?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The ectoparasites of introduced rodents in mainland New Zealand forests include several species of cosmopolitan flea that may be important in the population dynamics and future biocontrol of rodents. We describe a 2-rodent, 2-flea system that showed little change over 20 years. Ship rats (Rattus rattus) and house mice (Mus musculus) were snap trapped at fixed sites in the Orongorongo Valley, Wellington, for a study of their population ecology. The fleas Leptopsylla segnis and Nosopsyllus fasciatus were common on mice and rats respectively, and less common on the alternate hosts. Prevalence was described in relation to sex and age of the host, and to season and year. Male mice were more likely than females to carry L. segnis. Prevalence and intensity of infection mostly increased with age of host. Prevalence of both flea species showed modest seasonal variation, with a dip in autumn. Pregnant or lactating female rats and mice were less likely to have fleas than were non-breeding adult females. Prevalence did not vary positively with host density. We detected a slight overall increase in the prevalence of each flea species over the duration of the study. We conclude with some discussion of flea assemblages in New Zealand.
Introduction
Ectoparasites of rodents can be important as vectors or reservoirs of human diseases such as plague. The last recorded case of plague in New Zealand was in 1911 (MacLean Citation1955), and rodents are now mostly excluded from houses, limiting the spread of zoonotic diseases. However, introduced rodents play a significant and destructive role in New Zealand’s natural ecosystems, and their ectoparasites may in future be critical to the spread of both lethal and non-lethal biocontrol agents. A classic parallel case from Australia concerns myxomatosis and the rabbit flea (Spilopsyllus cuniculi (Dale): Pulicidae) (Sobey et al. Citation1973). Understanding the ecology of rodent ectoparasites in New Zealand is therefore potentially useful for predicting the transmission of microscopic pathogens that may have a role in rodent control.
The flea fauna of New Zealand is unusual in consisting largely of fleas that are associated with birds (Smit Citation1979; Pilgrim Citation1980, Citation1991). The mammal fleas are cosmopolitan or Australian species associated with mammals introduced to New Zealand, except for an endemic flea (Porribius pacificus Jordan: Ischnopsyllidae) associated with native species of bat. Host-flea associations have been reported by Tenquist and Charleston (Citation1981, Citation2001). The species of flea on small mammals in various parts of New Zealand have been reported in recent years (King and Moody Citation1982a; Gibson Citation1986; Efford et al. Citation1988; Murphy Citation1989; Roberts Citation1991a; Innes et al. Citation2001). The rodent fleas are from three genera in separate families: Nosopsyllus (Ceratophyllidae), Pygiopsylla (Pygiopsyllidae), and Leptopsylla (Leptopsyllidae).
We conducted a long-term snap-trapping study of rodents (ship rats Rattus rattus L. and house mice Mus musculus L.) in the forest of the Orongorongo Valley. For 20 years all fleas associated with them were collected as part of the autopsy procedures and later identified. Only two species of flea were recorded (Leptopsylla segnis (Schönherr) and Nosopsyllus fasciatus (Bosc.)). The study provided large samples and an opportunity to examine the changes in the flea populations in relation to the changes in the mouse and rat populations (Fitzgerald et al. Citation2004; Efford et al. Citation2006). The only other ectoparasite noted was the sarcoptid burrowing mite Notoedres muris (Megnin) on rats. They produce conspicuous lesions on the ears, tail, and scrotum. We did not attempt to include them in this study.
Materials and methods
Study area
The Orongorongo Valley is a steep-sided valley near Wellington, New Zealand, with a Field Station at 41°21'S, 174°58'E. Campbell (Citation1984) described the climate and vegetation. The valley is largely covered in evergreen temperate rainforest, comprising southern beech (mostly Nothofagus truncata (Colenso)), podocarps (Coniferales, especially Dacrydium cupressinum Lamb. and Prumnopitys ferruginea (D. Don)) and hardwood species (Metrosideros robusta A. Cunn., Melicytus ramiflorus J. R. Forst. & G. Forst., and Elaeocarpus dentatus (J. R. Forst. & G. Forst.)). Silver beech Nothofagus menziesii (Hook. f.) is dominant above 600 m.
The mean summer temperature (December–February) at the Field Station (130 m a.s.l.) is 15.9°C and mean winter temperature (June–August) is 7.5°C; frosts are uncommon. The annual rainfall over 30 years averaged 2370 mm, with monthly averages ranging from about 100 mm in January to 275 mm in July. The silver beech forest is cooler and moister than the lower forest.
Rattus rattus and Mus musculus were the only rodent species present; other small mammals were feral cats (Felis catus L.), the mustelids stoat (Mustela erminea L.) and weasel (M. nivalis L.), hedgehogs (Erinaceus europaeus L.), and brushtail possums (Trichosurus vulpecula (Kerr)). Fleas are seldom found on possums in New Zealand, the only record being of a cat flea (Ctenocephalides felis (Bouché)) (Tenquist and Charleston Citation1981).
Intensive studies in part of the long-term study area provided snapshots of absolute host densities. Between July and December 1977, the density of the mouse population was estimated to decline from 3.3 to 0.55 ha−1 (Fitzgerald et al. Citation1981). Later, in a live trapping study of R. rattus, their density was estimated as being in the range 5–9 ha−1 (Wilson et al. Citation2007).
Sampling
Rats and mice were snap-trapped on a ‘long line’ of 116 sites at 50-m intervals through the forest near the valley floor from August 1971 to May 1998 (Fitzgerald and Karl Citation1979, Figure 2; Efford et al. Citation2006). Southern beech trees were present at 34 sites, designated ‘beech’ sites, and absent at the 82 ‘podocarp’ sites (Fitzgerald et al. Citation2004). Rats and mice were also trapped on another line, of 36 trap sites, in silver beech forest (> 600 m a.s.l.) from November 1973 to August 1978. For 20 years, from the beginning of the study until May 1991, all fleas found during the autopsy procedures were preserved and identified.
At each trap site, one rat snap-trap and one mouse snap-trap were set under a metal cover. Traps were set for three consecutive nights in late February, May, August and November (summer, autumn, winter and spring). Rodents were collected from the traps each morning, sealed individually in plastic bags, and autopsied on the same day. Bodies were placed in an insect-killing jar with ethyl acetate for a few minutes to kill any fleas. Fleas were collected from the jar and by systematically searching the fur and stored in 70% alcohol. Each rodent was measured and weighed, and its reproductive state was determined. The skulls were saved for determining tooth wear.
Fleas were later prepared as microscope slides using the technique of Smit (Citation1954), modified slightly by R.L.C. Pilgrim (pers. comm.). Fleas were identified and their sex determined, following Smit (Citation1979). Slides were lodged in the Pilgrim flea collection in Te Papa Tongarewa, Museum of New Zealand, Wellington.
Mice were assigned to Lidicker’s (Citation1966) toothwear classes (see also Murphy and Nathan Citation2021) and rats to Karnoukhova’s (Citation1972) toothwear classes (see also Innes Citation2005). Toothwear classes represent unequal time intervals. The Lidicker scale may be calibrated approximately for Orongorongo Valley mice as follows (see Fitzgerald et al. Citation2004): class 1 = 0–1 month, 2 = 1–2 months, 3 = 2–4 months, 4 = 4–6 months, 5 = 6–8 months, 6 = 8–10 months, 7 = 10–14 months, and 8 = > 14 months. Calibration of the Karnoukhova scale is more problematic owing to the greater longevity of rats and to variation within the small known-age sample reported by Efford et al. (Citation2006 Appendix 1); tentatively: classes 1 & 2 = 0–2 months, 3 = 3–9 months, 4 = 10–18 months, 5–7 = > 18 months.
Statistical analysis
In our analyses ‘prevalence’ refers to the proportion of hosts carrying at least one flea, ‘intensity’ is the number of fleas per infected host, and ‘abundance’ is the number of fleas per host whether infected or not (Bush et al. Citation1997). Results are given as mean ± SE except as indicated. We used χ2 tests of homogeneity and t-tests to indicate the statistical support for simple comparisons. Binomial confidence limits of proportions were calculated using the Wilson score interval with continuity correction (Newcombe Citation1998). For annual analyses each ‘year’ was defined to include the four samples from November in the preceding calendar year to August in the current calendar year (Fitzgerald et al. Citation2004; Efford et al. Citation2006).
Trend in annual prevalence was assessed by fitting generalised linear models that controlled for the effects of host sex and toothwear age (as a linear predictor on the logit scale). Significance was assessed by a likelihood ratio test comparing a model with additive logit-linear year effect to a model with no year effect.
Aggregation was measured by the size parameter (k) of a negative binomial distribution fitted to the numbers of fleas on individual hosts by maximum likelihood (Venables and Ripley Citation2002). Small values of k imply high aggregation.
The capture rate of the rodent hosts was converted to a linear index of density that allowed for sprung traps and non-target captures (Fitzgerald et al. Citation2004):
where a0 traps were un-sprung, a1 traps were sprung or caught a non-target species, and a2 traps caught the target species. At the low capture rates that we mostly observed, this index is numerically close to the percentage trap success index with ad hoc adjustment for sprung traps (e.g. King Citation1982). We used the median index value of each host species (mice 2.533, rats 3.510) to separate times of ‘low’ and ‘high’ density. We used the function ‘glm.nb’ of Venables and Ripley (Citation2002) to perform a negative binomial regression of flea abundance on host age class and density index, thereby allowing for aggregation of fleas on hosts.
Results
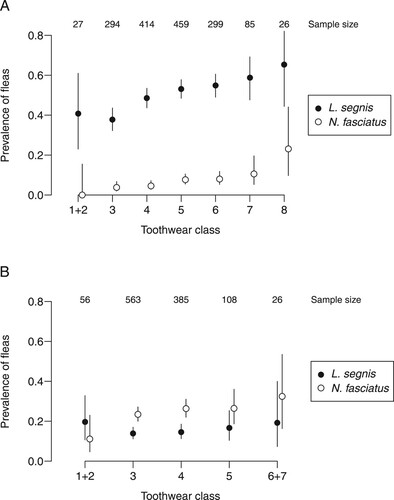
Between August 1971 and May 1991, flea checks were performed on 1150 rats and 1622 mice from the valley-floor long line and, between May 1974 and August 1978, on 15 rats and 155 mice from the higher-altitude trap line in silver beech. On the long line, 49% of mice and 15% of rats carried L. segnis, while 7% of mice and 25% of rats carried N. fasciatus (). The prevalence of fleas on mice and rats from the silver beech trap line was lower but followed a similar pattern (). The following results are for mice and rats from the long line except where it is stated otherwise.
Table 1. Fleas on rats (Rattus rattus) and mice (Mus musculus) snap-trapped in the Orongorongo Valley, Wellington, New Zealand 1971–1991.
Number of fleas on live and dead hosts
A small proportion (5%) of mice and rats were still alive when the snap traps were checked. These were killed and included in the sample. The majority were mice caught by the tail in rat traps. The abundance of fleas on live and dead hosts is summarised in . We used a one-sided t-test of the hypothesis that live hosts carried more fleas than dead hosts, separately for each host and flea species. In no case was there a significant difference (P ≥ 0.2). These data indicate that fleas did not leave their hosts quickly after they died (cf. King and Moody Citation1982a), and that our counts reflect flea loads on live animals.
Table 2. Numbers of fleas on live and dead hosts (1114 dead and 36 live rats, 1525 dead and 97 live mice).
Sex ratio of fleas
The sex ratio of L. segnis was strongly female-biased on both mice (61.2%, N = 3183; X2 = 161, 1 df, P < 0.001) and rats (72.1%, N = 377; X2 = 74, 1 df, P < 0.001). The observed sex ratio of N. fasciatus was also female-biased on both mice (53.5%, N = 129) and rats (62.9%, N = 808); the bias was not statistically significant for mice (X2 = 0.63, 1 df, P = 0.43), but was significant for rats (X2 = 54, 1 df, P < 0.001).
Distribution of number of fleas per host
The number of fleas per host was much more variable than expected from a Poisson distribution (i.e. variance much greater than mean) in all cases except the small sample of N. fasciatus from mice in silver beech (). Aggregation of fleas measured by the negative binomial parameter k was towards the high end of the range tabulated by Shaw et al. (Citation1998): a large proportion of the flea population was concentrated on a few host individuals (see also Krasnov Citation2008, p. 328).
Co-occurrence of flea species
The prevalence of fleas of either species was 51.3% for mice (N = 1622) and 35.2% for rats (N = 1151). Host individuals with fleas of one species were more likely to be infested with the other species (chi-squared test for homogeneity of contingency tables: mice X2 = 19.1, 1 df, P < 0.001; rats X2 = 19.8, 1 df, P < 0.001). This effect is explained, at least in part, by correlated effects on the two flea species of host sex and age, and seasonal variation, as we document below.
The effects of sex and age of host
Male mice were about 27% more likely to carry L. segnis fleas than were female mice (). The observed prevalence of L. segnis was about 22% greater among male than female rats, and lack of significance may merely be due to the lower overall prevalence of L. segnis in that species. The prevalence of N. fasciatus on mice, and of either flea species on rats, did not differ significantly with sex of host ().
Table 3. Sex differences in prevalence of fleas on mice and rats. 14 mice and 6 rats were not sexed. P is significance level of test for sex difference in prevalence.
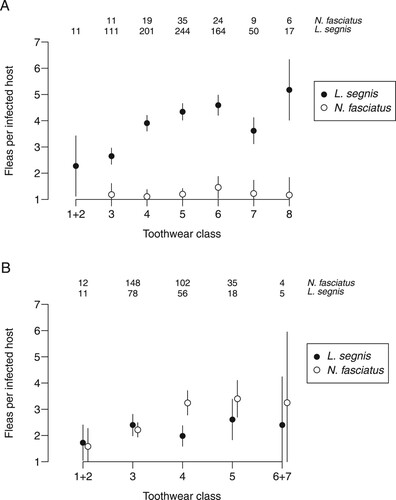
Prevalence trended upwards with age for each flea/host combination except for L. segnis on rats (). This was also true for the number of fleas per infected host with respect to L. segnis on mice and N. fasciatus on rats ().
Figure 1. Variation with age in the prevalence of fleas on mice A, and rats B. Bars indicate 95% binomial confidence intervals. Toothwear classes differ between mice and rats; 4 mice in toothwear class 1, and 9 rats in each of toothwear classes 1 and 7, were lumped with the adjacent class for plotting.
Effect of season and breeding status of host
The prevalence of both flea species was lowest in the autumn and highest in the spring or summer (). Breeding of rodents was largely restricted to spring and summer (Fitzgerald et al. Citation2004; Efford et al. Citation2006). We examined the breeding-season prevalence of fleas on subclasses of adult rodents (potentially mature mice in toothwear class 4 and above, and rats in toothwear class 3 and above). Breeding female mice and rats were less likely to carry L. segnis than non-breeding females (). No significant difference was detected with N. fasciatus, although the trend was similar ().
Table 4. Seasonal variation in prevalence of fleas on mice and rats. Samples were collected in February (summer), May (autumn), August (winter) and November (spring). Sample size N is the number of host individuals.
Table 5. Prevalence of fleas on mice and rats in the breeding season (spring and summer) in relation to sex and breeding status of the host.
Spatial variation in prevalence
The prevalence of L. segnis on mice was greater on the low-elevation long line than the high-elevation silver beech line (). There was also evidence for variation along the long line itself, with higher prevalence at ‘beech’ sites ().
Table 6. Prevalence of fleas on mice and rats caught at 82 ‘podocarp’ and 34 ‘beech’ trap sites on the long line (see text).
Annual variation in prevalence
Host density varied substantially over the 20-year sampling period. Mouse density showed irregular multiannual fluctuations and average rat density approximately doubled between the early and later years (). Too few hosts were trapped in some seasons to determine the prevalence of fleas, and we therefore plotted prevalence aggregated by year (). The prevalence of each flea species showed a weak tendency to increase over time on both hosts (likelihood ratio tests Appendix 1).
Figure 3. Annual variation 1972–1991 in the proportion of A, mice and B, rats with fleas in relation to a quarterly trap-catch index of the host population. Solid symbols Leptopsylla segnis, open symbols Nosopsyllus fasciatus. 95% binomial confidence intervals. N is the number of the host species sampled in each year. Analysis year runs from previous November to September. Breaks in the population index on the log scale indicate seasons in which no host was trapped. Dashed line separates nominal ‘low’ and ‘high’ values of the population index. Shaded band indicates 95% prediction interval for prevalence of dominant flea species (L. segnis on mice and N. fasciatus on rats).
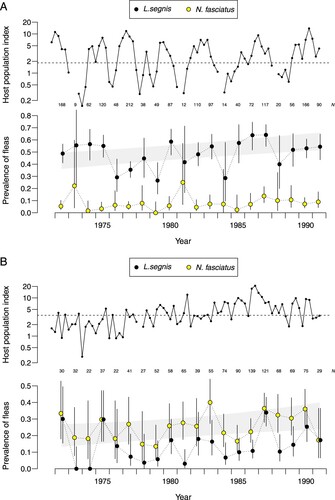
Samples from times of low and high host density did not differ significantly in flea prevalence (P > 0.15) except for L. segnis on mice for which there was a negative relationship (56.4% low, 46.7% high; P = 0.001). Periods of high host density had a preponderance of young hosts (Fitzgerald et al. Citation2004), so the density effect was possibly an artefact of increasing flea prevalence with age of host (). We performed a negative binomial regression of per capita L. segnis abundance on mouse age class and found that adding the host density index as a continuous predictor did not improve the model (likelihood ratio test X2 = 1.7, 1 df, P = 0.2; details in Appendix 2).
There was weak evidence that annual variation in the prevalence of L. segnis on mice was linearly correlated with that on rats (r = 0.37, P = 0.1). No correlation was apparent for N. fasciatus (r = 0.18, P = 0.4).
Discussion
We found only two species of flea on rodents in forest of the Orongorongo Valley, Leptopsylla segnis and Nosopsyllus fasciatus. In this ‘two rodent – two flea’ system there was a clear predominance of L. segnis on mice and of N. fasciatus on rats. The life histories of these two fleas are very different: L. segnis is ‘semi-sessile’, often referred to as semi-sedentary, remaining attached to the fur of the host for long periods, whereas N. fasciatus leaves the host after feeding, to conduct much more of its life cycle in litter or nesting material, as is more common among fleas (Krampitz Citation1980; see also Cole and Koepke in Gibson Citation1986; and Krasnov et al. Citation2004).
The majority of mice and more than a third of rats carried fleas. Prevalence remained high through large fluctuations in host density () and despite the low absolute density of hosts. Fleas can suppress the survival of rodent hosts in the laboratory (e.g. Devevey and Christie Citation2009). High flea prevalence in the wild therefore has the potential to affect host population dynamics, even without the potential for disease transmission.
Influence of host sex, age and season on flea infestation
Both season and host age (indexed by toothwear) had strong effects on prevalence. The two predictors are correlated because rodent breeding was seasonal (Fitzgerald et al. Citation2004; Efford et al. Citation2006). Post-breeding host populations in autumn (May) are dominated by recently recruited rats and mice with low flea prevalence (). The intuitive explanation is that each host accumulates fleas throughout its life, and having acquired fleas is unlikely to become flea-free, especially as habitual refuges and nest sites become infested.
We found a somewhat greater prevalence of fleas on male hosts than on female hosts, but the difference was pronounced only for L. segnis on mice (). Male-biased parasitism is widespread (Krasnov et al. Citation2012). The larger home ranges and greater activity of male mice may be a sufficient explanation (e.g. Fitzgerald et al. Citation1981).
Spatial variation
The prevalence of the dominant flea species on each host along the low elevation long line was greater at ‘beech’ trap sites than at ‘podocarp’ sites (). Mice from the high elevation silver beech trap line carried relatively few fleas (). Long-line beech sites, mostly with Nothofagus truncata, tended to be on drier slopes, but also showed a less complex forest structure than ‘podocarp’ sites (Campbell Citation1984). Differences in flea abundance may therefore be either a direct response to environmental conditions or a reflection of host behaviour in the differing habitats.
Sex ratio of fleas
Female fleas predominate in samples from their hosts in New Zealand (). A female bias is common in fleas (Krasnov et al. Citation2008, p. 93), although the sex ratio of L. segnis on M. musculus in California was only slightly female-biased (51% of 251 fleas; Linsdale and Davis Citation1956). Despite the substantial behavioural differences between L. segnis and N. fasciatus (Krampitz Citation1980), the overall sex ratios of fleas residing on the rodent hosts were similar (62.4% and 61.6% respectively in our study). Gibson (Citation1986) noted a male bias in 24 N. fasciatus collected from rat nests, but collections from host burrows are rarely male-biased (Krasnov et al. Citation2008, Table 7.2).
Table 7. Sex ratio of fleas on mice and rats in New Zealand (samples of 10 or more individuals).
Flea assemblages on rodent hosts in New Zealand
The lack of any other rodent flea species on the rodents of the Orongorongo Valley is unexplained; the rodent fleas Pygiopsylla hoplia Jordan & Rothschild, P. phiola Smit and Nosopsyllus londiniensis (Rothschild) have all been recorded within 20 km of the study area – P. hoplia in Stokes Valley, P. phiola at Lower Hutt and N. londiniensis in Stokes Valley and Belmont (Smit Citation1979; Pilgrim Collection Te Papa Tongarewa).
King and Moody (Citation1982a) reported rather similar rodent–flea relationships in Craigieburn Forest Park and the Eglinton and Hollyford Valleys in Fiordland National Park. At Craigieburn, of 97 mouse carcasses searched, 18 had fleas (43 L. segnis and one N. fasciatus). No rats were trapped there, but of 164 stoats, 12 had fleas (22 N. fasciatus and five L. segnis). However, in Fiordland National Park none of 480 mice, 20 rats and 368 stoats had L. segnis, while N. fasciatus was present on all host species. A possible explanation for the absence of L. segnis there may be provided by Krampitz (Citation1980, p. 377) who states that L. segnis ‘infests only synanthropic murines, and then only in restricted areas. Like its most important host, the house mouse, L. segnis is well adapted for residence in the drier parts of the world’ and ‘laboratory colonies do best in relatively low levels of humidity’. Average rainfall was 4250 mm in the Hollyford Valley and 2300 mm in the Eglinton Valley (King and Moody Citation1982b). However, the other New Zealand localities could barely be considered dry (Craigieburn 1450 mm (King and Moody Citation1982b), Pureora 1829 mm (King et al. Citation1996), and Orongorongo Valley 2370 mm (Campbell Citation1984)).
A three rodent – three flea system was recorded in Pureora Forest Park, central North Island, where an Australian rodent flea Pygiopsylla hoplia was present in addition to L. segnis and N. fasciatus (Innes et al. Citation2001). R. rattus had all three fleas, R. norvegicus had N. fasciatus and Pygiopsylla hoplia and M. musculus had L. segnis and N. fasciatus.
N. fasciatus is the most widespread flea of rodents in New Zealand, and it is also recorded on almost all the other species of small mammal in New Zealand – hedgehog, rabbit, cat, dog, stoat, ferret and weasel (Smit Citation1979). It was the predominant flea on stoats from New Zealand National Parks: 181 stoats yielded 670 rodent fleas; 99% were N. fasciatus, and the remainder were L. segnis (King and Moody Citation1982a). They suggested that stoats acquire N. fasciatus by using rat dens or when preying on rats. However, as Haddow et al. (Citation1983, pp. 118–119) noted, N. fasciatus ‘may parasitise virtually any host with which it comes in contact, whether rodent … insectivores, carnivores, man or birds’. Their list of host records from specimens in the Rothschild Collection, British Museum includes many species of rodent, especially murids, but also ‘birds like doves, swallows, thrushes and wrens’. The list of avian hosts of N. fasciatus in New Zealand is probably even more eclectic, including a dead albatross on Campbell Island, Apteryx australis Shaw, Columba livia Gmelin, Rhipidura fuliginosa (Sparrman), Leucocarbo colensoi (Buller), Gallirallus australis (Sparrman), Strigops habroptila G. R. Gray, and Sturnus vulgaris L. (Smit Citation1964, Citation1965, Citation1979; Pilgrim Collection, Te Papa Tongarewa Museum of New Zealand).
Comments on the flea fauna of offshore and outlying islands
In our two-rodent system, there was a clear predominance of L. segnis on mice and N. fasciatus on rats. This raises the possibility that fleas merely ‘spill over’ to the alternate host, but there is evidence from islands with restricted host faunas that N. fasciatus persists on mice in the absence of rats (Mana Island from 1977–1978 (Efford et al. Citation1988) to 1989 (Fitzgerald and Guo Citation1989), Allports Island (Murphy unpubl. in Murphy and Pickard Citation1990, p. 241), and Antipodes Island (Smit Citation1979, Pilgrim Collection Te Papa Tongarewa Museum of New Zealand)). The only evidence of which we are aware that a population of L. segnis may persist on rats in the absence of mice, comes from mouse-free islands in northern New Zealand – Korapuki Island (Smit Citation1979), where there was only Rattus exulans (Peale) (Towns and Broome Citation2003), Motukahaua (Gibson Citation1986) where there was only R. rattus (Innes Citation2005), and Great Mercury Island where there were both R. rattus and R. exulans (BMF pers. obs.).
R. exulans presumably arrived in New Zealand without the cosmopolitan rodent fleas: those fleas are not known from the eastern Pacific, although the data are sparse (Gibson Citation1986; Roberts Citation1991a, Citation1991b). Roberts (Citation1991c) advanced the hypothesis that the fleas of Rattus exulans on New Zealand islands where it is now the only rodent host had originally arrived with R. rattus, R. norvegicus or M. musculus that subsequently died out. However, spontaneous extinction of these cosmopolitan rodents has not been documented and, given the frequency with which N. fasciatus has been found on avian hosts (references above), it seems more likely to us that it hitched a ride on a bird. Starlings and gulls that nest or roost on small offshore islands would be candidates. Roberts’ hypothesis may apply in the case of L. segnis on Korapuki, or the fleas may have transferred to R. exulans from another source. However, the hypothesis may be difficult to test in future as rodents are eradicated from more and more islands to conserve the endemic fauna.
Appendices
Download MS Word (15.7 KB)Acknowledgements
We are indebted to the late Prof. R.L.C. Pilgrim for guidance on flea preparation and identification. We thank Te Papa Tongarewa Museum of New Zealand for access to the Pilgrim Siphonaptera Collection. We thank two reviewers for their comments.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data analysed in this paper are openly available in Zenodo at https://doi.org/10.5281/zenodo.8151598.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
References
- Bush AO, Lafferty KD, Lotz JM, Shostak AW. 1997. Parasitology meets ecology on its own terms: Margolis et al. revisited. The Journal of Parasitology. 83:575–583. doi:10.2307/3284227.
- Campbell DJ. 1984. The vascular flora of the DSIR study area lower Orongorongo Valley, Wellington, New Zealand. New Zealand Journal of Botany. 22:223–270. doi:10.1080/0028825X.1984.10425254.
- Devevey G, Christie P. 2009. Flea infestation reduces the life span of the common vole. Parasitology. 136:1351–1355. doi:10.1017/S0031182009990746.
- Efford MG, Fitzgerald BM, Karl BJ, Berben PH. 2006. Population dynamics of the ship rat Rattus rattus L. in the Orongorongo Valley, New Zealand. New Zealand Journal of Zoology. 33:273–297. doi:10.1080/03014223.2006.9518457.
- Efford MG, Karl BJ, Moller H. 1988. Population ecology of Mus musculus on Mana Island, New Zealand. Journal of Zoology. 216:539–563. doi:10.1111/j.1469-7998.1988.tb02450.x.
- Fitzgerald BM, Efford MG, Karl BJ. 2004. Breeding of house mice and the mast seeding of southern beeches in the Orongorongo Valley, New Zealand. New Zealand Journal of Zoology. 31:167–184. doi:10.1080/03014223.2004.9518370.
- Fitzgerald BM, Guo C. 1989. Notes on mice (Mus musculus) on Mana Island, May 1989. Unpublished report. Ecology Division Report No. 14. Department of Scientific and Industrial Research, Lower Hutt.
- Fitzgerald BM, Karl BJ. 1979. Foods of feral house cats (Felis catus L.) in forest of the Orongorongo Valley, Wellington. New Zealand Journal of Zoology. 6:107–126. doi:10.1080/03014223.1979.10428353.
- Fitzgerald BM, Karl BJ, Moller H. 1981. Spatial organization and ecology of a sparse population of house mice (Mus musculus) in a New Zealand forest. The Journal of Animal Ecology. 50:489–518. doi:10.2307/4070.
- Gibson RN. 1986. Some ectoparasites on rodents in New Zealand. I. Fleas (Insecta: Siphonaptera). Mauri Ora. 13:81–92.
- Haddow JF, Traub R, Rothschild M. 1983. Distribution of Ceratophyllid fleas and notes on their hosts. In: Traub R, Rothschild M, Haddow JF, editors. The Rothschild collection of fleas. The Ceratophyllidae: key to the genera and host relationships. Cambridge: M. Rothschild and R. Traub; p. 42–163.
- Innes JG. 2005. Ship rat. In: King CM, editor. The handbook of New Zealand mammals. 2nd ed. Melbourne: Oxford University Press; p. 187–203.
- Innes JG, King CM, Flux M, Kimberley MO. 2001. Population biology of the ship rat and Norway rat in Pureora Forest Park, 1983–87. New Zealand Journal of Zoology. 28:57–78. doi:10.1080/03014223.2001.9518257.
- Karnoukhova NG. 1972. Age determination of brown and black rats. Soviet Journal of Ecology. 2:144–147.
- King CM. 1982. Age structure and reproduction in feral New Zealand populations of the house mouse (Mus musculus), in relation to seedfall of southern beech. New Zealand Journal of Zoology. 9:467–480. doi:10.1080/03014223.1982.10423879.
- King CM, Innes JG, Flux M, Kimberley MO, Leathwick JR, Williams DS. 1996. Distribution and abundance of small mammals in relation to habitat in Pureora Forest Park. New Zealand Journal of Ecology. 20:215–240.
- King CM, Moody JE. 1982a. The biology of the stoat (Mustela erminea) in the National Parks of New Zealand VII. Fleas. New Zealand Journal of Zoology. 9:141–144. doi:10.1080/03014223.1982.10423843.
- King CM, Moody JE. 1982b. The biology of the stoat (Mustela erminea) in the National Parks of New Zealand I. General introduction. New Zealand Journal of Zoology. 9:49–55. doi:10.1080/03014223.1982.10423837.
- Krampitz HE. 1980. Host preference, sessility and mating behaviour of Leptopsylla segnis reared in captivity. In: Traub R, Starcke H, editors. Fleas. Proceedings of the International Conference on Fleas. Rotterdam: A.A. Balkema; p. 371–378.
- Krasnov BR. 2008. Functional and evolutionary ecology of fleas. A model for ecological parasitology. Cambridge (UK): Cambridge University Press.
- Krasnov BR, Bordes F, Khokhlova IS, Morand S. 2012. Gender-biased parasitism in small mammals: patterns, mechanisms, consequences. Mammalia. 76:1–13. doi:10.1515/mammalia-2011-0108.
- Krasnov BR, Khokhlova IS, Shenbrot GI. 2004. Sampling fleas: the reliability of host infestation data. Medical and Veterinary Entomology. 18:232–240. doi:10.1111/j.0269-283X.2004.00500.x.
- Krasnov BR, Shenbrot GL, Khokhlova IS, Hawlena H, Degen AA. 2008. Sex ratio in flea infrapopulations: number of fleas, host gender and host age do not have an effect. Parasitology. 135:1133–1141. doi:10.1017/S0031182008004551.
- Lidicker WZ Jr. 1966. Ecological observations on a feral house mouse population declining to extinction. Ecological Monographs. 36:27–50. doi:10.2307/1948487.
- Linsdale JM, Davis BS. 1956. Taxonomic appraisal and occurrence of fleas at the Hastings Reservation in Central California. University of California Publications in Zoology. 54:293–370.
- MacLean FS. 1955. The history of plague in New Zealand. New Zealand Medical Journal. 54(300):131–143.
- Murphy EC. 1989. The demography of an island and mainland population of house mice in the Marlborough Sounds, New Zealand. PhD thesis. Wellington: Victoria University.
- Murphy EC, Nathan HW. 2021. The house mouse. In: King CM, Forsyth DM, editors. The handbook of New Zealand mammals. 3rd ed. Melbourne: CSIRO Publishing; p. 161–240.
- Murphy EC, Pickard CR. 1990. The house mouse. In: King CM, editor. The handbook of New Zealand mammals. 1st ed. Auckland: Oxford University Press; p. 225–242.
- Newcombe RG. 1998. Two-sided confidence intervals for the single proportion: comparison of seven methods. Statistics in Medicine. 17:857–872. doi:10.1002/(SICI)1097-0258(19980430)17:8<857::AID-SIM777>3.0.CO;2-E.
- Pilgrim RLC. 1980. The New Zealand flea fauna. In: Traub R, Starcke H, editors. Fleas. Proceedings of the International Conference on Fleas. Rotterdam: A.A. Balkema; p. 173–184.
- Pilgrim RLC. 1991. Fleas. New Zealand Entomologist. 14:1–9. doi:10.1080/00779962.1991.9722604.
- Roberts M. 1991a. The parasites of the Polynesian rat within and beyond New Zealand. International Journal for Parasitology. 21:777–783. doi:10.1016/0020-7519(91)90145-W.
- Roberts M. 1991b. The parasites of the Polynesian rat: biogeography and origins of the New Zealand parasite fauna. International Journal for Parasitology. 21:785–793. doi:10.1016/0020-7519(91)90146-X.
- Roberts M. 1991c. Parasitological evidence for the presence of other rodent species on “kiore only” islands. Journal of the Royal Society of New Zealand. 21:349–356. doi:10.1080/03036758.1991.10420832.
- Shaw DJ, Grenfell BT, Dobson AP. 1998. Patterns of macroparasite aggregation in wildlife host populations. Parasitology. 117:597–610. doi:10.1017/S0031182098003448.
- Smit FGAM. 1954. Identification of fleas. In: Pollitzer R, editor. Plague: World Health Organization. Monograph Series No. 22, Annex 2; p. 648–682.
- Smit FGAM. 1964. Insects of Campbell Island. Siphonaptera. Pacific Insects Monograph. 7:330–334.
- Smit FGAM. 1965. Siphonaptera of New Zealand. Transactions of the Royal Society of New Zealand, Zoology. 7:1–50.
- Smit FGAM. 1979. The fleas of New Zealand (Siphonaptera). Journal of the Royal Society of New Zealand. 9:143–232. doi:10.1080/03036758.1979.10419413.
- Sobey WR, Adama KM, Johnston GC, Gould LR, Simpson KNG, Keith K. 1973. Macquarie Island: the introduction of the European rabbit flea Spilopsyllus cuniculi (Dale) as a possible vector for myxomatosis. Journal of Hygiene. 71:299–308. doi:10.1017/S0022172400022762.
- Tenquist JD, Charleston WAG. 1981. An annotated checklist of ectoparasites of terrestrial mammals in New Zealand. Journal of the Royal Society of New Zealand. 11:257–285. doi:10.1080/03036758.1981.10421840.
- Tenquist JD, Charleston WAG. 2001. A revision of the annotated checklist of ectoparasites of terrestrial mammals in New Zealand. Journal of the Royal Society of New Zealand. 31:481–542. doi:10.1080/03014223.2001.9517666.
- Towns DR, Broome KG. 2003. From small Maria to massive Campbell: forty years of rat eradications from New Zealand islands. New Zealand Journal of Zoology. 30:377–398. doi:10.1080/03014223.2003.9518348.
- Venables WN, Ripley BD. 2002. Modern applied statistics with S. 4th ed. New York (NY): Springer.
- Wilson DJ, Efford MG, Brown SJ, Williamson JF, McElrea GJ. 2007. Estimating density of ship rats in New Zealand forests by capture–mark–recapture trapping. New Zealand Journal of Ecology. 31:47–59.