citation2015
Type species
Telopathes magna MacIsaac and Best, in MacIsaac et al., Citation2013.
Diagnosis
Corallum sparsely branched to the second order, branching not restricted to one plane; branches directed distally. Stem and branches pinnulate. All pinnules simple, directed distally at an angle from the axis of the branch, in two anterolateral rows, arranged in either subopposite or alternate order. Pinnules up to 30 cm long. Spines simple, conical, smooth, and compressed laterally. Polyps elongate and arranged uniserially on branches and pinnules. Preserved polyps up to about 7 mm in transverse diameter.
Remarks
The genus is morphologically similar to Bathypathes and Stauropathes in having two rows of primary pinnules and no secondary pinnules. In both Bathypathes and Telopathes the pinnules can be either subopposite or alternately arranged; in Stauropathes they are suboppositely arranged. Bathypathes forms monopodial colonies (rarely branched due to injury) whereas mature Stauropathes and Telopathes are branched (young colonies, however, might be unbranched).
Distribution
The genus is known from the western North Atlantic, and now from the Tasman Basin.
Telopathes tasmaniensis n. sp.
(Figures 1, 14)
Material examined
Holotype. NIWA 15339, NZOI Stn Z15006, 44.330°S, 147.170°E, Tasman Basin (Australia), U Seamount, 1083 m, 27 January 1997 (schizoholotype USNM 1174698/SEM stub 286).
Type locality
Tasman Basin, (Australia), 44.330°S, 147.170°E, 1083 m.
Description
Colony monopodial (when young) to sparsely branched when mature; pinnulate, with simple pinnules arranged suboppositely. Spines smooth, rounded, bulbous; some with a distinct apical knob; polypar spines up to 0.076 mm tall. Polyps 5 mm in transverse diameter.
The holotype (A) is an incomplete colony, broken at the top and bottom. The holdfast was not collected. The length of the remaining part of the stem is about 53 cm, and its lower end is 4 mm in diameter. The stumps of pinnules are present almost to the proximal end of the stem; therefore, very little of the lower unpinnulated portion of stem is present. Branching is to the first order only; and only two branches are present. The longest pinnule is broken off at its distal end; the remaining section is about 12 cm long with a basal diameter of about 1 mm. The pinnules are arranged in two rows and also in subopposite pairs. The pinnules are spaced about 1 cm apart in each row, resulting in a total pinnular density of 12 per 5 cm along the stem and about 10 per 5 cm along the branch. The two rows of pinnules form a narrow interior angle of ∼30°, and the individual pinnules are inclined distally forming a distal angle of ∼60°.
Figure 14. Telopathes tasmaniensis n. sp., holotype NIWA 15339: A, entire corallum; B, sections of pinnules (B from schizoholotype, USNM 1174698/SEM stub 286).
The spines (B) are smooth, very rounded to bulbous; some with a distinct apical, cone-shaped knob; and some in the process of splitting and forming double spines. The spines are slightly taller on one side of the axis. On pinnules 0.033–0.39 mm in diameter, the polypar spines are up to 0.076 mm tall and the abpolypar spines are up to 0.056 mm tall. The spines are arranged in very distinct axial rows, six or seven of which are visible in lateral view. Within each row the spines are 0.10–0.21 mm apart, resulting in 6–7 per mm.
The polyps are arranged in a single row, mostly on the inner side of the pinnules. They are up to 5 mm in transverse diameter. The interpolypar distance is about 2 mm and consequently, there are slightly less than 2 polyps per cm.
Discussion
Telopathes tasmaniensis n. sp. is similar to T. magna, the only other species in the genus, in being sparsely branched with relatively long, simple, bilateral pinnules (12 cm or more vs. up to 30 cm in T. magna) and large polyps (5 mm in transverse diameter vs. 4–7 mm in T. magna). It differs from T. magna in several ways. Whereas the pinnules are subopposite in T. tasmaniensis, they can be either subopposite or alternating in T. magna. Although the polypar spines of the two species are similar in maximum size (0.076 mm vs. 0.068 mm), those in T. magna are conical and compressed laterally whereas those in T. tasmaniensis are bulbous.
Etymology
The species name tasmaniensis is in reference to the type locality, the Tasman Basin.
Distribution
Known only from the type locality, Tasman Basin.
Family Cladopathidae
Genus Heteropathes Opresko
Heteropathes Opresko, Citation2011: 67–68.
Type species
Heliopathes americana Opresko, Citation2003b (by original designation).
Diagnosis
Corallum monopodial (rarely branched) and pinnulate; pinnules in two lateral rows and in one or more anterior rows; lateral pinnules simple; arranged alternately; anterior pinnules of varying length, usually shorter than lateral pinnules and subpinnulate, with secondary and tertiary pinnules; polyps 5–6 mm in transverse diameter.
Remarks
The genus currently contains the species H. americana (Opresko, Citation2003b), H. pacifica (Opresko, 2005), and H. heterorhodzos (Forster Cooper, Citation1909). The first two species both have secondary pinnules that tend to occur in pairs, and tertiary pinnules on some secondary pinnules. Based on the illustrations provided by Forster Cooper (the type specimen is lost), H. heterorhodzos differs from the other two species by not having any tertiary pinnules and by having as many as six secondary pinnules all arising from about the same point on the anterior primary pinnules. These characteristics, if verified, would likely be sufficient to support placing the species in a different genus.
Heteropathes (as Heliopathes) was originally placed with Hexapathes Kinoshita, Citation1910, in the subfamily Hexapathinae (see Opresko Citation2003b, p. 524). However, recent studies (Brugler et al. Citation2013, and unpublished data) indicate that the genetic separation between these two genera and other genera in the Cladopathinae is insufficient to maintain recognition of the subfamilies. Heteropathes is morphologically very similar to the cladopathid genus Trissopathes. In both genera the lateral primary pinnules are simple whereas the anterior primary pinnules are subpinnulate. The major differences between the genera is in the orientation and number of orders of subpinnules on the anterior primary pinnules. In Trissopathes the anterior primary pinnules have only secondary pinnules and these are regularly arranged in subopposite (sometimes alternating) pairs which lie in a plane set perpendicular to the general direction of the stem or branch. In contrast, in Heteropathes (excluding H. heterorhodzos) tertiary pinnules are present on some secondaries, and the secondaries and tertiaries are not restricted to a single plane perpendicular to the stem.
Molecular phylogenetic reconstructions using mitochondrial markers such as cox3-cox1, nad5-nad2, and intergenic regions have failed to adequately separate some specimens identified as Heteropathes from specimens identified as Trissopathes (Brugler et al. Citation2013, and unpublished data). This may be due, in part, to the fact that juvenile forms of these two genera can be difficult to tell part. However, more extensive molecular studies are needed to fully evaluate the relationship between Heteropathes and Trissopathes.
Distribution
The genus is found both in the Pacific and in the Atlantic.
Heteropathes intricata n.sp.
(Figures 1, 15)
Material examined
Holotype. NIWA 19924, NZOI Stn X704, 35.775°S, 177.803°E, Havre Trough, 1476–1783m, 15 Febuary 1996 (schizoholotype USNM 1527056/SEM stub 470).
Type locality
Havre Trough, 35.775°S, 177.803°E,1476–1783m.
Description
Corallum monopodial and pinnulate. Primary pinnules arranged in four rows, two lateral and two anterior (to anterolateral), and in alternating pairs. Lateral primary pinnules simple; anterior primary pinnules complexly subpinnulate with secondary and tertiary pinnules. Anterior primary pinnules extending out horizontally at right angles to the general vertical direction of the stem; lateral primaries directed distally. Up to two pairs of secondary pinnules on the anterior primary pinnules. Secondary pinnules in each pair subopposite or slightly offset; proximal pair lying in a plane that is horizontal and at right angles to the general direction of stem; distal pair lying in a plane that is in the general direction of the stem. Up to two subopposite tertiary pinnules present on each proximal secondary pinnule; each pair lying in a plane in the general direction of the stem. Pinnular spines small, up to about 0.12 mm tall. Polyps unknown.
The corallum of the holotype (A) has a stem about 23 cm long and 0.8 mm in diameter above the base; the unpinnulated section is 1.4 cm long. The lateral primary pinnules are simple, mostly 1.5 cm long (maximum about 2 cm) and 0.32–0.36 mm in basal diameter. The anterior (anterolateral) primary pinnules are up to 2 cm long and about 0.3 mm in diameter slightly above the expanded base. The density of the lateral primary pinnules is 10–14 per 2 cm (total for both rows) and that of the anterior pinnules is 12–16 per 2 cm. The anterior pinnules extend out at nearly right angles to the stem (distal angle close to 90°); whereas the lateral pinnules are inclined distally relative to the stem (distal angle about 60°). The lateral pinnules are not strictly lateral in position; the interior angle formed by the two rows is about 220° near base of corallum, increasing to 300–330° on the upper parts of the corallum. The anterior primary pinnules are not strictly anterior in position; the interior angle between the two rows is very variable and ranges from about 30° to 90°.
Figure 15. Heteropathes intricata n. sp., holotype NIWA 19924: A, corallum; B, anterior primary pinnule and subpinnules; C, anterior primary pinnule and subpinnules; D, sections of posterolateral primary (left), anterolateral primary (centre), and secondary (right) pinnule (B–D from schizoholotype, USNM 1527056/SEM stub 470).

Subpinnules (B,C) are present only on the anterior primary pinnules. Up to two pairs of secondary pinnules occur on each anterior primary; the lowermost pair is subopposite, and together with the anterior primary pinnule, lie in a plane that is largely horizontal and at right angles to the direction of the stem. The distal pair of secondaries is also subopposite, but lie in a plane that is at right angles to the first pair and in the same general direction of the stem. One pair of subopposite tertiary pinnules can occur on the lowermost secondary pinnules and is aligned in a plane that runs in the same direction as the stem. Often one or more tertiary pinnules are missing and one secondary pinnule such that on many anterior primary pinnules there are only the two lowermost secondaries, one distal secondary, and one or no tertiary pinnules. The lowermost secondary pinnules generally originate 0.1 mm to not more than 0.5 mm above the point where the anterior pinnules connects with the stem. The distal pair of secondaries originates 0.5 to about 1 mm above the base. Fusions occur between some adjacent secondary and tertiary pinnules.
The pinnular spines (D) are simple, smooth, triangular to conical. and often with a very acute apex. Generally, the spines become taller and more distally inclined towards the distal end of the pinnules. In the subsample examined, the polypar spines on the proximal sections of the posterolateral primary pinnules are conical, up to about 0.12 mm tall, and directed distally; those on the proximal sections of the anterolateral primary pinnules are more triangular, 0.04–0.06 mm tall, and mostly at right angles to the axis. The spines on the secondary and tertiary pinnules are similar in size and shape to those on the anterolateral pinnules. The spines are arranged in irregular axial rows, three to six of which can be seen in lateral view; with 2–3 spines per mm in each row. The spines on stem are acute, triangular, and mostly 0.08–0.09 mm tall.
Polyps are not present on the holotype.
Discussion
Heteropathes intricata n. sp. resembles H. pacifica in the orientation of the subpinnules on the anterior primary pinnules. In both species the proximal pair of secondary pinnules tend to lie in a plane that is perpendicular to the stem, whereas the distal pair of secondary pinnules and the pairs of tertiary pinnules (when present) tend to lie in planes that are in the general direction of the stem. The pattern is more consistent and uniform in H. intricata than in H. pacifica; however, in both species individual secondary and tertiary pinnules can be missing in places. The species differ primarily in the length of the primary pinnules and the size of the spines. Even though the types of the two species are similar in size (length of stem 22 and 23 cm), the lateral primary pinnules in H. pacifica are more than twice as long as those in H. intricata (5.5 cm vs. 2 cm), and the anterior primary pinnules are less than half as long (0.7 cm vs. 2 cm). In H. intricata the pinnular spines are up to 0.12 mm tall whereas those in H. pacifica are not more than 0.07 mm.
Etymology
The species name intricata is derived from the Latin intricatus (complicated), in reference to the complex orientation of the subpinnules on the anterior primary pinnules.
Distribution
Known only from the type locality, the Havre Trough (, #9).
Conclusions
This paper is part of a larger study of the antipatharian fauna of the New Zealand region that began in 1999. In a preliminary survey of the NIWA collections, 58 species were documented and provisional identifications were presented (Cairns et al. Citation2009). Following more detailed examination, including evaluation of the microscopic skeletal elements of the corals by scanning electron microscopy, some of the identifications were updated, and it was determined that the NIWA collection included a relatively large number of new species. Seven of these species were described in an earlier paper (Opresko Citation2015) and ten additional ones are presented in this paper. Ongoing molecular and morphological studies indicate that there are more undescribed taxa in the NIWA collection (see for example, Brugler et al. Citation2015). The species list presented in Cairns et al. (Citation2009) will therefore require revision once all the NIWA material is studied. However, based on current published and unpublished data, a brief overview of antipatharian diversity in the New Zealand region can be given here, keeping in mind that the information is likely to change in the near future. A minimum of 84 species has up to now been documented in the NIWA collection, including representatives of all seven families. The most diverse family is the Schizopathidae with 31 species spread across 13 genera. The Antipathidae is represented by 17 species, the Myriopathidae by 13, the Aphanipathidae by 11, the Cladopathidae by five, the Stylopathidae by four and the Leiopathidae by three. In 2007 it was estimated that worldwide 235 species of antipatharians had been described up to that time (France et al. Citation2007). Since then 25 additional species have been named for a total of 260 species. The antipatharian fauna of the New Zealand region therefore represents approximately 32% of the total number of currently recognised species. This high diversity is in part a reflection of the fact that the NIWA collection includes both deepsea and relatively shallow-water species from subtropic to temperate to polar regions in the southern hemisphere. A direct comparison to other regions is not possible at this time because no other regional collection covers such a wide bathymetric and latitudinal range.
Acknowledgements
The author wishes to thank the funding agencies, and the participants in the research voyages and MPI Observers for their sample collection. Special thanks go to Dennis Gordon, Rob Stewart, Di Tracey, Sadie Mills, Kareen Schnabel, and Dean Stotter (all of NIWA) for their hospitality and assistance during numerous visits to Wellington. Samples from cruises TAN0205, and TAN0413 were supplied by the National Institute of Water and Atmospheric Research (NIWA) Invertebrate Collection and were collected as part of a research programme ‘Seamounts: their importance to fisheries and marine ecosystems’, undertaken by the NIWA and funded by the New Zealand Foundation for Research, Science and Technology (C01X0224 (SFAS033)) and with additional funding from the Ministry of Fisheries (project no. ZBD2004-01) and NOAA Satellite Operations Facility (NRAM053). Specimens from cruise TAN1213 were collected by NIWA during the Nascent Inter-Ridge Volcanic and Neotectonic Activity (NIRVANA) voyage, funded by the Ministry for Primary Industries, in collaboration with Auckland University, GNS Science (New Zealand), and the University of New Hampshire (USA) and with funding from the program ‘Impact of resources Use on Vulnerable Deep-sea Communities’ project (CO1X0906), funded by the Ministry of Business, Innovation & Employment. The author also wishes to thank S. Cairns, W. Keel, W. Moser, and K. Reed for their assistance during visits to the USNMNH. The photomicrographs were prepared in the SEM Laboratory of the USNMNH with the assistance of S. D. Whittaker, director of the SEM Lab. D.M. Opresko is a Research Associate of the USNMNH, and gratefully acknowledges that affiliation.
Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Funding
References
- Bo M, Barucca M, Biscotti MA, Brugler MR, Canapa A, Caneses S, Lo Iacono C, Bavestrello G. 2018. Phylogenetic relationships of Mediterranean black corals (Cnidaria: Anthozoa: Hexacorallia: Antipatharia): effects on classification within the order. Invertebrate Systematics. 32:1102–1110. doi: https://doi.org/10.1071/IS17043
- Brook G. 1889. Report on the Antipatharia. Reports of the scientific results of the voyage of the H.M.S. Challenger. Zoology. 32:1–222.
- Brugler MR, Opresko DM, France SC. 2013. The evolutionary history of the order Antipatharia (Cnidaria: Anthozoa: Hexacorallia) as inferred from mitochondrial and nuclear DNA: implications for black coral taxonomy and systematics. Zoological Journal of the Linnean Society. 169:312–361. doi: https://doi.org/10.1111/zoj.12060
- Brugler M, Voza T, Dawes C, Joseph C, Marin J, Bellaflores-Mejia N, Moaleman S, Paul L, Goldman SL, Alcendor R, et al. 2015. Molecular characterization of the black coral Telopathes cf. magna from deep waters around New Zealand, Antarctica (Ross and Somov Seas) and Hawai’i. Poster presented at the 6th International Symposium on Deep-Sea Corals held in Boston, MA (U.S.A.), 12–16 September 2016.
- Cairns SD, Gershwin L-A, Brook FJ, Pugh P, Dawson EW, Ocaña O, Vervoort W, Williams G, Watson JE, Opresko DM, et al. 2009. The Phylum Cnidaria: corals, medusae and hydroids. In: Gordon D, editor. New Zealand inventory of biodiversity, volume 1, Kingdom Animalia. Christchurch: Canterbury University Press; p. 59–101.
- Duchassaing P. 1870. Revue des Zoophytes et des Spongiaires des Antilles, Paris. 52 pp.
- Duchassaing P, Michelotti J. 1860. Mémoire sur les coralliaires des Antilles. Mém Reale Acad Sci Torino (Ser. 2). 19:279–365.
- Ehrenberg CG. 1834. Die Corallenthiere des rothen Meeres, physiologisch untersucht und systematisch verzeichnet. Berlin: Abhandlung Königlichen Akademie der Wissenschaften. p. 153–154.
- Esper EJC. 1796. Die Pflanzenthiere in Abbildungen nach der Natur mit Farben erleuchtet nebst Beschreibungen, Nürnberg.
- Forster Cooper CF. 1909. Reports of the Percy Sladen Trust expedition to the Indian Ocean. No. XVII. –Antipatharia. Transactions of the Linnean Society of London (Zool. Ser. 2). 12:301–323. doi: https://doi.org/10.1111/j.1096-3642.1909.tb00144.x
- France SC, Brugler MR, Opresko DM. 2007. Order Antipatharia. The Phylum Cnidaria: a review of phylogenetic patterns and diversity 300 years after Linnaeus. In: Zhang Z-Q, Shear WA, editors. Linnaeus Tercentenary: progress in invertebrate taxonomy. Zootaxa. 1668: 1–776.
- Gray JE. 1857. Synopsis of the families and genera of axiferous zoophytes or barked corals. Proceedings of the Zoological Society of London. 25:278–294.
- Kinoshita K. 1910. On a new antipatharian Hexapathes heterosticha n. gen. and n. sp. Annotationes Zoologicae Japonenses. 7:231–234.
- Lapian HFN, Barucca M, Bavestrello G, Biscotti MA, Bo M, Canapa A, Tazioli S, Olmo E. 2007. A systematic study of some black coral species (Antipatharia, Hexacorallia) based on rDNA internal transcribed spacer sequences. Marine Biology. 151:785–792. doi: https://doi.org/10.1007/s00227-006-0525-8
- Linnaeus C. 1758. Systema naturae per regna tria naturae. Editio decima, reformata. Tom 1. Holmiae, 824 pp.
- Lütken CF. 1872. Antipathes arctica, a new species of black coral (Antipathidae) from the Polar Seas. Ann Mag Nat Hist (Ser. 4). 10:77–83. [originally published in Oversigt over det Kongl Danske Vidensk Selsk Forhand. 1871, pp. 18–26]. doi: https://doi.org/10.1080/00222937208696650
- MacIsaac KG, Best M, Brugler MR, Kenchington ELR, Anstey LJ, Jordan T. 2013. Telopathes magna gen. nov., spec. nov. (Cnidaria: Anthozoa: Antipatharia: Schizopathidae) from deep waters off Atlantic Canada and the first molecular phylogeny of the deep-sea family Schizopathidae. Zootaxa. 3700:237–258. doi: https://doi.org/10.11646/zootaxa.3700.2.3
- Opresko DM. 2001. Revision of the Antipatharia (Cnidaria: Anthozoa). Part I. Establishment of a new family, Myriopathidae. Zoologische Mededelingen. 75:343–370.
- Opresko DM. 2002. Revision of the Antipatharia (Cnidaria: Anthozoa). Part II. Schizopathidae. Zoologische Mededelingen. 76:411–442.
- Opresko DM. 2003a. Redescription of Antipathes dichotoma Pallas, 1766 (Cnidaria: Anthozoa: Antipatharia). Zoologische Mededelingen. 77:481–493.
- Opresko DM. 2003b. Revision of the Antipatharia (Cnidaria: Anthozoa). Part III. Cladopathidae. Zoologische Mededelingen. 77:495–536.
- Opresko DM. 2004. Revision of the Antipatharia (Cnidaria: Anthozoa). Part IV. Establishment of a new family, Aphanipathidae. Zoologische Mededelingen. 78:209–240.
- Opresko DM. 2006. Revision of the Antipatharia (Cnidaria: Anthozoa). Part V. Establishment of the new family Stylopathidae. Zoologische Mededelingen. 80:109–138.
- Opresko DM. 2009. A new name for the Hawaiian antipatharian coral formerly known as Antipathes dichotoma (Cnidaria: Anthozoa: Antipatharia). Pacific Science. 63:277–291. doi: https://doi.org/10.2984/049.063.0209
- Opresko DM. 2011. Nomenclatural note: Heteropathes (Cnidaria: Anthozoa: Antipatharia: Cladopathidae): A new name for Heliopathes Opresko, 2003. Zootaxa. 2782:67–68. doi: https://doi.org/10.11646/zootaxa.2782.1.4
- Opresko DM. 2015. New species of black corals (Cnidaria: Anthozoa: Antipatharia) from New Zealand and adjacent regions. New Zealand Journal of Zoology. 42(3):145–167. doi: https://doi.org/10.1080/03014223.2015.1051550
- Opresko DM, Cairns SD. 1992. New species of black coral (Cnidaria: Antipatharia) from the northern Gulf of Mexico. Northeast Gulf Science. 12:93–97. doi: https://doi.org/10.18785/negs.1202.02
- Opresko DM, Tracey D, Mackay E. 2014. Antipatharia (black corals) for the New Zealand region. A field guide of commonly sampled New Zealand black corals including illustrations highlighting technical terms and black coral morphology. Wellington: National Institute of Water and Atmospheric Research.
- Pallas PS. 1766. Elenchus Zoophytorum Sistens Generum Adumbrationes Generaliores et Specierum Cognitarum Succinctas Descriptiones cum Selectis Auctorum Synonymis. Hagae-Comitum [The Hague]: Petrum van Cleef.
- Pax F. 1932. Beiträge zur Kenntnis der Japanischen Dörnchenkorallen. Zoologische Jahrbucher (Syst.). 63:407–450.
- Pourtalès LF. 1867. Contributions to the fauna of the Gulf stream at great depths. Bulletin of the Museum of Comparative Zoology. 1(6):103–142.
- Pourtalès LF. 1880. Zoological results of the Blake expedition to the Caribbean Sea. Bulletin of the Museum of Comparative Zoology. 6:113–118.
- Quattrini AM, Faircloth BC, Dueñas LF, Bridge TCL, Brugler MR, Calixto-Botía IF, DeLeo DM, Forêt S, Herrera S, Lee SMY, et al. 2017. Universal target-enrichment baits for anthozoan (Cnidaria) phylogenomics: new approaches to long-standing problems. Molecular Ecology Resources. 1–15. doi:10.1111/1755-0998.12736.
- Roule L. 1905. Description des Antipathaires et Cérianthaires Recueillis par S.A.S. le Prince de Monaco dans l'Atlantique Nord. (1886–1902). Résultats des Campagnes Scientifiques du Prince de Monaco, Fasc. 30:1–99.
- Silberfeld E. 1909a. Diagnosen neuer Japanischer Antipatharien aus der Sammlung von Herrn Prof. Doflein. Zoologischer Anzeiger. 34:760–763.
- Silberfeld E. 1909b. Japanische Antipatharien. Beitrage zur Naturgeschichte Ostasiens. Abhandlungen der Mathematisch-Physikalischen Klasse der Königlich Bayerischen Akademie der Wissenschaften. 7(Suppl. 1):1–30.
- Thomson JA, Simpson JJ. 1905. Report on the Antipatharia collected by Prof. Herdman at Ceylon, in 1902. Supplemental Report No. XXV to the Report to the Government of Ceylon on the Pearl Oyster Fisheries of the Gulf of Manaar, Part 4: 93–106.
- van Pesch AJ. 1914. The Antipatharia of the Siboga expedition. Siboga Expeditie Monographie. 17:1–258.
- Wagner D, Brugler MR, Opresko DM, France SC, Montgomery AD, Toonen RJ. 2010. Using morphometrics, in situ observations and genetic characters to distinguish among commercially valuable Hawaiian black coral species; a redescription of Antipathes grandis Verrill, 1928 (Antipatharia: Antipathidae). Invertebrate Systematics. 24:270–291. doi: https://doi.org/10.1071/IS10004
- Warner GF, Opresko DM. 2004. A new black coral (Anthozoa: Antipatharia) from a reef wall environment in Jamaica. Bulletin of Marine Science. 74:169–174.