 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Research on stoat diet composition in New Zealand has primarily focussed on consumption of indigenous fauna in largely unmodified landscapes. This study used stomach content and stable isotope (δ13C and δ15N) analysis to assess stoat diet in a highly modified agricultural landscape in Southland, New Zealand, focussing on stoat predation of the mallard duck. Stoats were captured in Lochiel, Southland during August–November 2016 and 2017. Stomach content analysis of 26 captured stoats revealed limited stoat predation of mallards (n = 1) and mallard eggs (n = 1). Using liver tissue, stable isotope mixing models suggested that bird eggs on average met between 73 and 85% of stoat metabolic requirements throughout the mallard breeding period. Furthermore, mixing model outputs suggested that bird eggs made up a substantial proportion (77–84%) of stoat assimilated diet early in the mallard breeding period, when mallard eggs are readily available. In contrast, isotope mixing models suggested that mallard ducks/ducklings did not make a large overall contribution to stoat diets (< 3%). This study shows that stoats are an egg predator in the Southland agricultural landscape and mallard eggs may contribute to stoat assimilated diet early in the mallard breeding season before alternative prey items become available.
Introduction
Introduced mammalian predators have been implicated in the decline of biodiversity worldwide (Blackburn et al. Citation2004; Doherty et al. Citation2016; Russell et al. Citation2016). In New Zealand, rats (Rattus norvegicus), cats (Felis catus), stoats (Mustela erminea), weasels (Mustela nivalis), mice (Mus musculus), ferrets (Mustela furo) and possums (Trichosurus vulpecula) were introduced from 1769 and have contributed to significant declines in endemic wildlife (King Citation2005; Wright Citation2017). Stoats have been identified as a predator of native fauna in forest (King and Moody Citation1982; Murphy and Dowding Citation1995), wetland (O’Donnell et al. Citation2015), alpine (Smith et al. Citation2005; McAulay Citation2019) and braided riverbed habitats (Murphy et al. Citation2004; Dowding et al. Citation2015).
In the agricultural plains near Lochiel (Southland), stoats are the most abundant mustelid (Southland Fish and Game, unpublished data) and whilst non-native avifauna and mammals are common, native avifauna are uncommon (pers. obs.). Common potential stoat prey items near Lochiel include non-native passerines, rodents, lagomorphs and Anseriformes, specifically, the mallard duck (Anas platyrhynchos). Predation of mallard hens and their ducklings and eggs is concerning for recreational gamebird hunters, waterfowl enthusiasts and Fish & Game New Zealand, the statutory managers of gamebird populations.
In New Zealand, mallards can initiate nests as early as mid-July, and will re-nest multiple times if their nest fails (Sheppard et al. Citation2019). The peak mallard nesting period is late August to September with the peak brood rearing period being September through to November (Sheppard Citation2017; Sheppard et al. Citation2019). Recent research in Waikato and Southland has revealed that some mammalian and avian species prey on nesting mallard hens, ducklings and eggs (Sheppard Citation2017). Radio tracking of 304 mallard hens revealed at least one depredation event occurred at 167 (39%) of 432 monitored nests, with unidentified predators removing or destroying all eggs from 18% of nests (Sheppard Citation2017). Furthermore, duckling survival rangedg from 16-30% and of 243 hens with internal transmitters, 21 (9%) were killed by predators whist nesting (Sheppard Citation2017). These predation and survival rates are of concern for Fish & Game New Zealand because some New Zealand regions are experiencing declining mallard populations (McDougall and Amundson Citation2017) and gamebird licence sales.
Both within New Zealand and internationally, stoats exhibit a wide dietary niche (King and Moody Citation1982; Alterio and Moller Citation1997; McDonald et al. Citation2000; Remonti et al. Citation2007) and will opportunistically exploit temporally available food sources (King Citation1983; Remonti et al. Citation2007; Smith et al. Citation2011). For gamebird managers the potential for opportunistic stoat predation on seasonally abundant food sources is concerning, because from August through to late-November mallard nests and ducklings (Garrick et al. Citation2017; Sheppard et al. Citation2019) may provide a temporal food source for stoats. Mallards typically nest on the ground within narrow, linear, unmanaged habitat (rank grass, shelterbelts) (Garrick Citation2016; Sheppard Citation2017). These habitat features are common foraging areas for stoats and feral cats in agricultural areas of Southern New Zealand (Alterio et al. Citation1998) and the ground nesting behaviour of mallards may make their nests particularly vulnerable to mammalian predators.
To date, studies on the foraging ecology of New Zealand stoats have used gut content analysis to infer diet and have focussed primarily on stoat predation of native fauna in intact forest, alpine, and riverbed habitat (King Citation1983; Murphy et al. Citation2004; Smith et al. Citation2005; Smith et al. Citation2008; Dowding et al. Citation2015). In this study, complementary stomach content and stable isotope (δ13 C and δ15 N) analysis was used to assess stoat diet in a highly modified agricultural landscape with the focus being predation of a nationally valued gamebird (Nugent Citation1992; Stewart and Garrick Citation2017). Using a dual stomach content and stable isotope approach can provide greater insight into the assimilated diet of a consumer because different tissue types have different isotopic turnover rates (days–months) (Tieszen et al. Citation1983; Vander Zanden et al. Citation2015). Typically, metabolically active tissues like liver have a faster turnover rate relative to less metabolically active tissues such as hair (Tieszen et al. Citation1983; Vander Zanden et al. Citation2015).
The aim of this study was to determine the extent to which mallard ducks, ducklings and eggs are consumed by stoats. This will help inform managers of the risk that stoats pose to mallards. Given the extent of mallard hen, duckling and nest predation observed in recent studies, it was hypothesised that mallards and mallard eggs would feature heavily in the diet of stoats during the mallard nesting and brood rearing period indicating that stoats are exploiting this seasonally abundant food source.
Materials and methods
Study area
Stoat trapping was conducted near Lochiel, Southland (46°11'36.87"S, 168°17'53.88"E). Surrounding land use consisted of intensive agriculture, specifically dairy cattle and sheep farmed on predominantly rye grass (Lolium perenne) pastures. The study area was exclusively private land scattered with several small (<1 ha) man-made ponds created for waterfowl habitat or to hold livestock waste (effluent ponds). The Oreti River lies to the west of the trapping site between 230 m – 3.6 km away, and numerous small streams and agricultural drains are scattered throughout the study area. The remaining land cover was limited to road verges or ditches of rank grass and shelterbelts of typically macrocarpa (Cupressus macrocarpa), gum tree (Eucalyptus spp.) or flax (Phormium tenax).
Stoat trapping
Stoat trapping was conducted in 2016 and 2017 between 8 August and 30 November; this coincided with the main mallard nesting and brood rearing period (Sheppard Citation2017). Stoats were trapped and killed using paired Mark IV Fenn traps set at either end of a wooden tunnel (length 800 mm, width 220 mm, height 180 mm). In the 2016 season, tunnels were baited with 20 g pieces of fresh rabbit, nailed to the roof of the trap tunnel. Towards the end of the 2016 season baits were changed to non-estrous female or adult male stoat bedding to try and lessen hedgehog (Erinaceus europaeus) bycatch. During the 2017 trapping season, non-estrous female and adult male stoat bedding was used exclusively to bait the traps.
Traps were placed along habitat features such as hedgerows, shelter belts and woodlots as anecdote suggests stoats may use these linear features as movement corridors. Traps were inconsistently spaced, ranging from 80 m – 1.2 km apart. Traps were checked every three or four days. Captured stoats were placed in labelled plastic bags and frozen for later analysis.
Prey source collection
Likely stoat prey sources were harvested from the study area for stable isotope analysis and included: adult mallard hens, mallard ducklings, mallard eggs, passerines (blackbirds (Turdus merula), song thrush (Turdus philomelos), common starling (Sturnus vulgaris)), passerine eggs, lagomorphs (European hares (Lepus europaeus), rabbits (Oryctolagus cuniculus)) and rodents (Norway rats and house mice) (King Citation2005).
Mallard hens from the study area were harvested with a shotgun at the end of the gamebird season (late July) (n = 4) or opportunistically as fresh roadkill during the breeding season (n = 4). Blackbirds (n = 2), thrushes (n = 3) and starlings (n = 2) that were caught as trap bycatch or freshly killed by vehicles were collected throughout the study period. Five passerine eggs were collected from five separate nests within the study area (blackbird egg n = 1, starling egg n = 2, thrush egg n = 2). European hares (n = 3) and common rabbits (n = 2) were either harvested as fresh roadkill or caught as bycatch in our trapping tunnels. Rats were caught as bycatch in the stoat trapping tunnels (n = 4) and mice were trapped using Victor® wooden mouse traps (n = 3). Freshly laid mallard eggs (n = 5) were harvested from five separate nests. Mallard nests were located using an indicating bird dog. Three class 1a ducklings and two class 1c ducklings (Gollop and Marshall Citation1954) were harvested by hand from within the study area. An invertebrate isotopic signature was not determined in this study as there was uncertainty about invertebrate availability as a prey item. Following the collection of the prey items, small pieces of muscle tissue were cut from the prey sources and frozen in labelled bags. Breast tissue was taken from the small birds and mallard hens whilst tissue from the hind legs was taken from all other prey items.
Stomach and stable isotope analysis
Trapped stoats had the contents of their stomachs and intestines removed for analysis. Using a dissecting microscope, stomach contents were identified visually by comparison with hair, feathers and shell fragments of known origin. Prey items were grouped according to the prey categories: mallard duck, mallard egg, small bird (passerines), small bird egg, lagomorph, rodent, vegetation, invertebrate, unidentified tissue and unidentified hair.
Following stomach content analysis, the liver was removed from each stoat for later determination of δ15N and δ13C. Although the isotopic turnover rates of stoat liver have not been determined, liver was the elective tissue because in general it is a fast-turnover tissue, representing the average diet several days before an animal's death (Dalerum and Angerbjörn Citation2005). Stoat liver, prey muscle tissue portions, mallard eggs (homogenised yolk and albumen) and small bird eggs (homogenised yolk and albumen) were individually dried at 70○C for 72 h. Following drying, stoat and prey tissue samples were individually ground with a mortar and pestle and placed in labelled 2 mL ependorff tubes. Before stable isotope analysis, lipids were extracted from stoat liver tissue, mallard eggs and small birds eggs with a 2:1 chloroform:methanol solution (Bligh and Dyer Citation1959). Lipids were extracted from these tissues because of high (>4) C/N ratios indicative of significant lipid content and because no suitable lipid normalisation equations were available (Ehrich et al. Citation2011). The C/N ratio of all muscle tissue was > 4, so lipid content in all prey muscle tissue was arithmetically corrected using a general mammal/bird specific lipid normalisation equation (Ehrich et al. Citation2011, Equation 3). Lipid normalisation equations provide an economic and convenient way to account for lower δ13C values because of lipid content whilst preserving δ15N integrity (Post et al. Citation2007; Ehrich et al. Citation2011). Samples for carbon and nitrogen isotope analysis were prepared by weighing 0.8 mg (± 0.08) samples of homogenised material into tin foil capsules and dried under vacuum overnight. Nitrogen and carbon isotopes were assayed by combusting whole material to N2 and CO2 gas in a Carlo Erba NC2500 elemental analyser (CE Instruments, Milan), using helium carrier gas enriched with oxygen. The gases were separated on a packed Porapak QS GC column and sent sequentially to the inlet of a Europa Scientific ‘20/20 Hydra’ (Europa Scientific, UK) isotope ratio mass spectrometer (IRMS), in continuous flow mode. Raw isotope ratios were normalised by three-point calibration to international scales using two IAEA (International Atomic Energy Agency) reference materials (USGS-40 and USGS-41) and a laboratory standard (EDTA-OAS), assayed with the unknown samples. δ13C and δ15N values of these standards are as follows: USGS-40 (−4.52, −26.24), USGS-41 (−47.57, −37.76), EDTA-OAS (−0.73, −38.52). Samples were processed at the Isotrace lab at the University of Otago, Dunedin, New Zealand.
The laboratory standard, EDTA-OAS (Elemental Microanalysis Ltd, UK) has multi-year and multi-laboratory calibration records against IAEA reference materials. EDTA-OAS was also used as a drift control material by assaying a pair of aliquots after every twelve samples of a batch. Instrumental drift corrections (when applied) were calculated from regression of the EDTA-OAS against time. Precision was assessed from the RMS difference between sequential duplicates (IANZ Citation2004) of every 10th sample by random inclusion of three true control materials chosen to mimic the nature of the sample materials. Expected precision for analysis of control materials is typically ± 0.2 ‰ for δ15N and ± 0.1 ‰ for δ13C. Isotopic ratios are then expressed as parts per thousand using the formula:where δX is δ15N or δ13C, and R is the respective 15N/14N or 13C/12C ratio or the sample being measured.
Data exploration
Prior to the development of mixing models, an isospace plot was produced and visually inspected to (1) confirm that consumer δ15N and δ13C values fell within the prey polygon in isospace (Ben-David and Flaherty Citation2012), (2) that prey and consumer sampling was sufficient and appeared biologically reasonably (Stock et al. Citation2018) and (3) to determine whether isotopic signatures of the prey groupings were sufficiently dissimilar (a criteria for the effective use of isotope mixing models (Phillips et al. Citation2014)). Where required, prey types were intuitively grouped (or ‘lumped’) with attention to retain biological meaning within the aggregated source (Stock et al. Citation2018). Students t-tests were used to confirm that δ15N and δ13C values differed among grouped samples (Zar Citation2010). A Holm adjustment was applied to P values to account for multiple comparisons (Holm Citation1979). Data analysis was carried out in R version 3.2.1.
Isotope mixing model
Stable isotope data were analysed using Bayesian isotope mixing model package MixSIAR (Stock and Semmens Citation2016) in the programme R (R Core Team Citation2017). An individual model was produced for each stoat. Informative priors were added to each model because they improve the accuracy of the mixing models and reduce the confidence intervals of dietary estimates, especially where prey categories are closely spaced or linearly aligned within the isospace (Moore and Semmens Citation2008; Derbridge et al. Citation2015). Informative priors were constructed using the percentage frequency of occurrence of each prey category, taken from stomach contents of the stoats caught in this study. To avoid an overly-informative alpha prior based on a small sample size (i.e. a limited number of stoat stomachs) informed priors were scaled to the weight of an uninformative prior, using the following formula:Mixing models produce the most reliable results when prey categories are pooled into broad categories of easily identified items within the stomach contents (Phillips et al. Citation2014). This pooling yields greater certainty from model estimates and narrower confidence intervals (Phillips Citation2012). Additionally, isotopically distinct prey items are criteria for the use of mixing models (Phillips et al. Citation2014). After data exploration (evaluating the position of prey sources in isospace), prey items were lumped into five prey categories, easily identified in stoat stomachs: lagomorph, rodent, small bird, mallard, and bird egg.
When applying the isotope mixing model, stoat specific δ15N and δ13C trophic enrichment factors (TEFs) were not available so the TEFs determined by Roth and Hobson (Citation2000) for adult red foxes (Vulpes vulpes) were used. A TEF of 3.4‰ (0.06 SEM) was applied for δ15N and 0.4‰ (0.063 SEM) for δ13C.
Results
Stomach content analysis
Twenty-six stoats (2016 n = 12, 2017 n = 14) were caught at the Lochiel study site during the mallard breeding period and their stomach contents were analysed (). Prey remains were found in all captured stoats. The eggs of small birds were the most frequently encountered prey item. Mallard feathers and tissue was found in one stoat and mallard egg was found in another stoat. For seven of the captured stoats the stomach contents could not be identified because the contents were too digested and did not exhibit remnants of feathers, hair, eggshell or invertebrate.
Table 1. Frequency and percent frequency of occurrence for prey items found in the stomachs of 26 stoats caught in Lochiel, Southland, New Zealand between 14 August and 24 November 2016 and 2017.
Stable isotopes and mixing model
All stoat liver δ15N and δ13C signatures, corrected by the trophic enrichment factors, fell within the prey polygon in isospace i.e. the space defined by the measured prey values (). This indicated good support to continue the proposed modelling approach (Smith et al. Citation2013). Stoat liver δ15N signatures ranged from 10.6‰ to 13.5 ‰ while δ13C signatures ranged from −27.2‰ to −24.7 ‰ ().
Figure 1. Isospace plot showing δ13C and δ15N values of stoat livers taken in Lochiel, Southland, in relation to their prey. Values are means ± 1 standard deviation for prey values. Stoat liver values have been corrected using tissue specific trophic enrichment factors.
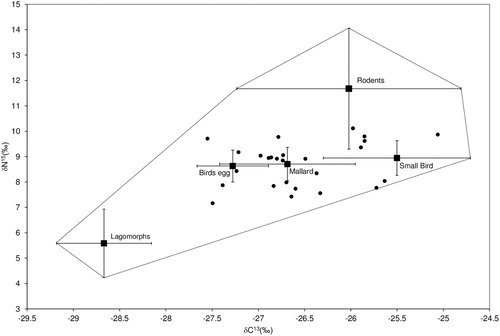
Table 2. Stable isotope values (δ15N, δ13C) of stoat liver tissue where stoats were captured between 14 August and 24 November 2016 and 2017 in Lochiel, Southland, New Zealand. Values for stoat liver are from lipid extracted tissue.
Five prey categories were formed as inputs for our isotope mixing model, - mallard (included mallard hen and duckling tissue), small birds, rodent (rats and mice), egg (mallard egg and small bird egg) and lagomorph (rabbit and hare) (Appendix 1). The mean δ13C value of rodents did not differ significantly from mallards, bird eggs or small birds (multiple comparison Holm test, P > 0.05, n = 10). However, the mean δ15N value of rodents did differ significantly from mallards, bird eggs and small birds (Holm test, P < 0.05, n = 3) which allowed for segregation amongst prey species in isospace. The δ15N and δ13C values for male and female stoats did not differ significantly (δ15N, male mean (SEM) = 12.1±(0.2), female mean (SEM) = 12.2±(0.2), t = 0.28, d.f. = 24, P = 0.78) (δ13C, male mean (SEM) = −26.4±(0.3), female mean(SEM), −25.9±(0.1) t = 1.45, d.f. = 24, P = 0.16).
Bird eggs were identified by our mixing models as the most important prey item, meeting on average between approximately 73 and 85% of stoat metabolic requirements throughout the mallard breeding period (, supplementary material (Figure S1)). However, the posterior distributions of prey categories ‘bird eggs’ and ‘small birds’ have a degree of correlation (R2 = −0.59) which may affect the ability of our models to accurately differentiate between these prey sources (Inger et al. Citation2010). Mixing models showed that on average, no more than 3% of stoat diet was comprised of mallard tissue (, supplementary material (Figure S1)).
Table 3. Estimated dietary proportions for individual stoats caught during the 2016 and 2017 mallard breeding season at Lochiel, Southland, New Zealand. Values are means ± 1 SD.
Discussion
This study has demonstrated that bird eggs are a readily consumed food item for stoats in the Southland agricultural environment. Remnants of bird eggs were found in one third of stoat stomachs and the isotope mixing models suggest that on average, bird eggs made up between approximately 73–85% of the individual metabolic requirements of stoats during the mallard breeding period. Stomach analysis revealed only two stoat stomachs contained mallard prey; one had consumed mallard egg and one had consumed mallard duckling tissue and feathers. However, isotope mixing model outputs revealed that bird eggs could make up a significant proportion (approximately 77–84%) of assimilated stoat diet early in the mallard breeding period (mid-August – early-September) when mallard egg availability is high and alternative bird egg (passerine) availability is low. In contrast, mixing model outputs suggested mallard ducks/ducklings made up a minor proportion of assimilated stoat diet.
The prevalence of bird eggs in the diet of the captured stoats was expected as eggs have been identified as a common stoat prey item (King Citation2005) and, when readily available, can make up a substantial proportion of stoat diet (Murphy et al. Citation2004; Dowding et al. Citation2015). The isotope mixing models could not differentiate between stoat consumption of mallard eggs and passerine eggs because the isotopic signatures of these two prey types did not differ sufficiently in isospace and therefore they had to be grouped as the prey category ‘bird eggs’. However, five stoats were captured early in the mallard nesting period (mid-August – early-September) (Sheppard Citation2017), before our first record of small bird egg consumption by a stoat (11 September) and well before the peak nesting period of blackbirds, song thrushes and European starlings (late September – October) in New Zealand (Gurr Citation1954; Flux Citation1966; Flux and Flux Citation1981; Bull and Flux Citation2006). As such, mallard eggs were the main egg type available during this time and were potentially consumed by these stoats as suggested by the isotope mixing models. The high bird egg dietary proportion estimates in these five stoats suggests that mallard eggs may be readily consumed by stoats and mallard nests may be vulnerable to stoat predation early in the breeding period when there are fewer alternative prey items (i.e. small bird eggs) available.
If stoats are consuming mallard eggs early in the breeding season, this is of concern for gamebird managers. Sheppard (Citation2017) found that mallard hens which initiate nests earlier in the breeding season are the experienced hens, which are more successful at raising ducklings relative to inexperienced hens. If stoats are preying upon mallard eggs during the early part of the peak nest period, as the stable isotope mixing models may suggest, stoats could be disturbing nests and removing eggs from the most successful mallard brood raisers and consequently stoat disturbance of early nests may have a disproportionately large effect on the mallard population.
Both the stomach content analysis and isotope mixing models indicate limited stoat consumption of mallard ducks or ducklings in the period of this study. The average contribution of mallards to stoat dietary estimates did not exceed 3% in mixing model outputs. This was surprising because in Sheppard’s (Citation2017) mallard hen radio tracking study, 16% of hens fitted with abdominal transmitters at the Southland study site were killed during the main nesting/brood rearing period (Southland Fish and Game, unpublished data). Furthermore, duckling survival was low, ranging from 16–30% depending on maternal experience. Theoretically, there should be many opportunities for stoats to prey upon or scavenge mallard hens or ducklings. There are several potential explanations for the limited evidence of mallard consumption by stoats. Firstly, it may be that there was an abundance of energetically less demanding prey items (i.e. eggs) available to stoats and they prefer to target these prey items; stoats have been known to preferentially select more energetically rewarding prey types as they become available (Smith et al. Citation2011). Secondly, stoats can exhibit surplus killing behaviour (Oksanen et al. Citation1985) and may be killing mallard hens and ducklings but not consuming them during the period represented by this study. Finally, it may be that an alternative predator, potentially feral cats, were responsible for the mallard hen and duckling predation outlined in Sheppard (Citation2017). Notably, feral cats are abundant in the immediate study area (minimum density estimate of 2.88 individually identifiable free-range cats per km2) (Southland Fish and Game, unpublished data) and cats are known to be duckling predators (Morgan Citation2002).
Mallard population models have revealed that mallard duckling and hen survival are the two most important variables influencing the New Zealand mallard population whilst nest survival is a second-tier variable (Sheppard Citation2017). As such, any mallard egg predation by stoats may negatively affect the mallard population to some extent, but this research indicates stoats are unlikely to be the most important mallard predator. Future research should assess the importance of mallard hens and ducklings in the diet of feral cats to determine whether mallards feature prominently in their diet.
There are two caveats associated with the stable isotope component of this work. Firstly, species specific lipid normalisation equations were not available to correct for lipid content in the prey muscle tissues. However, general mammal/bird lipid normalisation equations were used and provide an accurate and convenient way to account for lipids (Ehrich et al. Citation2011) whilst preserving δ15N integrity (Post et al. Citation2007). Secondly, species-specific (stoat) TEFs were not used when accounting for trophic fractionation in the isotope mixing models. This has incorporated an unknown amount of error into the mixing model outputs (Bond and Diamond Citation2011) and therefore dietary proportion estimates should be viewed as a first approximation. Despite this, the proxy TEFs utilised were of an obligate carnivore with a fast metabolic rate (McNab Citation1989), all consumer values fell within the prey isospace, and the results make biological sense. For example, when lagomorphs are abundant in a landscape they feature heavily in the diet of stoats (King and Moody Citation1982; Alterio and Moller Citation1997; Dowding and Elliott Citation2003). In the study area, lagomorph abundance is low (pers. obs., pers. comm. D Burgess, Environment Southland). Correspondingly, the mixing model results have shown lagomorphs to be unimportant prey items for Southland stoats during the mallard nesting and brood rearing period. These factors combined suggest that the TEFs utilised were suitable surrogates in the lack of stoat-specific factors and the study yields biologically reasonable results (Fry Citation2006; Smith et al. Citation2013; Stock et al. Citation2018).
This study has provided the first detailed insight into stoat foraging ecology in the Southland agricultural environment. While past research has focussed on stoats as predators of native species in relatively intact ecosystems, the goal of totally removing stoats from New Zealand (Department of Conservation Citation2016) has led to a large increase in predator trapping in non-native human modified habitat (Department of Conservation Citation2018a, Citation2018b; Glen et al. Citation2019). This study provides valuable contribution to our knowledge of stoat diets in an agricultural landscape and will help inform communities of likely effects of stoat control in these areas. While stoats were identified as a predator of bird eggs in these settings, results from this study indicate stoats are not heavily consuming mallard hens or ducklings. If confirmed, this would indicate that single species pest control may not achieve a desired outcome for fauna of interest. This has strong relevance to managers when deciding which predator guilds should be targeted for the benefit of species of interest.
In conclusion, this study indicates that stoats are an egg predator in the Southland agricultural landscape and mallard eggs may comprise a substantial proportion of assimilated diet early in the mallard breeding season. There was limited evidence that stoats consumed mallard ducks or ducklings which suggests that other predators, potentially cats, are more likely to be prolific mallard predators. Future research should use motion-activated cameras to confirm stoat predation of mallard nests, particularly early in the mallard breeding period and, quantify the prevalence of mallards in the diet of feral cats.
Supplementary material
Download PDF (1.9 MB)Acknowledgements
The authors wish to thank H & A Thomson, K McCallum, P Thomson, C & K McRae and J van Hout for access to their properties to capture stoats. The authors also thank two anonymous referees who provided valuable feedback on an earlier draft of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alterio N, Moller H. 1997. Diet of feral house cats Felis catus, ferrets Mustela furo and stoats M. erminea in grassland surrounding yellow-eyed penguin Megadyptes antipodes breeding areas, South Island. New Zealand Journal of Zoology. 243(4):869–877.
- Alterio N, Moller H, Ratz H. 1998. Movements and habitat use of feral house cats Felis catus, stoats Mustela erminea and ferrets Mustela furo, in grassland surrounding yellow-eyed penguin Megadyptes antipodes breeding areas in spring. Biological Conservation. 83(2):187–194.
- Ben-David M, Flaherty EA. 2012. Stable isotopes in mammalian research: a beginner's guide. Journal of Mammalogy. 93(2):312–328.
- Blackburn TM, Cassey P, Duncan RP, Evans KL, Gaston KJ. 2004. Avian extinction and mammalian introductions on oceanic islands. Science. 305(5692):1955–1958.
- Bligh EG, Dyer WJ. 1959. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology. 37(8):911–917.
- Bond AL, Diamond AW. 2011. Recent Bayesian stable-isotope mixing models are highly sensitive to variation in discrimination factors. Ecological Applications. 21(4):1017–1023.
- Bull PC, Flux JE. 2006. Breeding dates and productivity of starlings (Sturnus vulgaris) in northern, central, and southern New Zealand. Notornis. 53(2):208–214.
- Dalerum F, Angerbjörn A. 2005. Resolving temporal variation in vertebrate diets using naturally occurring stable isotopes. Oecologia. 144(4):647–658.
- Department of Conservation. 2016. [accessed 2019 Jan 4]. https://www.doc.govt.nz/news/media-releases/2016/predator-free-nz-2050-to-be-a-massive-team-effort/.
- Department of Conservation. 2018a. [accessed 2019 Jan 4]. https://www.doc.govt.nz/news/media-releases/2018/bold-predator-free-project-launched-in-hawkes-bay/.
- Department of Conservation. 2018b. [accessed 2019 Jan 4]. https://www.doc.govt.nz/news/media-releases/2018/$11.7-million-for-taranaki-predator-control/.
- Derbridge JJ, Merkle JA, Bucci ME, Callahan P, Koprowski JL, Polfus JL, Krausman PR. 2015. Experimentally derived δ13C and δ15N discrimination factors for gray wolves and the impact of prior information in Bayesian mixing models. PloS one. 10(3):e0119940.
- Doherty TS, Glen AS, Nimmo DG, Ritchie EG, Dickman CR. 2016. Invasive predators and global biodiversity loss. Proceedings of the National Academy of Sciences. 113(40):11261–11265.
- Dowding JE, Elliott MJ. 2003. Ecology of stoats in a South Island braided river valley. Contract report on Investigation 3405, New Zealand Department of Conservation, Wellington.
- Dowding JE, Elliott MJ, Murphy EC. 2015. Scats and den contents as indicators of the diet of stoats (Mustela erminea) in the Tasman Valley, South Canterbury, New Zealand. New Zealand Journal of Zoology. 42(4):270–282.
- Ehrich D, Tarroux A, Stien J, Lecomte N, Killengreen S, Berteaux D, Yoccoz NG. 2011. Stable isotope analysis: modelling lipid normalization for muscle and eggs from Arctic mammals and birds. Methods in Ecology and Evolution. 2(1):66–76.
- Flux JEC. 1966. Breeding of song thrushes and blackbirds at St. Arnaud, Nelson. Notornis. 13(3):42–149.
- Flux JEC, Flux MM. 1981. Population dynamics and age structure of starlings (Sturnus vulgaris) in New Zealand. New Zealand Journal of Ecology. 4:65–72.
- Fry B. 2006. Stable isotope ecology. New York, NY: Springer.
- Garrick E. 2016. Duckling survival and habitat selection of brood-rearing mallard (Anas platyrhynchos) females in Southland, New Zealand [master’s thesis]. Otago: University of Otago.
- Garrick EJ, Amundson CL, Seddon PJ. 2017. Duckling survival of mallards in Southland, New Zealand. Journal of Wildlife Management. 81(5):858–867.
- Glen AS, Perry M, Yockney I, Cave S, Gormley AM, Leckie C, Dickson R, Rakete-Stones W, Rakete-Stones P, Norbury GL, et al. 2019. Predator control on farmland for biodiversity conservation: a case study from Hawke’s Bay, New Zealand. New Zealand Journal of Ecology. 43(1):3358.
- Gollop JB, Marshall WH. 1954. A guide for aging duck broods in the field. St. Paul, MI: Mississippi Flyway Council.
- Gurr L. 1954. A study of the blackbird Turdus merula in New Zealand. Ibis. 96(2):225–261.
- Holm S. 1979. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 6:65–70.
- IANZ. 2004. Uncertainty of measurement, precision and limits of detection in chemical and microbiological testing laboratories. International Accreditation New Zealand, Auckland.
- Inger R, Jackson A, Parnell A, Bearhop S. 2010. SIAR v4 (Stable Isotope Analysis in R): an ecologist’s guide. [accessed 2018 Dec 1]. http://sethnewsome.org/sethnewsome/EE_files/SIAR%20for%20Ecologists.pdf.
- King CM. 1983. The relationships between beech seedfall and populations of mice, and the demographic and dietary responses of stoats, in three New Zealand forests. Journal of Animal Ecology. 52(1):141–166.
- King CM. 2005. The handbook of New Zealand mammals. 2nd edn. Melbourne: Oxford University Press.
- King CM, Moody JE. 1982. The biology of the stoat (Mustela erminea) in the National Parks of New Zealand II. Food habits. New Zealand Journal of Zoology. 9(1):57–80.
- McAulay JR. 2019. Using Stable Isotope Analysis to study the diet of stoats (Mustela erminea) in the alpine zone of New Zealand [master’s thesis]. Otago (NZ): University of Otago.
- McDonald RA, Webbon C, Harris S. 2000. The diet of stoats (Mustela erminea) and weasels (Mustela nivalis) in Great Britain. Journal of Zoology. 252(3):363–371.
- McDougall MB, Amundson CL. 2017. Harvest dynamics and annual survival of mallards and grey ducks. Journal of Wildlife Management. 81(3):449–460.
- McNab BK. 1989. Carnivore behavior, ecology, and evolution. Boston, MA: Springer. Chapter 12, Basal rate of metabolism, body size, and food habits in the order Carnivora; p. 335–354.
- Moore JW, Semmens BX. 2008. Incorporating uncertainty and prior information into stable isotope mixing models. Ecology Letters. 11(5):470–480.
- Morgan SA. 2002. Movements and hunting activity of house cats (Felis catus) living around Travis wetland, Christchurch, New Zealand [dissertation]. Canterbury, NZ: Lincoln University.
- Murphy EC, Dowding JE. 1995. Ecology of the stoat in Nothofagus forest: home range, habitat use and diet at different stages of the beech mast cycle. New Zealand Journal of Zoology. 19(2):97–109.
- Murphy EC, Keedwell RJ, Brown KP, Westbrooke I. 2004. Diet of mammalian predators in braided river beds in the central South Island, New Zealand. Wildlife Research. 31(6):631–638.
- Nugent G. 1992. Big-game, small-game, and gamebird hunting in New Zealand: hunting effort, harvest, and expenditure in 1988. New Zealand Journal of Zoology. 19(3-4):75–90.
- O’Donnell CF, Clapperton BK, Monks JM. 2015. Impacts of introduced mammalian predators on indigenous birds of freshwater wetlands in New Zealand. New Zealand Journal of Ecology. 39(1):19–33.
- Oksanen T, Oksanen L, Fretwell SD. 1985. Surplus killing in the hunting strategy of small predators. The American Naturalist. 126(3):328–346.
- Phillips DL. 2012. Converting isotope values to diet composition: the use of mixing models. Journal of Mammalogy. 93(2):342–352.
- Phillips DL, Inger R, Bearhop S, Jackson AL, Moore JW, Parnell AC, Semmens BX, Ward EJ. 2014. Best practices for use of stable isotope mixing models in food-web studies. Canadian Journal of Zoology. 92(10):823–835.
- Post DM, Layman CA, Arrington DA, Takimoto G, Quattrochi J, Montana CG. 2007. Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia. 152(1):179–189.
- R Core Team. 2017. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
- Remonti L, Balestrieri A, Prigioni C. 2007. Role of fruits in the diet of small mustelids (Mustela sp. from the western Italian Alps). European Journal of Wildlife Research. 53(1):35–39.
- Roth JD, Hobson KA. 2000. Stable carbon and nitrogen isotopic fractionation between diet and tissue of captive red fox: implications for dietary reconstruction. Canadian Journal of Zoology. 78(5):848–852.
- Russell JC, Jones HP, Armstrong DP, Courchamp F, Kappes PJ, Seddon PJ, Oppel S, Rauzon MJ, Cowan PE, Rocamora G, Genovesi P. 2016. Importance of lethal control of invasive predators for island conservation. Conservation Biology. 30(3):670–672.
- Sheppard JL. 2017. Breeding ecology and productivity of mallards and mallard-grey duck hybrids in New Zealand [dissertation]. Auckland, NZ: University of Auckland.
- Sheppard JL, Amundson CL, Arnold TW, Klee D. 2019. Nesting ecology of a naturalized population of Mallards Anas platyrhynchos in New Zealand. Ibis. 161(3):504–520.
- Smith DHV, Jamieson IG, Peach RME. 2005. Importance of ground weta (Hemiandrus spp.) in stoat (Mustela erminea) diet in small montane valleys and alpine grasslands. New Zealand Journal of Ecology. 29(2):207–214.
- Smith JA, Mazumder D, Suthers IM, Taylor MD. 2013. To fit or not to fit: evaluating stable isotope mixing models using simulated mixing polygons. Methods in Ecology and Evolution. 4:612–618.
- Smith DHV, Moller H, Wilson DJ, Murphy EC. 2011. Prey switching by stoats (Mustela erminea): a supplemental food experiment. Wildlife Research. 37(7):604–611.
- Smith DH, Wilson DJ, Moller H, Murphy EC, Pickerell G. 2008. Stoat density, diet and survival compared between alpine grassland and beech forest habitats. New Zealand Journal of Ecology. 32(2):166–176.
- Stewart C, Garrick E. 2017. Waterfowl hunter satisfaction and motivation in Southland, New Zealand. New Zealand: Southland Fish and Game. Unpublished report.
- Stock BC, Jackson AL, Ward EJ, Parnell AC, Phillips DL, Semmens BX. 2018. Analyzing mixing systems using a new generation of Bayesian tracer mixing models. PeerJ. 6:e5096.
- Stock B, Semmens B. 2016. MixSIAR GUI user manual. Version 3.1, (March), pp.1–59. Available at: https://github.com/brianstock/MixSIAR [Accessed February 22, 2018].
- Tieszen LL, Boutton TW, Tesdahl KG, Slade NA. 1983. Fractionation and turnover of stable carbon isotopes in animal tissues: implications for δ 13 C analysis of diet. Oecologia. 57(1-2):32–37.
- Vander Zanden MJ, Clayton MK, Moody EK, Solomon CT, Weidel BC. 2015. Stable isotope turnover and half-life in animal tissues: a literature synthesis. PLOS one. 10(1):e0116182.
- Wright J. 2017. Taonga of an island nation: saving New Zealand’s birds. Wellington: Parliamentary Commissioner for the Environment.
- Zar JH. 2010. Biostatistical analysis. 5th ed. New Jersey: Prentice Hill.
Appendix 1
Mean δ15N, δ13C and δ13C lipid corrected value of potential stoat prey items from Lochiel, Southland, New Zealand. Values presented as means ± one SEM with range in brackets. Values for mallard eggs and small bird eggs are from lipid extracted homogenised yolk and albumen.
