ABSTRACT
A robust understanding of an organism’s behavioural and ecological characteristics is an integral part of conservation; unfortunately, many of New Zealand’s native insect fauna still show a degree of data deficiency in these areas. Predator avoidance behaviours are one such area, and where there are colour morphs in New Zealand native and threatened insects, potential differences in the behaviour of these morphs can often be under investigated. The mountain stone wētā (Hemideina maori) possesses two distinct colour morphs, melanic and yellow, though the reason for this distinction is unclear. This study uses laboratory based assays to compare the behaviour of the morphs, including activity, refuge seeking, cohabitation, emergence and defensive behaviour. We observed emergence and cohabitation regularly, used video recordings to assay activity and refuge seeking behaviours, and measured defensive behaviours by probing individuals until a defensive response was displayed. Differences in all tested behaviours between colour morphs were non-significant; however, there were significant differences in defensive behaviour between sexes. We also discuss how defensive behaviours of H. maori compare with another tree wētā. Overall, the colour morphs in H. maori are similar in their predator responses and there may be a driving factor for melanism other than predation pressure.
urn:lsid:zoobank.org:pub:D54E864D-844F-4619-84E6-44BBAC1DE4E5
Introduction
Animals have evolved a diverse array of behaviours and morphological adaptations to cope with predation pressure. For example, cephalopods (octopi, squid, and cuttlefish) squirt ink in what is described as the Blanch-Ink-Jet Maneuver to aid in escaping predators (Caldwell Citation2005). Harvestmen (Opiliones) and some lizards exhibit autotomy, the ability to drop a body part, to escape predators (Clause and Capaldi Citation2006; Powell et al. Citation2021). Red flour beetles (Tribolium castaneum) feign their own death in the presence of predators, which has been found to increase their survival rates (Konishi et al. Citation2020). Beyond behaviour, animals also exhibit changes in morphology and physiology, such as increased body size and chemical defence (Witz Citation1990; Arbuckle and Speed Citation2015). For example, lubber grasshoppers (Romalea microptera) possess powerful toxins and their large size gives them a significant advantage against predators (Whitman and Vincent Citation2008). Crypsis (having a colouration that blends in with the surrounding in the environment) is also common (Brakefield Citation2009; Stevens and Merilaita Citation2011; Ruxton et al. Citation2018), as displayed by background matching of the black and white coloured New Zealand North Island lichen moth Declana atronivea (Mark et al. Citation2022). As animals evolve these morphological traits, they can also develop concomitant changes in behaviour (Brodie Citation1992; Kern et al. Citation2016).
Defensive behaviours, such as boldness and aggression, are often associated with melanic pigmentation in many taxa (Quesada and Senar Citation2007; Mafli et al. Citation2011; van den Brink et al. Citation2012; Schweitzer et al. Citation2015; San-Jose and Roulin Citation2018). In vertebrates, the melanocortin system has pleiotropic effects, meaning that some melanocortin receptors which control the expression of melanin in the skin may also be responsible for behavioural and physiological functions such as aggression, stress responses, and sexual traits (Ducrest et al. Citation2008). Changes in the activity of these receptors or the genes that encode them can therefore lead to covariation between pigmentation and behaviour (Ducrest et al. Citation2008). Although insects do not share this melanocortin system, they have underlying genetic mechanisms for both melanism and its pleiotropic effects. These mechanisms can vary between individual insects even within the same species and population (Wittkopp and Beldade Citation2009; San-Jose and Roulin Citation2018). There are myriad examples of associations between melanism and behaviour across insect taxa, including melanic facial spots associated with increased social dominance in paper wasps (Tibbetts and Dale Citation2004), increased mating success in melanic fruit flies (Singh Citation2015), and darker wing pigmentation in more territorial damselflies (Contreras-Garduño et al. Citation2006; Wittkopp and Beldade Citation2009).
Melanic colour polymorphisms can be found across New Zealand invertebrate taxa, including some species of spider wasps, stoneflies, and beetles (Harris Citation1988; Harris Citation2018; Foster et al. Citation2022). The mountain stone wētā (Hemideina maori: Orthoptera) is another example, being the only known species of tree wētā with both a common yellow and a melanic morph. Melanism has evolved multiple times across the species (King et al. Citation2003; King Citation2015) and in most populations there is a mix of melanic and yellow individuals (King Citation2015). The two morphs likely interbreed and are thus unlikely to be genetically divergent. Common explanations for melanic colour polymorphisms in insects, such as immune response, population density, diet, and levels of parasitism, have been ruled out in H. maori (Wilson and Reeson Citation1998; Wilson Citation2000; Wilson and Jamieson Citation2005; Hagen et al. Citation2006; Bindu et al. Citation2012; Dubovskiy et al. Citation2013). King and Sinclair (Citation2015) show that melanic morphs have less cuticular water loss than yellow morphs. However, melanic morphs are more commonly found at lower elevations (King Citation2015), which contradicts the expectation that melanic individuals should survive better at colder and higher elevations because they can reach temperature equilibrium faster than non-melanic individuals (King et al. Citation2003). Behavioural differences between the morphs are yet to be explored.
Although the evolutionary benefits of melanism in H. maori are still unknown, some studies have found melanism to be correlated with anti-predator behaviours in other species (Brodie Citation1992; Quesada and Senar Citation2007; Mafli et al. Citation2011; van den Brink et al. Citation2012; Schweitzer et al. Citation2015; San-Jose and Roulin Citation2018). Therefore, we are testing whether melanism is associated with antipredator behaviour in H. maori, by comparing the behaviour of the two colour morphs, yellow and melanic. While we are less likely to find behavioural differences between the morphs than if the morphs had been evolving independently for a long time, differences may still exist due to pleiotropy or linkage (Kern et al. Citation2016), or as a result of a selective pressure like predation (Brodie Citation1992; Kern et al. Citation2016). We also investigate differences in behaviour between sexes, to account for potential predation bias that is sometimes thought to occur in polygynous, sexually dimorphic genera like tree wētā (Kelly Citation2008; Wehi et al. Citation2011). We, therefore, examine activity, exploration, refuge-seeking, defensive behaviour, emergence, and cohabitation of wētā for both males and females of each colour morph.
Materials and methods
Study system
Hemideina maori are long-lived, flightless, and nocturnal Orthopterans that inhabit island rock outcrops (known as tors) and rock-under-rock spaces in New Zealand’s South Island, often at high elevations (Leisnham and Jamieson Citation2002). Most populations have primarily yellow to intermediate morphs (King et al. Citation2003), with only some having melanic individuals (; Neufeld and Leader Citation1998; Leisnham and Jamieson Citation2002). On the Rock and Pillar Range in Otago, melanic H. maori are distributed in a cline, with mainly yellow morphs at central and higher elevations, and a greater proportion of melanic wētā at southern and northern ends of the range, and at lower elevations (King et al. Citation1996; King et al. Citation2003). Other than the Rock and Pillar Range, melanic H. maori are also found on many other ranges, from Nelson down to Southland, with some populations at low elevations, including the islands of Mou Tapu and Mou Waho on Lake Wānaka (King et al. Citation2003; King Citation2015). Although predators are relatively few in the subalpine and alpine zones at present (O’Donnell et al. Citation2017), individuals across all populations display antipredator behaviour, such as stridulating (rasping of femur spines against the abdomen), gaping mandibles, and raising their hind legs (Field Citation2001). H. maori are also sexually dimorphic; males wield large mandibles with which they protect harems of up to ten females from other males (Koning and Jamieson Citation2001).
Sample collection and animal husbandry
We collected ten males and ten females of each colour morph (n = 40 in total), avoiding intermediate morphs, by carefully lifting large rocks on the 12th and 26th of January 2021, from the Rock and Pillar Conservation Area, Otago, New Zealand. We placed each wētā into a small (10.8 cm by 4.5 cm) vial with air holes drilled into the lid prior to transportation. The collected wētā were adults or subadults (5 melanic, 4 yelow).
On arrival at the animal laboratory in the Department of Zoology, University of Otago, we transferred wētā into tanks in an animal containment facility with an artificial day/night and climate cycle where they were housed for just over two months. The day/night cycle was set to 14:10 with a one hour dawn/dusk ramp, and on a reverse light cycle (dark at 14:00) to facilitate phenotyping. The temperature was 14 °C during the day and 8 °C at night, corresponding to the average summer conditions of the sampling region at the time of collection. Male-female pairs of the same morph were housed together in large enclosures (L 26 cm, W 14.5 cm, H 18 cm), that included a tile refuge (L 20 cm, W 10 cm, H 2.5 cm), a flax flower stem refuge, a water and a food dish, and a leafy stick of coprosma (Coprosma robusta). We provided carrot or apple and cat biscuits as food and replenished food as required. To maintain ambient humidity, the sides of each container were misted with water every three days, and frass was removed weekly.
Morphological data
Using a pair of electronic calipers (Kinchrome digital vernier caliper, no. 2313), we obtained measurements (measured in mm, accurate to 2 decimal places) for head length, head width, mandible length, pronotum length, right tibia length and right femur length for all wētā, and where applicable, ovipositor length for females (see Suppl. Mat. for means and standard deviations).
Behavioural phenotyping
We phenotyped wētā using five assays to gather behavioural data on emergence, cohabitation, activity/exploration, refuge-seeking, and defensive behaviour.
Defensive response
For the defensive assays, we used two (L 24 cm, W 24 cm, H 24 cm) arenas and a glass rod (L 25.5 cm, D 0.6 cm). We cleaned the rods and arenas before and after each test with 70% ethanol spray. We randomly selected one male and one female of each colour morphs at a time and placed them within the acclimation container at the centre of the arena. We left them undisturbed for a five-minute acclimation period. After five minutes, we removed the acclimation container from one of the wētā and used the glass rod to gently poke it on the right abdomen. Wētā were slowly poked until they displayed a defence response (Field Citation2001; Parli et al. Citation2020). Once a defence response was elicited, we stopped the trial and recorded the number of pokes as well as the type of response. We then cleaned the glass rod and repeated the process for the next wētā. Once completed, we tested the wētā again in the same trial order.
Emergence
We recorded emergence behaviour for 1–2 h after dark, on twelve different days over a one-month period. Under red light, we recorded whether wētā in each enclosure had emerged from their dark spaces (1 = emerged, 0 = not emerged).
Cohabitation
We recorded cohabitation behaviour once a week when enclosures were being cleaned. We recorded wētā location within the enclosure as occupying separate refuges (i.e. tiles or flax), the same refuge, or as touching in the same refuge.
Refuge-seeking, activity and exploration
We captured wētā behaviour using a camera (Sony HDR-CX110, frame rate of 60 fps), which we mounted above four arenas (L 24 cm, W 24 cm, H 24 cm) lined with black opaque plastic on the outside, to ensure wētā could not see each other during the trials. We wiped down the arenas with 70% ethanol spray between all phenotyping trials to eliminate any pheromone residue from earlier testing. We also lined the bottom of all the arenas with white paper towels and changed these between trials.
We conducted refuge-seeking trials between 9 and 12 pm with the lights on, using the same video and arena set-up as in the activity trials. We placed an artificial terracotta tile refuge (L 10 cm, W 7.6 cm, H 2.5 cm) against the middle of the bottom wall in all four arenas. We selected four trial wētā at random while ensuring we had one of each morph and sex included in every trial. Before starting, we weighed wētā with an electronic scale to obtain additional morphological data. We then transferred wētā into individual acclimation containers (H 10 cm, D 8.5 cm) covered with black duct tape. Acclimation containers were placed in the middle of their randomly assigned arena with one container per arena. We started the camera recording and left the wētā undisturbed in their acclimation chambers for five minutes. After five minutes elapsed we removed acclimation containers from all arenas and secured lids on the arenas to ensure wētā could not climb out. After removing the acclimation chambers, we left the room for another 20 min. We then returned wētā to their respective containers. removed the paper towels, and wiped down all four arenas with 70% ethanol spray. We placed new paper towels back in the arenas and the process was repeated for four new wētā.
We conducted activity trials under red light only (i.e. during the wētā’s night) and filmed used night mode. Four wētā were transferred into individual acclimation containers and placed in the middle of their respective randomly assigned arenas. We started the recording and left the wētā undisturbed for five minutes to acclimate. After five minutes, we removed the acclimation container and secured the arena lids. We left the room leaving the wētā undisturbed for a further 20 min. Following this period, we returned and transferred wētā back to their containers while also removing the paper towels and cleaning all arenas with 70% ethanol spray.
Refuge seeking, activity and exloration data were extracted using EthoVision XT behavioural quantification software (version 15, Noldus Information Technology). The refuge was added as a hidden zone in EthoVision with two entry/exit zones at both openings of the refuge. We measured refuge-seeking behaviour as latency to enter the refuge after removing the acclimation chamber.
Statistical analysis
All statistical analyses were conducted in R version 4.2.2 (R Core Development Team 2021). We analysed wētā behaviour using mixed effects models in the lme4 package (Bates et al. Citation2015). Wētā ID was a random effect in all models except for the cohabitation data, where ‘tank’ was used as a random effect instead. Relationships between dependent variables (behaviours including activity, refuge-seeking, defensiveness, emergence, and cohabitation) and fixed effects such as morph (melanic vs. yellow), sex (male vs. female), and trial (1 vs. 2) were tested for two and three-way interactions. Using the multimodal inference (MuMIn) package in R (Bartoń Citation2022), we narrowed down the best model for each behaviour based on model averaging with Akaike Information Criterion (). Right femur length was included as a covariate in our models. A principal component analysis done on the morphological measurements showed right hand femur length as explaining 50% of the variance for PC1 and 47% of the variance for PC2. Femur length has also been used as an indicator of body size in other wētā studies, e.g. Koning and Jamieson Citation2001; Jamieson Citation2002; Wehi and Hicks Citation2010. Femur length was significant in the refuge-seeking behaviour model but was non-significant in all other models and was therefore not included in the final models for other behaviours. We wanted to reduce the number of predictors in each model to preserve power, and therefore first examined ontogeny and location for each measure of behaviour using boxplots (see Suppl. Mat.) before excluding them as fixed effects for all behaviours. For assays in which two trials were conducted, we tested for repeatability using the rptR package (Stoffel et al. Citation2017). Activity (R = 0.012, CI = 0, 0.346), refuge-seeking (R = 0.214, CI = 0, 0.51), and defensive behaviour (R = 0, CI = 0, 0.056) assays were not repeatable, so ‘trial’ was therefore included in the models.
Table 1. Model outputs for statistical analyses of defensive response (number of pokes to the right side of the abdomen), emergence, activity (total distance moved) and refuge-seeking (latency to enter) behaviours of Hemideina maori. Morph (yellow or melanic), sex, and trial were included as fixed effects, with wētā ID included as a random effect. Variances, model estimates, test statistics (t)/Z values (Z), and significance levels (P) for these models were returned by either LMERs (linear mixed effects model) or GLMERS (generalised linear mixed effects model) with Poisson or Binomial error structures. Results are based on model selection through multimodal inference, hence not all fixed and interaction effects are presented. Significant values are in bold.
Emergence behaviour was quantified as natural emergence from refuges within the tanks over a 12-day period. We analysed emergence using a generalised linear mixed effects model (GLMM) with binomial error structure. Cohabitation behaviour was quantified as whether or not wētā were found in separate refuges or together over a total of 13 days. A GLMM with binomial structure was attempted for this data, but due to clustering of residuals and problems with overdispersion, a Fisher Exact Test was used instead. Total distance moved (cm) was the chosen variable to represent activity and was analysed using a linear mixed effects model (LMM). We created an exploration index by repeating the same LMM as for activity, but with the standard deviation of the distances moved within each zone instead of the total distance moved. We analysed refuge-seeking behaviour as both latencies to enter a refuge during the trial, and whether or not the wētā entered the refuge at all. Latency to enter the refuge was analysed using an LMM, while whether or not a wētā entered the refuge was analysed using a GLMM with binomial error structure. If the wētā failed to enter the refuge by the end of the fifteen-minute trial, the maximum value was added as their latency (900 s). We quantified defensive behaviour as the number of pokes in the abdomen required for wētā to give a response. This behaviour was analysed using a GLMM with Poisson error structure.
Results
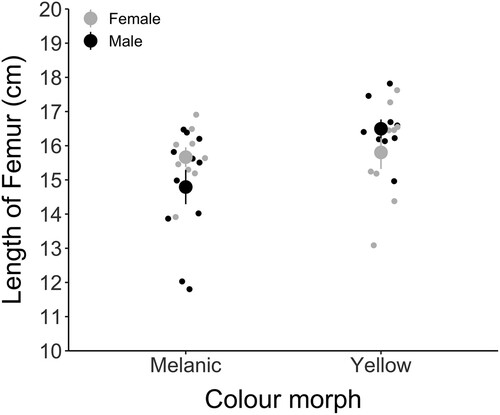
In the collected wētā, yellow Hemideina maori appeared to have longer femurs (16.15 ± 0.195) than their melanic counterparts (15.185 ± 0.228) (), although neither morph (LM: t = 0.23, P = 0.82) nor sex (LM: t = −1.514, P = 0.139) differed significantly in size and there was no interaction between morph and sex (LM: t = 1.872, P = 0.07).
Figure 2. The length of right hind femur (cm) for both melanic (nfemale = 9, nmale = 11) and yellow (nfemale = 9, nmale = 9) Hemideina maori, separated by sex. Means for females (shown in gray) and males (in black) and standard error of means are represented.
Defensive behaviour
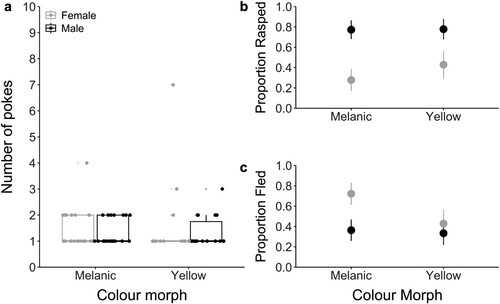
When we imitated a predator in the ‘poke’ test, defensive behaviours did not differ significantly between yellow and melanic morphs (GLMM: Z = 0.165, P = 0.869) or between sexes (GLMM: Z = −0.944, P = 0.345; A; ). Only four wētā took more than two pokes to respond, with the highest number of pokes being seven (mean = 1.444 ± 0.150). Some of the most common responses displayed by the wētā in the defensive trials were fleeing and rasping. For these behaviours, more males rasped than females (Z = 2.134, P = 0.033; B), and more females fled than males (Z = −2.217, P = 0.027; C). However, there was no significant difference between yellow and melanic morphs for both fleeing (Z = −1.602, P = 0.109) and rasping (Z = 0.781, P = 0.435) behaviours.
Figure 3. a) The number of pokes required to elicit a defensive response for melanic (nfemale = 9, nmale = 11) and yellow (nfemale = 7, nmale = 9) Hemideina maori. Medians are represented as horizontal bars, and the lower and upper hinges correspond to the first and third quartiles. The upper and lower whiskers extend to the largest and smallest values within 1.5 IQR (inter-quartile range) from the hinges. b) The proportion of wētā that exhibited rasping (defined as rubbing of femurs against abdomen to make a rasping sound). c) The proportion of wēta that fled (defined as running away from the stimulus). Means for females (shown in gray) and males (in black) and standard error of means are represented.
Emergence and cohabitation behaviour
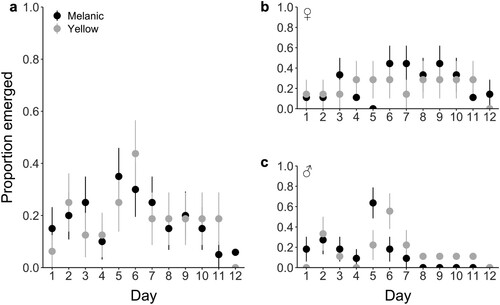
The greatest proportion of wētā emerged on days five and six, although the proportion of emergence did not increase over 0.5 for the entirety of the 12-day period (A). Emergence behaviour between yellow and melanic morphs did not differ significantly (GLMM: Z = 0.003, P = 0.998), although we did find a significant interaction between day and sex (GLMM: Z = −2.804, P = 0.005) which appeared to be driven by fewer males emerging over time than females (B,C). All other interactions were non-significant ().
Figure 4. a) The proportion of Hemideina maori that emerged within their tanks over a 12-day period after initial acclimation. Melanic wētā (nfemale = 9, nmale = 11) are shown in black, and yellow wētā (nfemale = 7, nmale = 9) are shown in gray. b) The proportion of female H. maori that emerged within their tanks, and c) The proportion of male H. maori that emerged within their tanks. Mean and standard error of means are represented.
Cohabitation behaviour also did not differ significantly between yellow and melanic morphs (Fisher Exact Test: P = 0.172). However, yellow wētā did appear to cohabitate more often (70.9% ± 5.5) than their melanic counterparts (60.7% ± 4.0) (Suppl. Mat.).
Activity and refuge-seeking behaviour
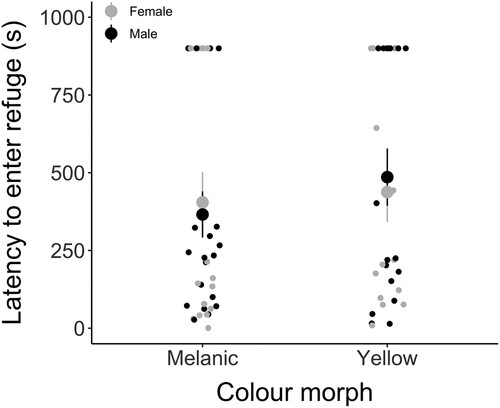
Latencies to enter a refuge did not differ significantly between yellow and melanic morphs (LMM: t = 0.034, P = 0.973; ) and there were no significant interactions between morph, sex or trial (). Size was a significant covariate (P = 0.008). We also ran a binomial model, in which we analysed refuge seeking behaviour as whether each wētā entered the refuge during the trial; this was non-significant between morphs (GLMM: Z = 0.424, P = 0.671).
Figure 5. Refuge-seeking behaviour (the latency to enter a refuge in seconds) for melanic (nfemale = 9, nmale = 11) and yellow (nfemale = 8, nmale = 9) Hemideina maori during 15-minute trials. If wētā failed to enter the refuge during the trial, latency was recorded as 900 s. Means for females (shown in gray) and males (in black) and standard error of means are represented.
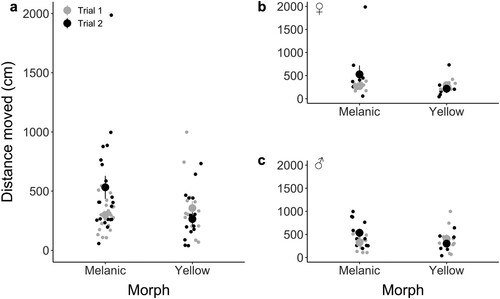
Distance moved (a measure of activity) did not differ significantly between yellow and melanic morphs (LMM: t = 0.202, P = 0.840), though we did detect a significant interaction between trial and morph (LMM: Z = −2.863, P = 0.004), such that melanic wētā were more active during the second trial (; ). For the exploration index (another measure of activity), trial was highly significant (t = −3.695, P < 0.001), but morph was not (t = −0.859, P = 0.391), and there was no significant interaction between trial and morph (t = −0.297, P = 0.767) or trial and sex (t = 1.796, P = 0.073).
Figure 6. a) Total distance moved (cm) by melanic (nfemale = 9, nmale = 11) and yellow (nfemale = 7, nmale = 9) Hemideina maori during 15-minute trials. b) Total distance moved by female wētā and c) total distance moved by male wētā. Plots separate trial one (gray) and two (black). Means and standard error of means are represented.
Discussion
This study found that wētā from melanic and yellow Hemideina maori colour morphs show similar patterns of activity, refuge-seeking, defensive, cohabitation, and emergence behaviours. However, some notable differences between males and females for certain defensive behaviours did emerge. Although the results do not support our initial hypothesis that melanism affects behaviour, the effects of melanism in invertebrates are highly variable across different species (Wittkopp and Beldade Citation2009; San-Jose and Roulin Citation2018). These results do, however, provide insight into anti-predator behaviours and our understanding of melanism in wētā, and provide further evidence that the melanism biosynthesis pathway does not always have pleiotropic effects on the behaviours of insects.
In this study, we did not detect any differences in emergence or refuge seeking behaviour between yellow and melanic H. maori morphs, but there was a significant interaction between day and sex for emergence behaviour – males tended to emerge less frequently over time than females. In contrast, H. crassidens female wētā tended to emerge less often in a similar laboratory setup (Parli et al. Citation2020). Wētā in this study also displayed shorter latencies to enter refuges (<500 s) than in H. crassidens wētā (>1000 s), despite using an identical phenotyping assay (Parli et al. Citation2020). For refuge-seeking behaviour, right femur length, as a proxy for size, was also a significant covariate; that is, larger wētā tended to take longer to enter the refuge. We speculate that this is because larger wētā may be bolder than smaller wētā and less likely to seek immediate shelter. Boldness has been shown to be correlated with size in other animals, including zebrafish and hermit crabs, with varying directions of effect (Kern et al. Citation2016; Briffa and Archer Citation2022).
Activity behaviours also did not differ significantly between morphs and sexes for wētā in this study, but melanic wētā did tend to move further, particularly in trial 2. In Parli et al. (Citation2020), wētā moved an average of 127 ± 25 cm total, whereas in our study wētā moved an average of 369 ± 49 cm total. This implies interspecies differences in Hemideina behaviour, with H. maori appearing to be more active and more skittish than H. crassidens in these studies. We hypothesise this could be due to differences in predation pressure and environment, but further studies are needed to understand the exact causes.
We did not find any evidence that defensive behaviours differ between yellow and melanic H. maori. However, wētā across both morphs and sexes responded to a ‘simulated predator’ probe after one poke on average, which was a much quicker response than in similar studies on H. crassidens where wētā responded on average after 3–6 pokes (Kelly et al. Citation2023), or 3–5 pokes (Parli et al. Citation2020). Interestingly, H. crassidens only fled 20% of the time in Parli et al.’s (Citation2020) study. This differed from these results with H. maori, where up to 80% of the wētā fled. We also found females fled more often than males, who were more likely to display rasping behaviour (). These results suggest that there is intraspecies variation between sexes for some defence behaviours in H. maori, as well as interspecies variation within the Hemideina genus. This provides a valuable insight that Hemideina spp. are variable in predator responses, and we therefore speculate that species may have different requirements when it comes to conservation, and any potential translocations.
Wētā high on the Rock and Pillars Range seem to have low rates of natural predation. Few introduced mammals are present, and there appears to be limited predation from native species such as skinks (Oligosoma spp.), nocturnal geckos (Hoplodactylus spp.) which only co-occur with wētā when their altitudinal limits overlap, and ruru (Ninox novaeseelandiae) (King et al. Citation2003). Because this study was unable to find differences between the anti-predator behaviours of melanic and yellow morphs in H. maori, and because predation pressure may also be limited, it appears unlikely that these colour polymorphisms are driven or maintained by predation, or antipredator behaviour. This contrasts with other studies where phenotypic variation in colour patterning among prey species can decrease the efficiency of predator attacks (Karpestam et al. Citation2016), and predators exploiting polymorphic prey may also have reduced performance in comparison to predators that prey on monomorphic populations (Poulton Citation1890; Bond and Kamil Citation2002). No evidence suggests that wētā are unpalatable, and it would be unusual for the darker morph of a polymorphic species to be distasteful, so aposematism is also highly unlikely (but see Foster et al. Citation2022). Nonetheless, we note that although H. maori are distributed across the South Island (Leisnham and Jamieson Citation2002), we collected wētā from one population only on the Rock and Pillar range. The overall sample size in this study is also relatively low, with ∼10 per morph per sex, but comparable to the previous studies with H. crassidens (Parli et al. Citation2020; Kelly et al. Citation2023). This low sample size could have obscured our ability to detect differences between melanic and yellow morphs, though we have been able to detect subtle differences in behaviour in prior studies (Parli et al. Citation2020; Kelly et al. Citation2023). Further sampling from more populations at a range of elevations, with different predation pressures, will provide a more robust picture of H. maori anti-predator behaviour.
In this study, most yellow wētā were found along Summit Road (elevation 1258–1275 m), and 80% of melanic wētā were recorded and collected from McPhees Rock at a slightly higher elevation (1295 m; Suppl. Material). This aligns with the hypothesis that melanic individuals might be more prevalent at higher elevations because melanic H. maori have reduced cuticular water loss in comparison to yellow morphs (King and Sinclair Citation2015) and might therefore have a selective advantage in desiccating environments (Parkash et al. Citation2008). However, we did not sample at the highest elevation (Summit Rock, 1450 m), and we note that King et al. (Citation2003) found melanic wētā to be at primarily lower elevations in the Rock and Pillar Range, which we did not search. We also found that the wētā we collected were similar in size across morph and sex, although yellow individuals tended to be slightly larger than melanics (). King and Sinclair (Citation2015) also found no difference in size between H. maori males and females, although in a study of sister species H. crassidens, females grew faster and to a larger size, and H. crassidens at higher elevations typically grew to a larger body size than those at lower elevations (Bulgarella et al. Citation2015). Because location also appeared to be non-significant for all behaviours (Suppl. Material), we did not include location as a fixed effect in any of the models.
The lack of repeatability of wētā behaviour in our trials was unexpected (cf. Parli et al. Citation2020). All models returned a very low repeatability index if not zero, and all confidence intervals contained zero, indicating that there were differences in the behaviour of individual wētā between trials. This contrasts with the findings of other studies on Hemideina spp., especially concerning defensive and emergence behaviours (Parli et al. Citation2020). It is unclear what drove the lack of repeatability, suggesting that further research on repeatability in tree wētā behaviour might be useful.
In conclusion, it remains unclear why the stone wētā Hemideina maori retains melanic and yellow morphs across some populations, but not others, and what the drivers of this polymorphism are. Despite anti-predator behaviour emerging as an unlikely driver of colouration differences, in this study we identified some interesting behaviours that differ between the sexes, and between other species in the genus. Although H. maori are currently considered non-threatened (Trewick et al. Citation2022), increasing impacts of climate change have the potential to shift their distributions and expose them to new predation threats. It is therefore valuable to know that melanic and yellow H. maori might respond similarly to predators and conservation efforts could be focused equally across morphs. Additionally, it appears that H. maori might respond differently to predation threats than H. crassidens and other related species, and thus have different conservation requirements. Further research could better establish these differences in predator response behaviours between species, which will provide a much greater understanding of their interactions, and how these ecosystems work. With the wide range of genetic mechanisms driving behaviour-morphology correlations in insects, this study may also be useful in cases where pleiotropic effects of pigmentation and other characteristics are being studied in invertebrate taxa.
Supplemental material
Download MS Word (2.6 MB)Acknowledgements
We thank Nat Lim, Tania King, and Myrene Otis for assistance with collecting the wētā. We also thank the Department of Zoology for animal housing support. LT and HD were supported by Centre for Sustainability Summer Scholarships at the University of Otago. CT was funded by a Te Ngaru Paewhenua Summer Scholarship. PMW was funded by Rutherford Discovery Fellowship LCR-14-001.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data is available at https://doi.org/10.17605/OSF.IO/PMXF4
.Additional information
Funding
References
- Arbuckle K, Speed MP. 2015. Antipredator defenses predict diversification rates. Proceedings of the National Academy of Sciences. 112(44):13597–13602. doi:10.1073/pnas.1509811112.
- Bartoń K. 2022. MuMIn: Multi-model inference [Internet]. [Accessed 2023 February 9]. https://cran.r-project.org/web/packages/MuMIn/index.html.
- Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. Journal of Statistical Software. 67(1):1–48. doi:10.18637/jss.v067.i01.
- Bindu TN, Balakrishnan P, Sudheendrakumar VV, Sajeev TV. 2012. Density-dependent polyphenism and baculovirus resistance in teak defoliator, Hyblaea puera (Cramer). Ecological Entomology. 37(6):536–540. doi:10.1111/j.1365-2311.2012.01389.x.
- Bond AB, Kamil AC. 2002. Visual predators select for crypticity and polymorphism in virtual prey. Nature. 415(6872):609–613. doi:10.1038/415609a.
- Brakefield PM. 2009. Crypsis. In: Encyclopedia of Insects. Elsevier; [Accessed 2023 Feb 3]; p. 236–239. doi:10.1016/B978-0-12-374144-8.00071-0.
- Briffa M, Archer R. 2022. Size specific boldness associated with differences in resource requirements and habitat use: a cross-sectional study in hermit crabs. Current Zoology. zoac049. doi:10.1093/cz/zoac049.
- Brodie ED. 1992. Correlational selection for color pattern and antipredator behavior in the garter snake. Thamnophis ordinoides Evolution. 46:1284–1298. doi:10.1111/j.1558-5646.1992.tb01124.x.
- Bulgarella M, Trewick SA, Godfrey AJR, Sinclair BJ, Morgan-Richards M. 2015. Elevational variation in adult body size and growth rate but not in metabolic rate in the tree wētā Hemideina crassidens. Journal of Insect Physiology. 75:30–38. doi:10.1016/j.jinsphys.2015.02.012.
- Caldwell RL. 2005. An observation of inking behavior protecting adult octopus bocki from predation by green turtle (Chelonia mydas) hatchlings. Pacific Science. 59(1):69–72. doi:10.1353/psc.2005.0004.
- Clause AR, Capaldi EA. 2006. Caudal autotomy and regeneration in lizards. Journal of Experimental Zoology. 305A(12):965–973. doi:10.1002/jez.a.346.
- Contreras-Garduño J, Canales-Lazcano J, Córdoba-Aguilar A. 2006. Wing pigmentation, immune ability, fat reserves and territorial status in males of the rubyspot damselfly, Hetaerina americana. Journal of Ethology. 24(2):165–173. doi:10.1007/s10164-005-0177-z.
- Dubovskiy IM, Whitten MMA, Kryukov VY, Yaroslavtseva ON, Grizanova EV, Greig C, Mukherjee K, Vilcinskas A, Mitkovets PV, Glupov VV, Butt TM. 2013. More than a colour change: insect melanism, disease resistance and fecundity. Proceedings of the Royal Society B. 280(1763):20130584. doi:10.1098/rspb.2013.0584.
- Ducrest A, Keller L, Roulin A. 2008. Pleiotropy in the melanocortin system, coloration and behavioural syndromes. Trends in Ecology & Evolution. 23(9):502–510. doi:10.1016/j.tree.2008.06.001.
- Field LH. 2001. The biology of wētās, king crickets and their allies, 1st ed. UK: CABI Publishing. doi:10.1079/9780851994086.0000.
- Foster BJ, McCulloch GA, Foster Y, Kroos GC, Waters JM. 2022. Ebony underpins Batesian mimicry in an insect melanic polymorphism. bioRxiv, June 16, 2022. doi:10.1101/2022.06.13.495778.
- Hagen SB, Sørlibråten O, Ims RA, Yoccoz NG. 2006. Density-dependent melanism in winter moth larvae (Lepidoptera: Geometridae): A countermeasure against parasitoids? Environmental Entomology. 35(5):1249–1253. doi:10.1093/ee/35.5.1249.
- Harris A. 2018. Fauna of New Zealand 12: pompilidae (insecta: hymenoptera). DSIR; [Accessed 2023 Feb 1]. doi:10.7931/J2/FNZ.12.
- Harris AC. 1988. Cryptic colouration and melanism in the sand-burrowing beetle Chaerodes trachyscelides (Coleoptera: Tenebrionidae). Journal of the Royal Society of New Zealand. 18(3):333–339. doi:10.1080/03036758.1988.10426474.
- Jamieson IG. 2002. The relationship between male head size and harem size in the sexually dimorphic mountain stone weta, Hemideina maori. Ecological Entomology. 27:41–48. doi:10.1046/j.0307-6946.2001.00382.x.
- Karpestam E, Merilaita S, Forsman A. 2016. Colour polymorphism protects prey individuals and populations against predation. Scientific Reports. 6(1):22122. doi:10.1038/srep22122.
- Kelly CD. 2008. Identifying a causal agent of sexual selection on weaponry in an insect. Behavioral Ecology. 19(1):184–192. doi:10.1093/beheco/arm121.
- Kelly M, Wehi PM, Johnson SL. 2023. Behavioural differences in predator aware and predator näive Wellington tree wētā, Hemideina crassidens. Current Opinions in Insect Science. 3:10058. doi:10.1016/j.cris.2023.100058.
- Kern EMA, Robinson D, Gass E, Godwin J, Langerhans RB. 2016. Correlated evolution of personality, morphology and performance. Animal Behaviour. 117:79–86. doi:10.1016/j.anbehav.2016.04.007.
- King KJ. 2015. Phylogeography, physiology and the evolution of melanism in the alpine tree wētā, Hemideina maori [dissertation], University of Otago, Dunedin, New Zealand. http://hdl.handle.net/10523/6547.
- King KJ, Sinclair BJ. 2015. Water loss in tree weta (Hemideina): adaptation to the montane environment and a test of the melanisation–desiccation resistance hypothesis. Journal of Experimental Biology. 218(13):1995–2004. doi:10.1242/jeb.118711.
- King TM, Kennedy M, Wallis GP. 2003. Phylogeographic genetic analysis of the alpine weta, Hemideina maori: evolution of a colour polymorphism and origins of a hybrid zone. Journal of the Royal Society of New Zealand. 33(4):715–729. doi:10.1080/03014223.2003.9517755.
- King TM, Wallis GP, Hamilton SA, Fraser JR. 1996. Identification of a hybrid zone between distinctive colour variants of the alpine weta Hemideina maori (Orthoptera: Stenopelmatidae) on the Rock and Pillar range, southern New Zealand. Molecular Ecology. 5(4):583–587. doi:10.1111/j.1365-294X.1996.tb00350.x.
- Koning JW, Jamieson IG. 2001. Variation in size of male weaponry in a harem-defence polygynous insect, the mountain stone wētā Hemideina maori (Orthoptera: Anostostomatidae). New Zealand Journal of Zoology. 28(1):109–117. doi:10.1080/03014223.2001.9518261.
- Konishi K, Matsumura K, Sakuno W, Miyatake T. 2020. Death feigning as an adaptive anti-predator behaviour: Further evidence for its evolution from artificial selection and natural populations. Journal of Evolutionary Biology. 33(8):1120–1128. doi:10.1111/jeb.13641.
- Leisnham PT, Jamieson IG. 2002. Metapopulation dynamics of a flightless alpine insect Hemideina maori in a naturally fragmented habitat: Metapopulation of a flightless insect. Ecological Entomology. 27(5):574–580. doi:10.1046/j.1365-2311.2002.00452.x.
- Mafli A, Wakamatsu K, Roulin A. 2011. Melanin-based coloration predicts aggressiveness and boldness in captive eastern Hermann’s tortoises. Animal Behaviour. 81(4):859–863. doi:10.1016/j.anbehav.2011.01.025.
- Mark CJ, O’Hanlon JC, Holwell GI. 2022. Camouflage in lichen moths: Field predation experiments and avian vision modelling demonstrate the importance of wing pattern elements and background for survival. Journal of Animal Ecology. 91(12):2358–2369. doi:10.1111/1365-2656.13817.
- Neufeld DS, Leader LP. 1998. Freezing survival by isolated Malpighian tubules of the New Zealand alpine wētā Hemideina maori. Journal of Experimental Biology. 201(2):227–236. doi:10.1242/jeb.201.2.227.
- O’Donnell C, Weston K, Monks J. 2017. Impacts of introduced mammalian predators on New Zealand’s alpine fauna. New Zealand Journal of Ecology. 41(1):1–22. doi:10.20417/nzjecol.41.18.
- Parkash R, Ramniwas S, Rajpurohit S, Sharma V. 2008. Variations in body melanization impact desiccation resistance in Drosophila immigrans from Western Himalayas. Journal of Zoology. 276(2):219–227. doi:10.1111/j.1469-7998.2008.00478.x.
- Parli A, Besson A, Wehi P, Johnson S. 2020. Sub-lethal exposure to a mammalian pesticide bait alters behaviour in an orthopteran. Journal of Insect Conservation. 24(3):535–546. doi:10.1007/s10841-020-00222-6.
- Poulton EB. 1890. The colours of animals, their meaning and use, especially considered in the case of insects. New York: D. Appleton and Company. doi:10.5962/bhl.title.11353.
- Powell EC, Painting CJ, Hickey AJ, Machado G, Holwell GI. 2021. Diet, predators, and defensive behaviors of New Zealand harvestmen (Opiliones: Neopilionidae). The Journal of Arachnology. 49(1):122–140. doi:10.1636/JoA-S-20-002.
- Quesada J, Senar JC. 2007. The role of melanin- and carotenoid-based plumage coloration in nest defence in the Great Tit. Ethology. 113(7):640–647. doi:10.1111/j.1439-0310.2007.01364.x.
- Ruxton GD, Allen WL, Sherratt TN, Speed MP. 2018. Avoiding attack: the evolutionary ecology of crypsis, aposematism, and mimicry, 2nd ed. Oxford: Oxford University Press.
- San-Jose LM, Roulin A. 2018. Toward understanding the repeated occurrence of associations between melanin-based coloration and multiple phenotypes. The American Naturalist. 192(2):111–130. doi:10.1086/698010.
- Schweitzer C, Motreuil S, Dechaume-Moncharmont F-X. 2015. Coloration reflects behavioural types in the convict cichlid, Amatitlania siquia. Animal Behaviour. 105:201–209. doi:10.1016/j.anbehav.2015.04.024.
- Singh S. 2015. Changes in body melanisation and not body size affect mating auccess in Drosophila immigrans. In: Chakravarthy AK, editor. New horizons in insect science: towards sustainable pest management. New Delhi: Springer India; p. 27–38. doi:10.1007/978-81-322-2089-3_3.
- Stevens M, Merilaita S2011. Animal camouflage: mechanisms and function. Cambridge: Cambridge University Press.
- Stoffel MA, Nakagawa S, Schielzeth H. 2017. rptR: repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecology and Evolution. 8(11):1639–1644. doi:10.1111/2041-210X.12797.
- Tibbetts EA, Dale J. 2004. A socially enforced signal of quality in a paper wasp. Nature. 432(7014):218–222. doi:10.1038/nature02949.
- Trewick SA, Hegg D, Morgan-Richards M, Murray T, Watts C, Johns PM, Michel P. 2022. Conservation status of Orthoptera (wētā, crickets and grasshoppers) in Aotearoa New Zealand, 2022. Wellington: Department of Conservation, Te Papa Atawhai.
- van den Brink V, Dolivo V, Falourd X, Dreiss AN, Roulin A. 2012. Melanic color-dependent antipredator behavior strategies in barn owl nestlings. Behavioral Ecology. 23(3):473–480. doi:10.1093/beheco/arr213.
- Wehi PM, Hicks BJ. 2010. Isotopic fractionation in a large herbivorous insect, the Auckland tree weta. Journal of Insect Physiology. 56:1877–1882. doi:10.1016/j.jinsphys.2010.08.005.
- Wehi PM, Nakagawa S, Trewick SA, Morgan-Richards M. 2011. Does predation result in adult sex ratio skew in a sexually dimorphic insect genus?: Sex ratio and predation in weta. Journal of Evolutionary Biology. 24(11):2321–2328. doi:10.1111/j.1420-9101.2011.02366.x.
- Whitman DW, Vincent S. 2008. Large size as an antipredator defense in an insect. Journal of Orthoptera Research. 17(2):353–371. doi:10.1665/1082-6467-17.2.353.
- Wilson GC, Jamieson IG. 2005. Does melanism influence the diet of the Mountain Stone wētā Hemideina maori (Orthoptera: Anostostomatidae)? New Zealand Journal of Ecology. 29(1):149–152.
- Wilson K. 2000. How the locust got its stripes: the evolution of density-dependent aposematism. Trends in Ecology & Evolution. 15(3):88–90. doi:10.1016/S0169-5347(99)01687-0.
- Wilson K, Reeson AF. 1998. Density-dependent prophylaxis: evidence from Lepidoptera-baculovirus interactions? Ecological Entomology. 23(1):100–101. doi:10.1046/j.1365-2311.1998.00107.x.
- Wittkopp PJ, Beldade P. 2009. Development and evolution of insect pigmentation: Genetic mechanisms and the potential consequences of pleiotropy. Seminars in Cell & Developmental Biology. 20(1):65–71. doi:10.1016/j.semcdb.2008.10.002.
- Witz BW. 1990. Antipredator mechanisms in arthropods: A twenty year literature survey. The Florida Entomologist. 73(1):71. doi:10.2307/3495331.

