ABSTRACT
When resources such as mates or mating sites are limited, selection drives the evolution of complex and frequently violent fighting behaviour. Contest outcome is determined by an individual’s resource holding potential (RHP) in comparison to that of their opponent. During contests individuals may assess only their own RHP, or they may mutually assess both their own and their opponent’s RHP to determine the effort they allocate to a contest. Male giraffe weevils (Lasiorhynchus barbicornis) bear an elongated rostrum used as a weapon during intense fights over access to females. We used sequential analysis to target the fine-scale structure of contests and phases of contest escalation to determine the assessment strategy used by rival male giraffe weevils in the wild. We found significant non-random temporal structure in behavioural transitions in all transition matrices, and winner and loser transition matrices were significantly different. Contest de-escalation was extremely rare compared to escalation and winners and losers used very different behaviours during contests overall. This study provides support for the sequential assessment model of mutual assessment by male giraffe weevils during competitive interactions. It also demonstrates the utility of sequential analysis in making sense of the often complex interactions occurring in wild insect populations.
Introduction
Animal contests over resources are frequently violent and costly, involving ritualised and complex behaviours. Due to the high potential costs of fighting, selection should favour an animal’s ability to assess the relative likelihood that it will succeed in a contest. This may be achieved if an animal can assess the difference between themselves and their opponent’s resource holding potential (RHP) to help it decide when to withdraw from a contest (mutual assessment) (Parker Citation1974; Maynard Smith Citation1976). Models of mutual assessment include the asymmetric war of attrition (Maynard Smith Citation1976; Parker and Rubenstein Citation1981; Hammerstein and Parker Citation1982), and the sequential assessment model (Enquist and Leimar Citation1983, Citation1990). In contests best explained by these models, an individual must be able to gain enough information about their opponent to assess their RHP (Briffa and Elwood Citation2009) and several recent studies have debated the ability of many species to do this (Elwood and Arnott Citation2012, Citation2013; Fawcett and Mowles Citation2013). Alternatively, if an individual has awareness of its own RHP, but no gauge of its opponent’s, the strategy is termed ‘self-assessment’ (Taylor and Elwood Citation2003), and in such cases, individuals may choose to retreat from a contest based on an internal threshold. Self-assessment is described by several models including the energetic war of attrition (Mesterton-Gibbons et al. Citation1996; Payne and Pagel Citation1996), and the cumulative assessment model (Payne Citation1998), furthermore a recent meta-analysis of contests found that self assessment is the dominant strategy used among animals (Pinto et al. Citation2019). Both mutual and self assessment strategies allow the eventual loser to retreat from a fight, rather than persisting until it reaches a maximum cost threshold (Briffa and Elwood Citation2009).
Each contest model has its own predictions, which are useful when trying to distinguish among them. The cumulative assessment and energetic war of attrition models, which both fall under the category of self assessment, predict that both escalation and de-escalation is possible during contest phases, whereas the sequential assessment model under mutual assessment does not predict any de-escalation (Arnott and Elwood Citation2009; Briffa and Elwood Citation2009). This is because in contests where self assessment occurs the intensity of behaviours can move up and down as individuals aren’t collecting information about their rivals, however if opponents are using mutual assessment then there is no de-escalation as only behaviours of increasing intensity provide additional useful information of an opponent’s RHP (Green and Patek Citation2018). Finally, matching of contest behaviours between individuals in a fight is not expected for the sequential assessment model or the energetic war of attrition, however it is predicted under the cumulative assessment model (Arnott and Elwood Citation2009; Briffa and Elwood Citation2009).
Typically, the relationship between contest duration or escalation (as a proxy for contest cost) and the RHP of both the winner and loser are also used to differentiate between assessment strategies (Taylor and Elwood Citation2003; Morrell et al. Citation2005; Prenter et al. Citation2006; Reichert and Gerhardt Citation2011; McGinley et al. Citation2015). Under self-assessment contest duration increases with an increase in the RHP of both contest losers and winners, whereas under mutual assessment the same is found for contest losers but contest winners show the opposite relationship, a decrease in contest duration with increasing RHP (Arnott and Elwood Citation2009). However, these methods may not enable complete differentiation between some assessment strategies, especially when differences in RHP between individuals are small (Taylor and Elwood Citation2003; Briffa Citation2008; Brandt and Swallow Citation2009). Therefore, an additional approach has been suggested, which incorporates details of contest structure to assist in distinguishing between contest models (Enquist et al. Citation1990; Payne Citation1998; Briffa and Elwood Citation2000b; Fawcett and Mowles Citation2013; Mesterton-Gibbons and Heap Citation2014). A sequential temporal analysis may be useful when fights progress through a series of ‘choreographed’ stages and levels of intensity (Payne Citation1998; Briffa and Elwood Citation2000a; Jennings et al. Citation2005; Morrell et al. Citation2005; Stuart-Fox Citation2006; Green and Patek Citation2018). Sequential analysis provides quantitative temporal and structural information on escalation patterns, the intensity of behaviours, transition frequencies, and behavioural matching between opponents that allow researchers to distinguish between contest models (Gottman and Roy Citation1990; Chen et al. Citation2002, Citation2020; Egge et al. Citation2011; Camerlink et al. Citation2016).
Here, we use sequential analyses to investigate contest assessment strategies in the New Zealand giraffe weevil Lasiorhynchus barbicornis (Coleoptera: Brentidae). Male giraffe weevils bear an extremely elongated rostrum, which is used as a weapon during fights to gain mating opportunities (Meads Citation1976; Painting and Holwell Citation2013, Citation2014a), and is under directional sexual selection (LeGrice et al. Citation2019). Small males also employ sneaking behaviour as an alternative reproductive tactic to avoid agonistic interactions with larger males (Painting and Holwell Citation2014b). Prior field observations of giraffe weevils provided evidence that males use mutual assessment during contests (Painting and Holwell Citation2014c). The size of winners and the relative size difference between contestants related negatively to both contest duration and the probability of escalation, with escalation more likely to occur between individuals of a similar size. Along with a lack of evidence for de-escalation during contests these data provided support for the sequential assessment model. However, the overall relationship between contest duration and winner/loser size was weak and a large number of very brief agonistic interactions between males made it difficult to determine meaningful patterns when using contest duration as a proxy for contest cost. Furthermore, the absence of quantitative data on contest structure and fine-scale transitions between behaviours limited our understanding of how closely giraffe weevil fights resemble those predicted by various contest models. Here, we apply sequential analyses to test the prediction that giraffe weevil fights are best modelled by the sequential assessment model, demonstrating the value of combining sequential analysis with other techniques to study contest dynamics in a wild insect population.
Materials and methods
Study site
Matuku Reserve (36° 51.92′S, 174° 28.32′E) is an area of native coastal broadleaf forest located in west Auckland, New Zealand, which hosts a large giraffe weevil population (Painting and Holwell Citation2014a). During March 2014, we filmed male-male interactions on two karaka trees (Corynocarpus laevigatus), which hosted large aggregations of giraffe weevils.
Field observations
On the day prior to filming, we captured, measured, and marked all male giraffe weevils at each site. We used Rok digital callipers to measure total body length (terminal end of elytra to tip of mandibles) to the nearest 0.01 mm, which was previously established to be a useful estimation of male RHP (Painting and Holwell Citation2014b). We used coloured Queen Bee marking paints (Lega, Italy) to uniquely mark individuals and allow identification by sight during observations (Painting and Holwell Citation2014a). To avoid disturbance during filming days, we marked and measured any newly arrived males after each filming bout concluded.
We filmed at each site for up to two hours per day, avoiding rain and wind during which giraffe weevils typically hide (Painting and Holwell Citation2014a). We set up a video camera (Canon Legria FS306) and tripod one metre away from the aggregation and avoided any sudden movements to prevent disturbance. We used a method of ‘sampling all occurrences of some behaviours’ (Altmann Citation1974), to film all male-male interactions as they occurred within the aggregation, with multiple film clips taken over the entire filming session as interactions between males occurred. Over the filming period, we included males ranging from 17.82 to 85.71 mm body length, which spans almost the full body-size range of this species (Painting and Holwell Citation2014a). The largest size difference between two males we recorded was 49.52 mm and the smallest 0.42 mm, with an average difference of 12.73 ± SD 11.17 mm.
Within aggregations, male giraffe weevils guard females, and when not guarding will use either fighting or sneaking behaviour to attempt to gain access to females (Painting and Holwell Citation2014b). Encounters between two males occur when one male walks through an aggregation and detects the presence of another male who is typically guarding a female. We started filming when an individual began to approach another male, but before any of the highly focused behaviour was displayed, including antennal beating and orientation (). We stopped filming when the interacting individuals moved >10 cm away from each other (or one male retreated), and did not approach each other again within five seconds after which reengagement was highly unlikely to occur. We terminated filming if no further fighting behaviours occurred after approach (e.g. one of the two males walked away), more than two individuals became involved in the fight, or when a male attempted to sneak rather than fight. We determined that a male was sneaking if he did not engage in any contest-initiating behaviours towards the other male and tucked himself around the side of a female or crouched low against the tree upon approach to avoid detection by the opponent (Painting and Holwell Citation2014c).
Table 1. Ethogram of agonistic behaviours employed during male-male interactions.
Video analysis
We recorded and scored 102 male-male interactions using the software program Solomon Coder. Two fights were excluded from further analyses because the contest outcome was unclear, leaving 100 scored recordings for 63 different males.
The first 10 videos were used to develop an ethogram of agonistic behaviours exhibited during interactions between male giraffe weevils (, ), although some rare behaviours observed in subsequent videos were later added, including climbing on, and picking up. We grouped these behaviours into three broad categories of low intensity, high intensity, and conflict-ending behaviours. Low intensity behaviours comprised comparatively slow rates of movement and typically occurred prior to any vigorous physical engagement. High intensity behaviours had comparatively fast rates of movement and involved vigorous, physical engagement with their opponent. Finally, conflict-ending behaviours comprised the final behaviour displayed by an individual at the end of a contest. Not all behaviours scored were mutually exclusive, although no more than two behaviours were allowed to co-occur. For example, antennal beating often occurred when an individual investigated and/or oriented towards another male. Therefore, we quantified co-occurring behaviours using different scoring methods (durations or frequencies) to allow comparison of time and energy allocations between contest winners and losers. In the example above therefore, antennal beating was recorded as a frequency (number of beats) while the individual was displaying orienting behaviour which was recorded as a duration. We also recorded whether an individual was guarding a female prior to engaging in a contest.
Statistical analyses
To determine if there was a difference in the allocation of time to individual competitive behaviours (excluding walking/approaching and contest resolution behaviours) between winners and losers, we calculated the maximum, minimum, mean, and coefficients of variation of behaviour durations and frequencies. We also included the total number of behaviours, body size, relative body size, and guarding status (guarder vs intruder). To determine the relationship between each behaviour and contest outcome (winner or loser) we used binary generalised linear regression models (GLMs). Ideally, we would have used generalised linear mixed models (GLMM) to account for repetition of focal individuals in the data. However, for the majority of behaviours there were a large number of interactions during which the behaviour was never observed, making application of a GLMM extremely problematic. Therefore, we removed all subsequent data for an individual so only their first interaction was analysed in the GLMs.
Climbing on, crouching, picking up/tossing, raking, biting, pawing, and pushing behaviours were excluded from analyses as there were too few occurrences of these behaviours in either the winner or loser categories, preventing the calculation of sensible model coefficients. We log transformed all behaviours to reduce heteroscedasticity in the model residuals. Effects sizes (odds ratios) and 95% confidence intervals were calculated for each model (Nakagawa and Cuthill Citation2007). Means are reported ± standard deviations. All analyses were conducted in R 2.15.3 (R Development Core Team, 2013).
Sequential analysis
We translated behavioural sequences into single-order Markov chain matrices, one for contest winners, one for losers, and a full matrix including both winner and loser transitions. These included the transitional probabilities (frequencies) with which any behaviour immediately followed another. Behaviours transitioned into were placed in matrix columns with the behaviours they transitioned immediately from in matrix rows. Thus the count for any cell in the matrix table indicated the number of times a behaviour in its corresponding column transitioned from the corresponding row. These matrices enabled the analysis of temporal structure in behavioural sequences during a contest, to determine if these proceed in a structured pattern that is significantly different from random.
We included 14 behaviours in the transition matrices. Biting, kicking, and antennal beating co-occurred with other behaviours and were consequently scored as frequencies, meaning they could not be included in sequential analyses. Raking, although recorded as a frequency due to the very consistent and brief time dedicated to each rake, was able to be included in the sequential analysis because it always occurred as a discrete behaviour. To visualise these sequential patterns, we constructed kinematic diagrams based on these matrices, including all transitions that occurred with a greater than 10% probability. We calculated Tukey-Freeman deviates at the α = 0.05 level for each transition matrix to determine those transitions that occurred more or less frequently than expected, using freely available Java Applets (http://caspar.bgsu.edu/~software/java/Applets.html). We used chi-squared tests to test for temporal structure in each transition matrix. We applied Monte Carlo permutation methods (n = 9999 replicates) due to violation of the assumptions for expected cell values for the original chi-squared test with no less than 20% of the expected cells values being less than five. To determine if conflict-ending behaviours, used to distinguish between winners and losers, were the determining factor of significant structure in the matrices, we removed these behaviours from the matrices and ran the chi-squared tests again.
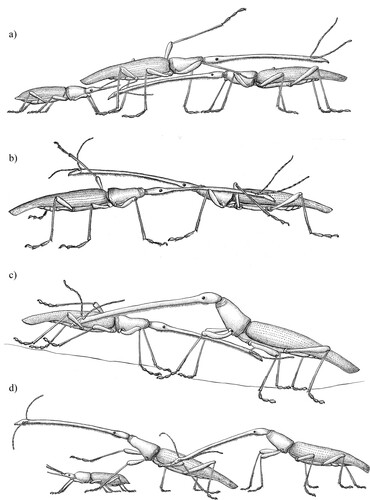
Figure 1. Examples of some competitive behaviours used by male giraffe weevils during contests. A, Paw: A male (centre) guarding a female (left) has both front legs raised and moving in a vigorous pawing action, contacting with the intruder male (right); B, Grapple: Two males line up with their rostrums held along the length of the other’s body and hooked under the middle leg; C, Rake: A male (right) uses his rostrum in a pulling action both downward and towards himself against a competitor male (left); D, Bite: A male (right) bites the middle leg of a male (centre) who is guarding a female (left).
Finally, to determine whether transition sequences of contest winners and losers differed significantly, we used a Mantel permutation test (n = 9999 replicates) looking for significant correlation between their respective matrices. We validated the result of this test with a chi-squared test using the same Monte Carlo permutation method as above with the matrix data arranged into two vector columns, one each for contest winners and losers with each row representing a single behavioural transition. Again, we ran both these tests including and excluding conflict ending behaviours.
Results
The full kinematic diagram, including information for both contest winners and losers, revealed an extensive repertoire of behaviours used within contests, with numerous, frequent transitions (). Generally, low intensity behaviours were used more frequently in contests compared to high intensity behaviours, however the highest transition probabilities occurred over a range of different behavioural intensities and conflict ending behaviours. Transition to the conflict ending behaviour ‘retreat’ (exclusive to contest losers) followed from multiple different behaviours, compared to pursuing behaviour (exclusive to contest winners) that only had one transition to it (from ‘climb on’) that occurred with a probability greater than 10% ().
Figure 2. Kinematic diagram describing a first order Markov chain analysis of competitive interactions for winner and loser giraffe weevil males (N = 63 individuals, 100 contests) for all transitions that occurred 10% or more of the time. Arrow thickness relates to transition frequency between behaviours (see legend). Box size is proportional to the frequency that each behaviour occurred. Light grey boxes represent low intensity behaviours, dark grey boxes, high intensity behaviours, and white boxes, conflict resolution behaviours.
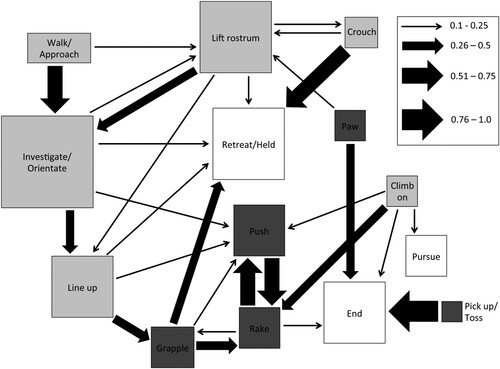
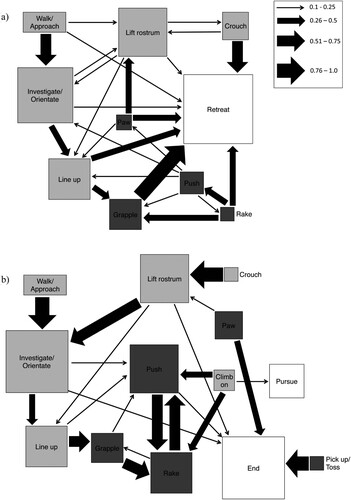
Figure 3. Kinematic diagrams describing a first order Markov chain analysis of interactions between male giraffe weevils shown for contest A, losers and B, winners for all transitions between behaviours that occurred 10% or more of the time. Arrow thickness relates to transition frequency between behaviours (see legend). Box size is proportional to the frequency with which each behaviour occurred. Light grey boxes represent low intensity behaviours, dark grey boxes, high intensity behaviours, and white boxes, conflict resolution behaviours.
Size and behavioural differences between winners and losers
Contest winners were significantly larger than losers (winners: 48.81 ± 16.09 mm; losers: 36.85 ± 12.91 mm; Binary GLM: χ2 = 7.4, p = 0.006, odds ratio [95% CI] = 9.22 [2.04–52.34]). Relative size difference within pairs was also a significant predictor of contest outcome (Binary GLM: χ2 = 10.8, p = 0.001, odds ratio [95% CI] = 1.74 [1.35–2.68]). However, within winner and loser groups we found wide variation and considerable overlap in body length (winner: maximum = 85.71 mm, minimum = 24.50 mm, CV = 0.33; loser: maximum = 84.75 mm, minimum = 17.82 mm, CV = 0.35), demonstrating that males of all sizes engage in fights. Finally, being a guarding male (as opposed to being the intruder) had a significant effect on contest outcome (Binary GLM: χ2 = 5.9, p = 0.02, odds ratio [95% CI] = 4.79 [1.46–19.04]).
On average, contest winners used a higher number of competitive behaviours during an interaction compared to losers, although this difference is not statistically significant (, 3). The competitive behaviour most commonly used by contest winners was beating of antenna (82% individuals), while the most common behaviour of losers was investigating/orientating towards their opponent (66% individuals). The necessity of behavioural matching during both lining up and grappling behaviours, means these occur at the most similar frequencies for winners and losers compared to any other behaviour ().
Table 2. Comparison of competitive behaviours used by contest winners and losers.
Of the 13 behaviours scored, four of these were used almost exclusively by either contest winners or losers. Eventual contest losers never used climbing on, and/or picking up/tossing their opponent behaviours during contests. Additionally, we only observed two contest losers raking, and in both occasions they only raked at their opponent once. This is compared to 41% of eventual contest winners that raked their opponent an average of 4.3 times (). Finally, 26% of losers used crouching during a contest, but only one eventual contest winner ().
Behaviours uncommonly used by contest losers and more frequently by contest winners included biting (5% of losers, 26% of winners), pawing (5% of losers, 19% of winners), and pushing (6% of losers, 51% of winners) (). On average, contest winners would bite their opponents a greater number of times and invested more time in pawing behaviour during contests (). All other competitive behaviours did not differ between contest winners and losers ().
Table 3. GLMs comparing investment in competitive behaviours between winners and losers, and overall number of behaviours executed.
Therefore, competitive behaviours in giraffe weevils are either almost mutually exclusive between contest winners and losers, or are used similarly between them.
Sequential analysis
Sequential analysis using first-order Markov chains revealed a significant non-random structure in behavioural transitions in the full matrix constructed of both contest winners and losers (Chi-square test: χ2 = 1834.5, P < 0.001, Table S1). We also found significant non-random variation in separate matrices constructed for contest losers (χ2 = 1387.6, P < 0.001, Table S2), and winners (χ2 = 1429.4, P < 0.001, Table S3). We repeated analyses with the final conflict resolution behaviours removed to test if these behaviours were significantly contributing to the non-random transition structure observed. However, both matrices were still significantly non-random in structure (winners χ2 = 1145.4, P < 0.001; losers χ2 = 1078.5, P < 0.001).
To determine if behavioural matching occurred between competitors during contests, we compared transition frequencies for winners and losers, and found no significant correlation for the complete matrices (Mantel test: Z = 653, P = 0.201), or with conflict ending behaviours removed (Z = 653, P = 0.223). This result was confirmed using a chi-squared test, which found a significant difference in the structure of winner and loser first-order transition matrices (Chi-square test: χ2 = 764.3 P = 0.004), and again when conflict resolution behaviours were removed (χ2 = 663.9, P < 0.001). In all of the matrices we found transitions between low intensity behaviours occurring regularly with significant and positive Tukey-Freeman deviates (Tables S1-S3), and winners (Table S3) produced a larger number of significant transitions compared to losers (Table S2). Losers used high intensity behaviours infrequently, rarely transitioning between high intensity behaviours, and never significantly and more frequently than expected (A; Table S2). However, contest winners used high intensity behaviours just as frequently as low intensity, and transitioned frequently between different high intensity behaviours with several high intensity transitions occurring significantly more often than expected due to chance including raking to pushing, pushing to raking, and grappling to raking (B; Table S3).
Contest escalation from low intensity to high intensity competitive behaviours rarely occurred more frequently than by chance. Only one significant transition was found for contest losers, from lining up to grappling behaviour (Table S2). Significant transitions for contest winners also included lining up to grappling, along with investigating/orientating toward their opponent to pawing behaviour (Table S3). The transition between lining up and grappling is the only significant pathway used by both contests winners and losers to transition from low to high intensity competitive behaviours. Furthermore, this is the only evidence for behavioural matching during competitive interactions. We found no significant contest de-escalation transitions for contest losers, and only one marginally significant transition for contest winners from pawing behaviour to rostrum lifting (Table S2, S3). However, this transition occurred only three times (Table S3), therefore, de-escalation within contests is rare in comparison to all other transition types. The most frequent and varied transition for contest losers occurs to the conflict ending behaviour retreat, including all of the greatest transition probabilities, except walking/approach and pushing (A).
Discussion
Through the use of sequential analysis, our study has provided support for the sequential assessment model for describing contest assessment strategy in giraffe weevils. A previous study on contest behaviour in giraffe weevils inspected the relationships between contest duration and winner/loser size, testing the predictions of the main competitive assessment models (Painting and Holwell Citation2014c). Although this study found evidence for mutual assessment, best described by the sequential assessment model, the relationships between contest duration and competitor size were weak, making it difficult to draw strong conclusions from these data. In our current study, by employing a fine-scale investigation of behavioural transitions and specifically testing key assumptions about contest structure, we found no evidence for behavioural matching or contest de-escalation. We were therefore able to confirm that the best model to describe contest behaviour among male giraffe weevils is the sequential assessment model.
Overall behavioural matching between contest winners and losers was absent, consistent with previous findings (Painting and Holwell Citation2014c), ruling out the energetic war of attrition model as a self-assessment strategy for this species as one of its key assumptions is behavioural matching during all stages of a contest (Mesterton-Gibbons et al. Citation1996; Payne and Pagel Citation1996). Both the cumulative assessment model and sequential assessment model do not require behavioural matching, and are instead identified by the presence or absence of de-escalation during contests (Enquist and Leimar Citation1983; Payne Citation1998). The sequential assessment model predicts that only escalation occurs during contests (Enquist and Leimar Citation1983, Citation1990), whereas de-escalation is permitted (but not required) under the cumulative assessment model (Payne Citation1998). De-escalation in giraffe weevil contests was found to be very rare, and only used occasionally by winners when transitioning from pawing behaviour to lifting their rostrum, most likely in an attempt to monitor the retreat of the rival losing male. We therefore suggest that giraffe weevils use sequential assessment during contests, and confirm that the application of sequential analysis increases our understanding of competitive behaviour and assessment in giraffe weevils. This is consistent with findings from other studies that have included contest structure in their analyses to determine assessment strategy (Morrell et al. Citation2005; Egge et al. Citation2011; Green and Patek Citation2018). We found that the competitive behaviours employed by male giraffe weevils can largely be divided between those that do not differ in use or frequency between winners and losers, and those that are used almost exclusively by one or the other.
Additionally, winners used on average a greater number of competitive behaviours during a contest, providing more evidence against behavioural matching. Typically, studies will simply compare behavioural transitions between winners and losers based on first-order Markov chains (Chen et al. Citation2002; Egge et al. Citation2011). However, to correctly determine if matching occurs, the type, frequency and intensity of the behaviours also need to be evaluated (Stuart-Fox Citation2006; Smallegange et al. Citation2007). Here we evaluated the rates and durations of individual competitive behaviours, in addition to differences in behavioural transitions between winners and losers. Behavioural matching was not found when comparing the transition matrices of contest winners and losers. The likely driver of this difference was that five out of the 13 behaviours were either mutually exclusive or almost mutually exclusive to either winners (climbing on, picking up/tossing, raking, biting, pawing, pushing) or losers (crouching). Five out of six of these behaviours used by contest winners were high intensity behaviours. Therefore, this tendency for only eventual contest winners to use several high intensity behaviours could be used as a predictor of contest outcome. Antenna beating during contests was used by both winners and losers, but more frequently by winners. Antenna beating might be used as a signal to an opponent, providing a measure of aggression, fighting ability, or the willingness of an individual to persist in a fight, possibly reducing the probability of fight escalation (Tinbergen Citation1953; Maynard Smith Citation1982; Moynihan Citation1982; Hofmann and Schildberger Citation2001).
We found that, on average, contest winners engaged in more competitive behaviours during a contest, and more behaviours of high intensity than losers. Sequential assessment is considered an efficient strategy among the game theory models because individuals are able to retreat from a contest at any stage, allowing for an effective avoidance of possible costs incurred during fighting (Enquist and Leimar Citation1983; Enquist et al. Citation1990; Arnott and Elwood Citation2009). In giraffe weevils, the large number of significant transitions made amongst low intensity behaviours or phases would allow low-cost assessment and rapid retreat of a smaller opponent before any significant fitness costs were incurred. Additionally, the sequential assessment model dictates that throughout an interaction, information is continuously gathered on relative RHP, and the more behavioural phases transitioned through and the higher the intensity level, the better the information gathered (Enquist and Leimar Citation1983; Enquist et al. Citation1990; Arnott and Elwood Citation2009). Considering this process for giraffe weevils, during the early stages of a contest a large size difference would be easily determined and the contest terminated rapidly. Also, larger males do not incur an energetic or lifespan trade-off with their size and weapon size which provides further reasoning for the willingness of contest winners, which are typically larger, to readily engage in more costly behaviours (LeGrice et al. Citation2019; Somjee et al. Citation2021).
If the size of competitors is more similar, then the frequent transitions observed within low intensity behaviours could allow for further assessment of rival RHP and lead to prolonged contest engagement by the smaller male (Enquist and Leimar Citation1990). If both contestants are still not able to determine an obvious size difference between them, only then will a switch to high intensity agonistic behaviours occur.
The difference in behaviour between contest winners and losers may suggest the presence of motivational asymmetry. For example, major male morphs of the stag beetle Cyclommatus mniszechi engage more rapidly in aggressive fighting behaviours compared to minor morphs, most likely because their greater energetic investment in large weapons means that withdrawing from a contest is more costly to them (Chen et al. Citation2020). Aside from assessing a difference in RHP, during a contest winners and losers might be differentially motivated based on their previous contest experience and/or mating opportunities. One possibility is the presence of a winner-loser effect, where a male that has won previous contests has an increased chance of winning contests in the future, and vice versa (Hsu et al. Citation2006). For example, in a controlled study, male water skinks (Eulamprus quoyii) that had previously won contests were more likely to initiate and win staged contests in the future (Kar et al. Citation2016). In our field study, we were not able to control for the influence that previous fights may have had on future interactions, and it is possible that any two males had encountered each other prior to our observations. Controlled studies taking place in a laboratory environment could be used in the future to determine if a winner effect is present in this species.
Elwood and Arnott (Citation2012) have argued that assumptions are frequently made about an animal’s cognitive ability to assess themselves in relation to their opponent during contests, and suggest that we sometimes imbue our study species with unrealistic decision-making capabilities. Despite these arguments, we do not believe that assigning a mutual assessment strategy to giraffe weevils is unrealistic or that we overestimate their cognitive capacity because mutual assessment can sometimes be achieved through very simple mechanisms (Enquist and Leimar Citation1983; Fawcett and Mowles Citation2013), described by Elwood and Arnott (Citation2012) as ‘mutual assessment without comparison’. For example, prior to transitioning to grappling behaviour, rival giraffe weevils line up, holding their rostrums along the length of their opponent’s body. Based simply on where their rostrum extends to along their opponent’s body, an individual could immediately gauge the difference between themselves and their opponent (Painting and Holwell Citation2014c). Further, the posterior end of male elytra is spatulate, forming a large ‘dip’, and may serve as an additional gauge of opponent length. When one weevil was not able to grasp at this ‘dip’ with their mandibles they would usually retreat from a contest, however, if able to grasp they would often use this hold to then ‘rake’ their opponent to try to dislodge them (RLG personal observations). This would allow giraffe weevils to use mutual assessment, without needing to separately assess themselves and their opponent’s size and process a comparative measure, a more cognitively complex process.
We have demonstrated in this study that sequential analysis techniques can be applied to wild populations successfully. Rarely has the competitive assessment strategy of an invertebrate been studied in the wild, especially without manipulation of the studied population (Pratt et al. Citation2003; Morrell et al. Citation2005; Bolton et al. Citation2013; Lopes Junior and Peixoto Citation2013). As far as we are aware, the use of sequential analysis has never been applied to observations of contests between wild animals. Along with previous studies conducted in the laboratory (Chen et al. Citation2002; Egge et al. Citation2011; Green and Patek Citation2018), we have demonstrated the utility of sequential analysis and suggest that future studies incorporate this technique when trying to distinguish among models of competitive assessment.
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed.
Acknowledgements
We would like to thank John Staniland and Forest and Bird Waitakere branch for allowing us to conduct our research at Matuku Reserve. We also thank all of the field assistants involved in this project; J. Le Grice, R. Le Grice, I. Le Grice, S. Wallace, A. Wallace, L. Powell, E. Edney-Brown, V. Lee, L. Zellner, K. Paler, P. Mill, A. Hildreth, P. Parmar, J. Brock, A. Zank, and F. Jacomb. We also thank Emma Scheltema for her wonderful illustrations.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability
Data associated with this study is deposited at 10.6084/m9.figshare.22207954
Additional information
Funding
References
- Altmann J. 1974. Observational study of behavior: sampling methods. Behaviour. 49(3/4):227–266. doi:10.1163/156853974X00534.
- Arnott G, Elwood RW. 2009. Assessment of fighting ability in animal contests. Animal Behaviour. 77(5):991–1004. doi:10.1016/j.anbehav.2009.02.010.
- Bolton J, Backwell PR, Jennions MD. 2013. Density dependence and fighting in species with indeterminate growth: a test in a fiddler crab. Animal Behaviour. 85(6):1367–1376. doi:10.1016/j.anbehav.2013.03.028.
- Brandt Y, Swallow JG. 2009. Do the elongated eye stalks of Diopsid flies facilitate rival assessment? Behavioral Ecology and Sociobiology. 63(8):1243–1246. doi:10.1007/s00265-009-0774-x.
- Briffa M. 2008. Decisions during fights in the house cricket, Acheta domesticus: mutual or self assessment of energy, weapons and size? Animal Behaviour. 75(3):1053–1062. doi:10.1016/j.anbehav.2007.08.016.
- Briffa M, Elwood RW. 2000a. Analysis of the finescale timing of repeated signals: does shell rapping in hermit crabs signal stamina? Animal Behaviour. 59(1):159–165. doi:10.1006/anbe.1999.1273.
- Briffa M, Elwood RW. 2000b. The power of shell rapping influences rates of eviction in hermit crabs. Behavioral Ecology. 11(3):288–293. doi:10.1093/beheco/11.3.288.
- Briffa M, Elwood RW. 2009. Difficulties remain in distinguishing between mutual and self-assessment in animal contests. Animal Behaviour. 77(3):759–762. doi:10.1016/j.anbehav.2008.11.010.
- Camerlink I, Arnott G, Farish M, Turner SP. 2016. Complex contests and the influence of aggressiveness in pigs. Animal Behaviour. 121:71–78. doi:10.1016/j.anbehav.2016.08.021.
- Chen S, Lee AY, Bowens NM, Huber R, Kravitz EA. 2002. Fighting fruit flies: a model system for the study of aggression. Proceedings of the National Academy of Sciences. 99(8):5664–5668. doi:10.1073/pnas.082102599.
- Chen Z-Y, Hsu Y, Lin C-P. 2020. Allometry and fighting behaviour of a dimorphic stag beetle Cyclommatus mniszechi (Coleoptera: Lucanidae). Insects. 11(2):81. doi:10.3390/insects11020081.
- Egge AR, Brandt Y, Swallow JG. 2011. Sequential analysis of aggressive interactions in the stalk-eyed fly Teleopsis dalmanni. Behavioral Ecology and Sociobiology. 65(2):369–379. doi:10.1007/s00265-010-1054-5.
- Elwood RW, Arnott G. 2012. Understanding how animals fight with Lloyd Morgan's canon. Animal Behaviour. 84(5):1095–1102. doi:10.1016/j.anbehav.2012.08.035.
- Elwood RW, Arnott G. 2013. Assessments in contests are frequently assumed to be complex when simple explanations will suffice. Animal Behaviour. 86(5):e8–e12. doi:10.1016/j.anbehav.2013.09.006.
- Enquist M, Leimar O. 1983. Evolution of fighting behaviour: decision rules and assessment of relative strength. Journal of Theoretical Biology. 102(3):387–410. doi:10.1016/0022-5193(83)90376-4.
- Enquist M, Leimar O. 1990. The evolution of fatal fighting. Animal Behaviour. 39(1):1–9. doi:10.1016/S0003-3472(05)80721-3.
- Enquist M, Leimar O, Ljungberg T, Mallner Y, Segerdahl N. 1990. A test of the sequential assessment game: fighting in the cichlid fish Nannacara anomala. Animal Behaviour. 40(1):1–14. doi:10.1016/S0003-3472(05)80660-8.
- Fawcett TW, Mowles SL. 2013. Assessments of fighting ability need not be cognitively complex. Animal Behaviour. 86(5):e1–e7. doi:10.1016/j.anbehav.2013.05.033.
- Gottman JM, Roy AK. 1990. Sequential analysis: a guide for behavioural researchers. New York: Cambridge University Press.
- Green P, Patek S. 2018. Mutual assessment during ritualized fighting in mantis shrimp (Stomatopoda). Proceedings of the Royal Society B: Biological Sciences. 285(1871):20172542. doi:10.1098/rspb.2017.2542.
- Hammerstein P, Parker GA. 1982. The asymmetric war of attrition. Journal of Theoretical Biology. 96(4):647–682. doi:10.1016/0022-5193(82)90235-1.
- Hofmann HA, Schildberger K. 2001. Assessment of strength and willingness to fight during aggressive encounters in crickets. Animal Behaviour. 62(2):337–348. doi:10.1006/anbe.2001.1746.
- Hsu Y, Earley RL, Wolf LL. 2006. Modulation of aggressive behaviour by fighting experience: mechanisms and contest outcomes. Biological Reviews. 81(1):33–74. doi:10.1017/S146479310500686X.
- Jennings DJ, Gammell MP, Payne RJH, Hayden TJ. 2005. An investigation of assessment games during fallow deer fights. Ethology. 111(5):511–525. doi:10.1111/j.1439-0310.2005.01068.x.
- Kar F, Whiting MJ, Noble DW. 2016. Influence of prior contest experience and level of escalation on contest outcome. Behavioral Ecology and Sociobiology. 70(10):1679–1687. doi:10.1007/s00265-016-2173-4.
- LeGrice RJ, Tezanos-Pinto G, de Villemereuil P, Holwell GI, Painting CJ. 2019. Directional selection on body size but no apparent survival cost to being large in wild New Zealand giraffe weevils. Evolution. 73(4):762–776. doi:10.1111/evo.13698.
- Lopes Junior RS, Peixoto PEC. 2013. Males of the dragonfly Diastatops obscura fight according to predictions from game theory models. Animal Behaviour. 85(3):663–669. doi:10.1016/j.anbehav.2012.12.033.
- Maynard Smith J. 1976. Evolution and the theory of games: in situations characterized by conflict of interest, the best strategy to adopt depends on what others are doing. American Scientist. 64(1):41–45.
- Maynard Smith J. 1982. Do animals convey information about their intentions? Journal of Theoretical Biology. 97(1):1–5. doi:10.1016/0022-5193(82)90271-5.
- McGinley RH, Prenter J, Taylor PW. 2015. Assessment strategies and decision making in male–male contests of Servaea incana jumping spiders. Animal Behaviour. 101:89–95. doi:10.1016/j.anbehav.2014.12.014.
- Meads MJ. 1976. Some observations on Lasiorhynchus barbicornis (Brentidae: Coleoptera). New Zealand Entomologist. 6(2):171–176. doi:10.1080/00779962.1976.9722234.
- Mesterton-Gibbons M, Heap SM. 2014. Variation between self- and mutual assessment in animal contests. The American Naturalist. 183(2):199–213. doi:10.1086/674443.
- Mesterton-Gibbons M, Marden JH, Dugatkin LA. 1996. On wars of attrition without assessment. Journal of Theoretical Biology. 181(1):65–83. doi:10.1006/jtbi.1996.0115.
- Morrell LJ, Backwell PRY, Metcalfe NB. 2005. Fighting in fiddler crabs Uca mjoebergi: what determines duration? Animal Behaviour. 70(3):653–662. doi:10.1016/j.anbehav.2004.11.014.
- Moynihan M. 1982. Why is lying about intentions rare during some kinds of contests? Journal of Theoretical Biology. 97(1):7–12. doi:10.1016/0022-5193(82)90272-7.
- Nakagawa S, Cuthill IC. 2007. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biological Reviews. 82(4):591–605. doi:10.1111/j.1469-185X.2007.00027.x.
- Painting CJ, Holwell GI. 2013. Exaggerated trait allometry, compensation and trade-offs in the New Zealand giraffe weevil (lasiorhynchus barbicornis). PLoS ONE. 8(11):e82467. doi:10.1371/journal.pone.0082467.
- Painting CJ, Holwell GI. 2014a. Exaggerated rostra as weapons and the competitive assessment strategy of male giraffe weevils. Behavioral Ecology. 25(5):1223–1232. doi:10.1093/beheco/aru119.
- Painting CJ, Holwell GI. 2014b. Flexible alternative mating tactics by New Zealand giraffe weevils. Behavioral Ecology. 25(6):1409–1416. doi:10.1093/beheco/aru140.
- Painting CJ, Holwell GI. 2014c. Observations on the ecology and behaviour of the New Zealand giraffe weevil (Lasiorhynchus barbicornis). New Zealand Journal of Zoology. 41(2):147–153. doi:10.1080/03014223.2013.854816.
- Parker GA. 1974. Assessment strategy and the evolution of fighting behaviour. Journal of Theoretical Biology. 47(1):223–243. doi:10.1016/0022-5193(74)90111-8.
- Parker GA, Rubenstein DI. 1981. Role assessment, reserve strategy, and acquisition of information in asymmetric animal conflicts. Animal Behaviour. 29(1):221–240. doi:10.1016/S0003-3472(81)80170-4.
- Payne RJH. 1998. Gradually escalating fights and displays: the cumulative assessment model. Animal Behaviour. 56(3):651–662. doi:10.1006/anbe.1998.0835.
- Payne RJH, Pagel M. 1996. Escalation and time costs in displays of endurance. Journal of Theoretical Biology. 183(2):185–193. doi:10.1006/jtbi.1996.0212.
- Pinto NS, Palaoro AV, Peixoto PE. 2019. All by myself? Meta-analysis of animal contests shows stronger support for self than for mutual assessment models. Biological Reviews. 94(4):1430–1442. doi:10.1111/brv.12509.
- Pratt AE, McLain DK, Lathrop GR. 2003. The assessment game in sand fiddler crab contests for breeding burrows. Animal Behaviour. 65(5):945–955. doi:10.1006/anbe.2003.2152.
- Prenter J, Elwood RW, Taylor PW. 2006. Self-assessment by males during energetically costly contests over precopula females in amphipods. Animal Behaviour. 72(4):861–868. doi:10.1016/j.anbehav.2006.01.023.
- Reichert MS, Gerhardt HC. 2011. The role of body size on the outcome, escalation and duration of contests in the grey treefrog, Hyla versicolor. Animal Behaviour. 82(6):1357–1366. doi:10.1016/j.anbehav.2011.09.019.
- Smallegange IM, Sabelis MW, van der Meer J. 2007. Assessment games in shore crab fights. Journal of Experimental Marine Biology and Ecology. 351(1–2):255–266. doi:10.1016/j.jembe.2007.07.004.
- Somjee U, Powell EC, Hickey AJ, Harrison JF, Painting CJ. 2021. Exaggerated sexually selected weapons maintained with disproportionately low metabolic costs in a single species with extreme size variation. Functional Ecology. 35(10):2282–2293. doi:10.1111/1365-2435.13888.
- Stuart-Fox D. 2006. Testing game theory models: fighting ability and decision rules in chameleon contests. Proceedings of the Royal Society B: Biological Sciences. 273(1593):1555–1561. doi:10.1098/rspb.2006.3468.
- Taylor PW, Elwood RW. 2003. The mismeasure of animal contests. Animal Behaviour. 65(6):1195–1202. doi:10.1006/anbe.2003.2169.
- Tinbergen K. 1953. Fighting and threat in animals. New Biology. 14:9–24.
