Abstract
Studies of the biogeography of Cladocera in New Zealand lakes are rare and only a few species of littoral chydorid Cladocera have been documented. Consequently, a number of issues relating to their taxonomy and systematics have yet to be resolved. The majority of chydorid Cladocera in the northern hemisphere have been given new names after a detailed investigation of their morphology and genetic variability. However, knowledge of their inter-hemispheric distribution is poor. It is likely that many species of chydorid Cladocera in the southern hemisphere, including in the New Zealand lakes, will show significant genetic variability from their northern hemisphere counterparts, although this has not yet been investigated. Here we review the taxonomy and biogeography of the New Zealand chydorid Cladocera. This is important when we consider the limited knowledge of New Zealand cladocerans and the consequent difficulties encountered by freshwater ecologists and paleolimnologists when using them to identify rapid environmental changes in the southern hemisphere.
Introduction
New Zealand has an extensive network of freshwater systems comprising streams, braided and meandering rivers, mountain and lowland lakes and a wide range of wetland complexes across the coastal margins that embodies a unique ecosystem and biodiversity in the southern hemisphere. However, compared to their terrestrial counterparts, freshwater ecosystems in New Zealand have been relatively under-studied, with the focus on vertebrate fish to date (McDowall Citation1990). The macro- and micro-invertebrates, including the cladocerans, have received a very little attention so the biogeography and taxonomy of these organisms are poorly understood in New Zealand. The poor state of cladoceran taxonomy in New Zealand has made it difficult to apply them to understanding lacustrine ecosystems and the records of global environmental change they contain.
Chydorids are a distinct group of organisms within the family Chydoridae. They span a wide range of habitats including littoral mud, submerged vegetation, sandy substrata and open lake environments (Smirnov Citation1971; Whiteside Citation1974). They feed at the substrate–water interface where they obtain food by scraping algae and detritus from surfaces over which they crawl or from sediments in which they burrow (Fryer Citation1974; Frey Citation1976; Whiteside & Swindoll Citation1988). Many taxa within the family Chydoridae are important indicators for temperature, lake water pH and nutrient dynamics (Whiteside & Swindoll Citation1988). The taxa differ greatly with seasons and habitat types (Whiteside Citation1974; Whiteside et al. Citation1978). Taxa such as Graptoleberis testudinaria Fischer, 1848 and Alona affinis Leydig, 1860 prefer plant macrophytes submerged, while other taxon such as Monospilus dispar Sars, 1862 prefers sandy substrata (Dole-Olivier et al. Citation2000).
A strong response of chydorid assemblages to environmental gradients such as surface water temperatures and total phosphorus (TP) in European mountain lakes was reported by Lotter et al. (Citation1997). In Europe, relatively warm-climate-preferring taxa belonging to small Alona groups (e.g. Alona guttata Sars, 1862) were predominantly recorded from the low-altitude temperate regions (Korhola Citation1999), while the cold-climate-preferring taxa such as Acroperus harpae Baird, 1836 and Alona affinis Leydig, 1860 predominantly occurred in sub-arctic and high-altitude sites (Hofmann Citation2000). Temperature also plays a profound role in the life history of these cladocerans (Nevalainen & Luoto Citation2010). Adult females produce a large number of parthenogenetic females during spring (Frey Citation1982a). However, under adverse climatic conditions (e.g. a dry period) gamogenesis occurs when females produce haploid reproductive or resting eggs (Frey Citation1982a).
Recently, species-specific temperature optima and the tolerance range of chydorid Cladocera has been investigated in Europe for control of gamogenesis. Using a weighted averaging (WA) approach, Alonella excisa Fischer, 1854 and Graptoleberis testudinaria Fisher, 1848 showed increased temperature optima ranges compared with Alonella nana Baird, 1850 and Chydorus sphaericus Müller, 1776 for gamogenesis (Nevalainen & Luoto Citation2010). Depending on climate, weather conditions and topography such as mountains, drylands and wetlands the resting eggs are transported widely via rivers, lake systems and even by birds, mammals and insects and by wind (Maguire Citation1963; Figuerola & Green Citation2002). Consequently, those species which were able to adapt the new environment over million years may have established their populations widely across hemispheres.
Until recently, the New Zealand chydorid Cladocera had been considered to be ubiquitous species based on their morphological resemblance to their northern hemisphere counterparts (Frey Citation1982b ,Citation1987). However, Korovchinsky (Citation2006) argued that most chydorid Cladocera described previously from New Zealand lakes should not be considered as cosmopolitan species. As a result of confusion that exists in the taxonomy and biogeography of these organisms, the use of chydorid cladocerans as proxy indicators of change in New Zealand lakes has faced difficulties.
The geological history of New Zealand could have played a major role in speciation of chydorid Cladocera, thereby reducing the cosmopolitanism. The influence of continental break-up, creation of oceanic barriers for transport of resting eggs as well as rapid tectonic movements in the region should have been the major factors in evolution of cladocerans in New Zealand and the increased tendency to endemism due to speciation (Frey Citation1995).
At present, only 40–45% of cladoceran species are identified confidently, as taxonomic issues have yet to be resolved with the family Chydoridae (Forrò et al. 2008). Several revisions to the taxonomy and systematics of the chydorid Cladocera have been made (Frey Citation1987; Korovchinsky Citation1996 ,Citation1997; Kotov Citation2009) based on analyses of both modern (Sinev Citation2004) and fossil (Frey Citation1987) cladoceran morphological variability in body parts and ontogenic change (Sacherova & Hebert Citation2003). However, pertinent taxonomic studies in New Zealand lakes are few. The present review will contribute to our understanding of the status of the taxonomy and biogeography of the chydorid Cladocera in New Zealand. This information is crucial for their application to studies of local and regional environmental change over varying time scales.
Methods of collecting intact and fossil chydorid Cladocera
Modern and fossil chydorid Cladocera need to be collected from lakes and reservoirs using a variety of methods for biogeographical and taxonomic investigations. Whiteside (Citation1974) used Tuffy balls (plastic mesh balls) manufactured for scrubbing dishes and placed them in submerged vegetation. Once the balls were sufficiently colonized by algae and bacteria (ca 5 days), a wide range of chydorid species used these balls as their food-habitats. Hann (Citation1995) used a downing box sampler, a rigid plastic enclosure, for sampling these biota. The organisms were collected by lowering the sampler into the water and cutting the stems of macrophytes containing the chydorids. Funnel-shaped nets or sweep nets and pattern samplers, usually with 100 µm size, have also been used frequently for sampling littoral chydorids (e.g. Whiteside Citation1974; Kattel et al. Citation2006; Nevalainen & Luoto Citation2010).
The taxonomy of recent and ancient chydorid cladocerans improved significantly once the remains of cladocerans in lake sediment surface and core samples began to be analysed (Frey Citation1960a). Surface and long sediment cores are collected using a variety of techniques. A simple gravity-operated sampling device has been commonly used for recovery of lacustrine sediments (Glew & Last Citation2001). Following the subsampling of the sediment core, a few grams (ca 1–3 g) of sediments are treated with 10% KOH solution with ca 200 mL water. The solution is placed on a hotplate at 60°C for at least three-quarters of an hour. The treated sample is then cleaned thoroughly before being prepared for a high-resolution slide for taxonomic analysis. The chydorid body parts such as head shield, post abdomen, carapace, ephippium and post-abdominal claws are then examined under a microscope at 100× and 400× magnification. Based on morphological characters of the body parts, the taxonomy of chydorids is identified (Frey Citation1986; Korhola & Rautio Citation2001; Kattel & Augustinus Citation2010).
However, recently, DNA analysis of chydorid Cladocera has become more important since the accuracy of the taxonomy of these animals has increased significantly using these analyses (Sacherova & Hebert Citation2003). These molecular studies suggest that there should be two to four times more cladoceran species than currently documented worldwide (Adamowicz & Purvis Citation2005; Forró et al. Citation2008) and the New Zealand chydorid Cladocera should be no exception to this.
Global status of the biogeography and taxonomy of chydorid Cladocera
The chydorid Cladocera were first described by OF Müller in 1769. He focused mainly on chydorid genera such as Eurycercus, Camptocercus, Alona, Pleuroxus and Chydorus (Müller Citation1785). Over the next hundred years or so, study of the taxonomy of the chydorid Cladocera was confined to Europe, where the systematics of most genera became well established (Frey Citation1995). In the later part of the nineteenth century, the taxonomic focus shifted to species level in both Europe and overseas. In the 1860s, GO Sars described more than 16 species of chydorid Cladocera from Norway only (Sars Citation1993). Later, Birge (Citation1918) described several chydorid species from different parts of the world including North America, South America, Sri Lanka and Australia. Although the taxonomic study of the chydorid Cladocera was extended globally, most species recorded outside Europe were described as though they were European species. A significant breakthrough in examination of differences in genera and species of the chydorid cladoceran extant between Europe and other continents occurred only in the later part of the twentieth century. DG Frey and NN Smirnov made significant contributions in this regard; for example, their clarification of the confusion between the genus Eurycercus in Europe and Sayca in Australia and New Zealand (Frey Citation1971). The interhemispheric taxonomic variation of genera Chydorus, Pleuroxus and Ephemeroporus has also been updated (Frey Citation1982b; Smirnov Citation2007) and species within the genus Alona have been comprehensively described (Sinev Citation2004; Van Damme et al. Citation2010). Nevertheless, understanding of the taxonomy and systematics of New Zealand chydorid Cladocera is still poor.
Biogeography and taxonomy of New Zealand chydorid Cladocera
Until recently, the New Zealand chydorid Cladocera were little documented due to use of the northern hemisphere taxonomy. Hence the taxonomy and systematics of most species of New Zealand chydorid Cladocera require a comprehensive review to be able to assess the number of endemic species in New Zealand lakes. The majority of studies of New Zealand lake biota have focused on general distribution patterns of zooplankton groups (e.g. cladocerans, copepods, rotifers, etc.) (Forsyth & McCallum Citation1980; Greenwood et al. Citation1999; Hall & Burns Citation2003) or genera (e.g. Bosmina, Ceriodaphnia, Daphnia, Alona, Chydorus, etc.) (Chapman & Green Citation1999; Burger et al. Citation2002; Schallenberg et al. Citation2003) across the seasons and habitat types, and trophic interactions such as fish vs zooplankton (Jeppesen et al. Citation1997 ,Citation2000), or zooplankton vs phytoplankton (James & Forsyth Citation1990). However, comprehensive study of chydorid cladocerans is lacking, including well-defined taxonomy to species level and their temporal and spatial distribution patterns. The records of chydorid cladocerans identified to species level are crucial for understanding species-specific preferences for structural habitats, their geographical distribution as well as species optima and tolerance to a variety of physicochemical variables (e.g. temperature, TP, salinity) in New Zealand lakes (). DG Frey studied the biogeography and taxonomy of the New Zealand chydorid Cladocera based on their remains in sediment from North Island lakes (Frey Citation1991a). Significant morphological variability was encountered such as differences in body size, head pores and postabdominal teeth from their northern hemisphere counterparts and argued for the presence of increasingly endemic species adapted for New Zealand climates (Frey Citation1971 ,Citation1991a).
Fig. 1 Map illustrating the locations of previously studied sites of cladoceran zooplankton in New Zealand lake and river systems. (a) Lake Oneopoto (paleolake) North Island (Kattel & Augustinus Citation2010); (b) Lake Ohau and Tekapo South Island (Burns et al. Citation1984).
There are eight species of endemic chydorid Cladocera in New Zealand lakes (Chapman & Lewis Citation1976; Frey Citation1995). Australian lakes have a far higher number of endemic species (N=41) (Smirnov & Timms Citation1983; Frey Citation1991a ,Citation1995). However, there are many similarities between the chydorid Cladocera present in Australia and New Zealand. For example, the polyembroynic genus Saycia occurs only in Australia and New Zealand, and the genus Pleuroxus are also abundant in Australia and New Zealand lakes (Frey Citation1993a ,Citationb; Shiel & Dickson Citation1995).
Variability in morphology of some species of chydorid Cladocera between New Zealand and Australia has also been reported. The New Zealand species of Saycia, Saycia cooki novaezealandie Frey, Citation1971, has been shown to be different from its Australian counterpart, Saycia cooki cooki King, 1866 (). S. cooki novaezealandie have more preanal teeth on the post-abdomen and have a shorter posterior margin relative to the length of the animal (Frey Citation1971).
Table 1 List of New Zealand (as well as southern Australia and Tasmania) chydorid Cladocera reported in various literature.
The extended latitudinal variation of New Zealand lakes between North and South Islands further suggests that several species of New Zealand chydorid Cladocera may be endemic. Latitudinal influence on speciation of New Zealand chydorid Cladocera has not been documented. However, given the separation of North and South Islands as well as extreme climatic variation between far north (sub-tropical) and far south (sub-antarctic), speciation within the genera between two islands can also be a part of future investigations as Frey (Citation1971) reported differences in the endemic species of the genus Saciya between Australia and New Zealand.
Some species-rich genera, e.g. Pleuroxus, Chydorus, Alona and Biapertura, have an inter-hemispheric distribution, with Chydorus dominant in North America and Biapertura dominant in Australia and New Zealand (Frey Citation1995). Studies of modern chydorid Cladocera in New Zealand lakes by Davids et al. (Citation1987) and Burger et al. (Citation2002) documented relatively wide distribution of Biapertura affinis Leydig, 1860. The use of genus Biapertura is different with respect to location of their collection. For example, a similar species has been documented as Biapertura macrocopa in Australia (Sinev Citation2004). From a maar lake in Auckland, New Zealand, Kattel & Augustinus (Citation2010) reported large Alona-like species with a resemblance to the northern hemisphere species Alona affinis Leydig, 1860. However, it is possible that this specimen belongs to the Biapertura group of the southern hemisphere. Headshields of large Alona have been recorded from surface sediment samples from a lowland lake L0424 in the South Island of New Zealand (a).
Fig. 2 Headshields of some chydorid Cladocera recorded by GRK from the surface sediment samples of various lakes from South Island, New Zealand. Specimens are yet to be identified fully. (a) Lake L0424 (lowland grassland); (b) Lake L0407 (lowland forest); (c) Lake L0405 (lowland forest); (d) Lake L0412 (upland grassland); (e) Lake L0423 (lowland shrubland); (f) Lake L0419 (rocky catchment). Please refer to the text for details.
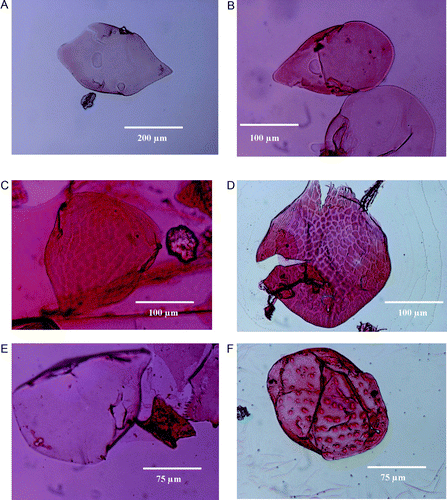
The headshields contained in the core sediment sample from the Auckland maar and the surface sediment samples from the South Island lake are significantly different with respect to the number of headpores. Unlike the headpores of Alona (Biapertura) affinis Leydig, 1860 (Sinev Citation1997), the Alona headshield from the South Island lake sediment has one median pore and two lateral pores connecting with a curve-like structure (a). Confirmation of the correct identification of large Alona including Alona affinis Leydig, 1860 and Biapertura affinis Leydig, 1860 through DNA analysis is required. Sacherova & Hebert (Citation2003) also showed that A. affinis is always genetically divergent from other Alona species.
The genus Ephemeroporus is present in both Australian and New Zealand lakes (). However, the species documented in New Zealand lakes may not be correct. For example, Burns et al. (Citation1984) reported the distribution of Ephemeroporus barroisi Richard, 1894 in a New Zealand lake (). E. barroisi was previously described as Pleuroxus barroisi or Chydorus barroisi (Frey Citation1982b). There are other closely related species described within the barroisi complex such as hybridus and tridentatus. Based on a distinct, single, large median pore in instar I, Ephemeroporus was described as different from the Chydorus genus. Some species such as tridentatus are present in Australian lakes (Frey Citation1982b; Shiel & Dickson Citation1995). A headshield of Chydorus like Cladocera has been recorded from the surface sediment sample of a lowland forested lake, L0407, from the South Island (b). This specimen is possibly the headshield of the species of Ephemeroporus. However, the distribution of E. barroisi in New Zealand lakes is poorly known.
Similarly, the taxonomy of Chydorus sphaericus Müller, 1776, a species regarded as cosmopolitan and present at nearly all latitudes and elevations, needs to be reconsidered for New Zealand. Until recently C. sphaericus was one of the commonly documented chydorid Cladocera in New Zealand lakes (Burns et al. Citation1984; Kattel & Augustinus Citation2010). However, Frey (Citation1980a) argued that C. sphaericus may have two species groups, each with an unknown number of species worldwide. A comprehensive revision for sphaericus complex of Chydorus in New Zealand is required through DNA analysis.
Frey (Citation1995) argued that chydorid Cladocera in New Zealand and Australian lakes must have evolved much later than their northern hemisphere counterparts, and the separation of Gondwana from Pangea ca 180 million years ago might have played a critical role, with the expanding oceans between them gradually creating a barrier to passive dispersal of cladoceran eggs. Consequently, the speciation of the chydorid Cladocera in the southern hemisphere has become significantly different from that of northern hemisphere taxa. For example, the chydorid genus Bryospilus () occurs in wet forests of Puerto Rico, Venezuela, Cameroon and New Zealand (Frey Citation1980b). The species Bryospilus bifidus Frey, 1980 is endemic to New Zealand and recorded in Nothofagus forest litter from the South Island (Frey Citation1980b). Morphologically, the genus Bryospilus closely resembles the genus Monospilus (Frey Citation1980b). However, the unique microhabitat structure of Bryospilus today suggests that these two genera must have adapted separately over a long period of time.
Bryospilus in New Zealand lakes is particularly important for understanding the changing climates and vegetation that accompanied the cycles of glaciation during the Quaternary. Monospilus dispar Sars, 1862 is widely distributed in Europe, preferring mainly littoral sandy/muddy substrata (Kattel et al. Citation2006). Chiambeng & Dumont (Citation2005) recorded M. dispar in the rain forests of Cameroon and argued that this species may have been able to survive the post-Miocene cooling and aridification in Africa. Records of M. dispar from New Zealand lakes are rare, although a headshield of a Monospilus-like genus has been recorded from the surface sediment sample from a lowland forest lake L0405 in the South Island (c). Whilst the taxonomy of Monospilus is yet to be established, it is important that the spatial and temporal distribution patterns of Monospilus and Bryospilus in New Zealand lakes are known so that they can provide information regarding the major climatic events and ecological shifts in the past that forced these two species to adapt separately.
Adamowicz & Sacherova (Citation2006) hypothesized that a directional evolution of chydorid Cladocera occurred using loss or gain of morphological characters in phylogeny-based methods. They argued that both loss and gain of structures during evolution may influence the probable direction of the ecological shifts (Adamowicz & Sacherova 2006). The morphological (taxonomic) species concept considers that species may be separated by means of ecological adaptation as well as by reproductive isolation (Korovchinsky Citation1996). However, it appears that factors including micro-habitat structure and body character evolve together for adaptation to climates which then determine the large-scale ecological shifts in some species of chydorid Cladocera. Nevertheless, some species remain unchanged. For example, Graptoleberis testudinaria Fischer, 1848 are widely distributed in both hemispheres and have changed little in New Zealand lakes (). They commonly colonize structural habitats of aquatic macrophytes and no differences have been reported in complex morphological character and species variation (Fryer Citation1968).
Cladoceran such as Alonella excisa Fischer, 1854 are also widely distributed in both hemispheres and display no taxonomic confusion. Headshields of A. excisa have been recorded from surface sediment samples collected from a lowland lake, L0412, in the South Island of New Zealand (d). However, unlike Alonella, some other chydorid cladocerans such as Pleuroxus and Alona that are found in both hemispheres have evolved as complex groups of genera in the southern hemisphere, including New Zealand and Australia.
Pleuroxus group
The genus Pleuroxus is one of most complex genera amongst the chydorid Cladocera (Frey Citation1993a ,Citationb). They have two distinct groups, aduncus and laevis. The P. aduncus group is not fully related to Pleuroxus in Australia and New Zealand as it may have several subspecies within it (Frey Citation1993a). Some New Zealand literature published has cited Pleuroxus aduncus Jurine, 1820 incorrectly (e.g. Green Citation1966). Frey (Citation1993b) proposed sub-division of the genus Pleuroxus into Tylopleuroxus for the southern hemisphere and Picripleuroxus for the northern hemisphere. However, Smirnov et al. (Citation2006) rejected Frey's subgeneric classification method and proposed using a genetically advanced method such as DNA markers in order to construct a consistent phylogenetic tree for this genus. Recently, surprising results have emerged from South Africa on Pleuroxus-like chydorids (Smirnov Citation2007) indicating that Australia and New Zealand may not be the exception in having Pleuroxus-like chydorids. Now several new subspecies within the Pleuroxus genus have been discovered in the Southern Hemisphere (Smirnov Citation2007). Currently, two Pleuroxus species, both belonging to aduncus complex, have been described from New Zealand lakes, namely: Pleuroxus hastirostris Sars, 1904 and Pleuroxus hevenacus Frey, 1991 ().
Alona group
One of the largest chydorid subfamilies, Aloninae, has been reviewed several times (e.g. Flösner & Frey 1970; Frey Citation1959 ,Citation1987 ,Citation1988 ,Citation1991b; Sinev Citation2001 ,Citation2004; Van Damme et al. Citation2010). Most species within this subfamily have been removed or given new names after investigation of their taxonomy and systematics (e.g. Sinev Citation2004; Van Damme et al. Citation2010). Alona Baird, 1843 is a large lump genus within which Aloninae from many parts of the world have been placed (Fryer Citation1968; Frey Citation1995; Korovchinsky Citation2006; Van Damme et al. Citation2010). Until recently, 14 Aloninane genera have been considered valid for taxonomic identification (Van Damme et al. Citation2010). Hence, the phylogenetic position of many Aloninae is yet to be resolved and the genus Alona Baird, 1843 is likely to have many endemic species within it. More importantly, the holarctic (distribution across the continents) speciation of southern hemisphere Alona has not been adequately substantiated (Frey Citation1982c).
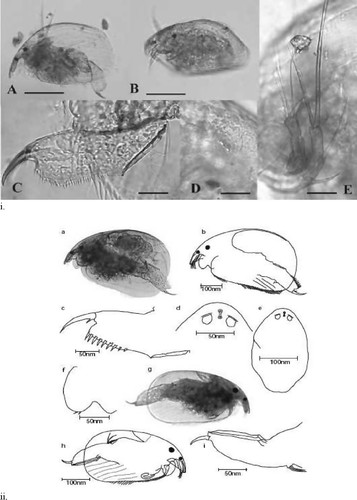
Morphological investigations, as well as DNA analyses, have yet to be undertaken on individual Alona populations. For example, Sinev (Citation2004) proposed that the previously documented species Alona macrocopa needs to be placed in a new genus, Armatalona, within the subfamily Alonanie (, (i)). DNA analysis of New Zealand Alona by Sacherova & Hebert (Citation2003) suggests that the phylogeny of Alona is more complex than that of other chydorids. Furthermore, a mitochondrial study by Sacherova & Hebert (Citation2003) on the phylogenetic history of 37 chydorid species collected from many parts of the world, including New Zealand, suggests that the existing taxonomic system is not accurate.
Fig. 3 Two members of the subfamily Aloninae. (i) Armatalona macocopa Sars, 1894 and (ii) Alona setigera Brehm, Citation1931 commonly present in New Zealand lakes. (i) Armatalona macrocopa Sars, 1894. A. lateral view; B. dorso lateral view; C. post abdomen; D. head pores; E. antennae II. scale bar: A–B = 0.1 mm, C–E = 0.02 mm (adopted after Sinev Citation2007). (ii) Alona setigera Brehm, Citation1931. a, b, general view of adult female; c, postabdomen female; d, headpore female; e, headshield female; f, labrum; g, h, general view of male; i, postabdomen male (adopted after Santos-Wisniewski et al. Citation2001).
The record of small Alona such as Alona guttata Sars, 1862 in Auckland maar lake sediments suggests that they have been present in New Zealand for at least the past 30 cal kyr BP and became dominant during the early Holocene (Kattel & Augustinus Citation2010). Both modern and fossil populations of Alona guttata Sars, 1862 have been recorded in regions with sub-zero temperatures such as Arctic Greenland (Frey Citation1991b) and the alpine Himalayas (Swar & Fernando Citation1979; Fernando & Kanduru Citation1984), mild northern and southern temperate regions of the USA (Harmsworth Citation1968) and New Zealand (Burger et al. Citation2002; Kattel & Augustinus Citation2010), as well as in the tropics of Brazil (Santos-Wisniewski et al. Citation2001). Fryer (Citation1968) reported that, unlike other small Alona, A. guttata occurs in both large lakes and small ponds and is tolerant of a wide range of physicochemical conditions. Schallenberg et al. (Citation2003) recorded Alona sp. (possibly a member of the small Alona group) from the Lake Waihola, a lowland shallow coastal, regularly saline-influenced lake in South Island, New Zealand, indicating an increased tolerance to salinity by these biota.
There are many similarities between the morphology of Alona guttata Sars, 1862 and another small Alona, Alona rectangula Sars, 1862 (Fryer Citation1968). For example, the shape and size of the carapace, head shield and the number of median pores present in these species are similar. Even within small geographic areas of Europe sub-species of Alona guttata have been reported by Kurz (Frey Citation1962 ,Citation1988). Consequently, along with the other small Alona, including A. rectangula, the subspecies A. guttata (e.g. A. guttata var tuberculata Kurz, 1874) makes identification of small Alona more difficult (Frey Citation1960a ,Citationb). The global distribution of small Alona requires further investigation.
The distribution of Alona rectangula Sars, 1862 has not been adequately documented from New Zealand lakes. A. rectangula has been incorrectly documented from sub-Antarctic islands, with the actual species inhabiting these islands being Alona weinecki Studer, 1878 (Frey Citation1988). Sacherova & Hebert (Citation2003) argued that A. rectangula is restricted to Europe and a very similar species, Alona circumfimbriata n. sp., is restricted to North America. According to these authors, there is no evidence of A. rectangula being present in New Zealand lakes, and they suggest that there may be more sub-genera present within the genus Alona.
Some workers suggest that the small Alona recorded in New Zealand lakes could be Alona setigera Brehm, Citation1931 (). Brehm (Citation1931) described A. setigera, a subspecies of A. guttata. However, unlike in A. guttata (Sinev Citation1999a), there are only two median pores in A. setigera and Brehm's description is incorrect (Sinev Citation1999a; Santos-Wisniewski et al. Citation2001; (ii)). However, the configuration of the lateral pores of A. setigera resembles that of the northern hemisphere species Alona costata Sars, 1862 or Alona rustica Scott, 1895, consisting of short transverse slits and pocket-like structures retained under the lateral pores (Flössner & Frey Citation1970; Sinev Citation1999b). Two different headshields of small Alona (e,f), possibly from Alona setigera Brehm, Citation1931, were recorded from the surface sediment samples of two South Island lakes, L0423 and L0419. However, neither of the headshields have pocket-like structures retained under the lateral pores and the shape and size of these headshields also vary significantly. An inventory of Cladocera in Australia proposed that A. guttata should come under the genus Biapertura and the species A. setigera should also be Biapertura setigera (Smirnov & Timms Citation1983). An extensive review of their morphology and genetic variability is required in order to verify the existence of endemic populations of chydorid Cladocera either in the form of Alona or in the form of Biapertura in New Zealand lakes.
Most chydorid Cladocera, including the small Alona group, may have adapted very well to New Zealand environments. The two small Alona, Aramatalona macrocopa and Alona setigera, were widely recognized as southern hemisphere chydorids, possibly endemic to New Zealand and Australia (Smirnov & Timms Citation1983; Smirnov Citation1989; Sinev Citation2007). However, both species have recently been recorded in the tropics of Thailand and Brazil (; Santos-Wisniewski et al. Citation2001; Sinev Citation2007). What makes small Alona Baird, 1843 (A. guttata, A. macrocopa and A. setigera) highly tolerant to a wide range of climates is not clear. However, given their adaptation to cold, hot, dry and humid climates (Harmsworth Citation1968; Swar & Fernando Citation1979; Fernando & Kanduru Citation1984; Frey Citation1991b), they appear to have been able to adapt to the glacial–interglacial cycles that dominated the Quaternary.
As the temperature of much of New Zealand during the Last Glacial Maximum (ca 18–28 cal kyr BP) was like today in the sub-Antarctic regions, it is likely that the most cold-tolerant chydorid Cladocera would have undergone a reproductive phase (Frey Citation1982a) during spring which may have then supported continuous and viable populations of most species of small Alona despite the harsh climate. In addition, all members of the chydoridae live in a broad range of habitats, including the littoral zone, associated with macrophytes and organic-rich sediments, sand and boulders (e.g. Fryer Citation1968 ,Citation1974; Kattel et al. Citation2006).
The range in size and diversity of feeding behaviour of Anomopods may have played a significant role in niche separation as well as speciation over the Cenozoic (Fryer Citation1968 ,Citation1995). Galaxias fish fossils discovered in a Miocene lake in Otago (Lee et al. Citation2007) further support the existence of lake ecosystems in New Zealand for millions of years, in which the chydorid Cladocera should have played an important bottom-up role in food web dynamics. However, the debate regarding biogeography, taxonomy and systematics of chydorid Cladocera is still unresolved.
Future prospects for New Zealand chydorid Cladocera research
New Zealand scientists face difficulties in using cladocerans to assess ecological and environmental changes in New Zealand due to poor knowledge of their taxonomy and biogeography. This is important as they are one of the significant bio-indicators of global change from lacustrine environments (Lotter et al. Citation1997; Battarbee Citation2000; Kattel & Augustinus Citation2010). In contrast with the situation in the northern hemisphere, study of the chydorid Cladocera is in its infancy in New Zealand. Until recently, most chydorid Cladocera recorded as both modern and fossil assemblages in New Zealand lakes were regarded as cosmopolitan species due to the use of taxonomic keys based on northern hemisphere taxa. Some members of the chydorid subfamily Aloninae, small Alona (A. guttata, A. setigera) for example, appear to be well distributed in New Zealand lakes and elsewhere. However, studies such as that by Frey (Citation1995) suggest that following the breakup of Gondwana, the New Zealand chydorid Cladocera may have evolved differently from their northern hemisphere counterparts. Consequently a significant variability in speciation has occurred in some members of the Chydoridae.
One of the important requirements for improving the taxonomy and biogeography of chydorid Cladocera is to examine the consequences of the geological history of New Zealand on adaptation of these animals. A detailed morphological character of body parts of extant and fossil chydorid cladocerans would continue to improve taxonomy and help understanding of the biogeography and ecological adaptation of these animals in New Zealand's freshwater environments. However, taxonomic interpretation based on DNA analysis of both fossils and extant chydorid cladocerans can be the only reliable option to optimize our efforts in global environmental change research for various time scales through the use of these animals.
Acknowledgements
We would like to acknowledge two anonymous reviewers. Their constructive suggestions were significant in improving the quality of this paper. We are also grateful to Marcus Vandergoes of GNS Science for providing surface sediment samples of South Island lakes, and the School of Environment at the University of Auckland, New Zealand, for provision of laboratory facilities and assistance. This work was supported by the University of Auckland Research Fund (3608532) Award to PCA.
References
- Adamowciz , SJ and Purvis , A . 2005 . How many branchiopod crustacean species are there? Quantifying the components of underestimation . Global Ecology and Biogeography , 14 : 455 – 468 .
- Adamowciz SJ , Sacherova V 2006 . Testing the directionality of evolution: the case of chydorid crustaceans . Journal of Evolutionary Biology 19 : 1517 1530 , doi: doi:10.1111/j.1420-9101.2006.01127.x .
- Alonso M 1996 . Crustacea Branchiopoda . Serie Fauna Iberica , 7 . CSIC , Madrid .
- Battarbee , RW . 2000 . Palaeolimnological approaches to climate change, with special regard to the biological record . Quaternary Science Reviews , 19 : 107 – 124 .
- Birge EA 1918 . The water fleas (Cladocera) . In : HP Ward , GC Whipple Freshwater biology . New York , Wiley . 676 740 .
- Brehm , V . 1931 . Cladoceran aus Neuseeland . Archiv fur Hydrobiologie , 23 : 491 – 501 .
- Burger , DF , Hogg , ID and Green , JD . 2002 . Distribution and abundance of zooplankton in the Waikato River, New Zealand . Hydrobiologia , 479 : 31 – 38 .
- Burns , CW , Butler , MI and Cuttance , PM . 1984 . Invertebrates, macroalgae, and chemical features in Morainic Ponds near Lakes Tekapo and Ohau, including new distribution records of crustacea . New Zealand Journal of Marine and Freshwater Research , 18 : 197 – 210 .
- Byars , JA . 1959 . A freshwater pond in New Zealand . Australian Journal of Marine and Freshwater Research , 11 : 222 – 240 .
- Chapman , MA and Lewis , MH . 1976 . An introduction to the Freshwater Crustacea of New Zealand , Auckland : Collins .
- Chapman , MA and Green , JD . 1999 . Crustacean zooplankton communities in New Zealand lake during four decades of trophic change . New Zealand Journal of Marine and Freshwater Research , 33 : 361 – 373 .
- Chiambeng D , Dumont HJ 2005 . The Branchiopoda (Crustacea: Anomopoda, Ctenopoda and Cyclestherida) of the rain forests of Cameroon, West Africa: low abundances, few endemics and a boreal-tropical disjunction . Journal of Biogeography 32 : 1611 1620 , doi: 10.1111/j.1365-2699.2005.01280.x .
- Davids , C , Stop , M and De Groot , CJ . 1987 . The cladoceran of the littoral Zone of Lake Marsseveen . Aquatic Ecology , 21 : 71 – 79 .
- Dole-Olivier , M , Galassi , DMP and des Châtelliers , MC . 2000 . The biology and ecology of lotic microcrustaceans . Freshwater Biology , 44 : 63 – 91 .
- Fernando , CH and Kanduru , A . 1984 . Some remarks on the latitudinal distribution of the Cladocera in the Indian subcontinent . Hydrobiologia , 113 : 69 – 76 .
- Figuerola , J and Green , AJ . 2002 . Dispersal of aquatic organisms by waterbirds; a review of past research and priorities for future studies . Freshwater Biology , 47 : 483 – 494 .
- Flössner , D and Frey , DG . 1970 . A morphological comparison of European and North American populations of Alona rustica Scott and Alona costata Sars (Cladocera, Chydoridae) . Limnologica (Berlin) , 7 : 325 – 337 .
- Forró , L , Korovchinsky , NM , Kotov , AA and Petrusek , A . 2008 . Global diversity of cladocerans (Cladocera; Crustacea) in freshwater . Hydrobiologia , 595 : 175 – 184 .
- Forsyth , DJ and McCallum , ID . 1980 . Zooplankton of Lake Taupo . New Zealand Journal of Marine and Freshwater Research , 14 : 65 – 69 .
- Frey , DG . 1959 . The taxonomic and phylogenetic significance of the headpores of the Chydoridae (Cladocera) . Internationale Revue der Gesamten Hydrobiologie , 44 : 27 – 50 .
- Frey , DG . 1960a . On the occurrence of cladoceran remains in lake sediments . Proceedings of the National Academy of Sciences of the United States of America , 46 : 917 – 920 .
- Frey , DG . 1960b . The ecological significance of cladoceran remains in lake sediments . Ecology , 41 : 684 – 699 .
- Frey , DG . 1962 . Cladocera from Eemian Interglacial of Denmark . Journal of Palaeontology , 36 : 1133 – 1154 .
- Frey , DG . 1971 . Worldwide distribution and ecology of Eurycercus and Saycia (Cladocera) . Limnology and Oceanography , 16 : 254 – 308 .
- Frey , DG . 1976 . Interpretation of Quaternary paleoecology from Cladocera and midges, and prognosis regarding usability of other organisms . Canadian Journal of Zoology , 54 : 2208 – 2226 .
- Frey , DG . 1980a . On the plurality of Chydorus sphaericus (O.F. Müller) (Cladocera, Chydoridae) and designation of a neotype from Sjaelsø, Denmark . Hydrobiologia , 69 : 83 – 123 .
- Frey , DG . 1980b . The non-swimming chydorid Cladocera of wet forests, with descriptions of a new genus and two new species . Internationale Revue der Gesamten Hydrobiologie , 65 : 613 – 641 .
- Frey , DG . 1982a . Contrasting strategies of gamogenesis in northern and southern populations of Cladocera . Ecology , 63 : 223 – 241 .
- Frey , DG . 1982b . Relocation of Chydorus barroisi and related species (Cladocera, Chydoridae) to a new genus and description of two new species . Hydrobiologia , 86 : 231 – 269 .
- Frey , DG . 1982c . Questions concerning cosmopolitanism in Cladocera . Archiv für Hydrobiologie , 93 : 484 – 502 .
- Frey , DG . 1986 . “ Cladocera analysis ” . In Handbook of Holocene palaeoecology and palaeohydrology , Edited by: Berglund , BE . 667 – 692 . Chichester : John Wiley & Sons .
- Frey , DG . 1987 . The taxonomy and biogeography of the Cladocera . Hydrobiologia , 145 : 5 – 17 .
- Frey , DG . 1988 . Alona weinecki Studer on the Subantarctic Islands, not Alona rectangula Sars (Chydoridae, Cladocera) . Limnology and Oceanography , 33 : 1386 – 1411 .
- Frey , DG . 1991a . The species of Pleuroxus and of three related genera (Anomopoda, Chydoridae) in southern Australia and New Zealand . Records of the Australian Museum , 43 : 291 – 372 .
- Frey , DG . 1991b . First subfossil records of Daphnia headshields and shells (Anomopoda, Daphniidae) about 10,000 years old from northmost Greenland, plus Alona guttata (Chydoridae) . Journal of Paleolimnology , 6 : 193 – 197 .
- Frey , DG . 1993a . Species of Pleuroxus (Anomopoda: Chydoridae) from the subantartic islands and southernmost South America: a partial unravelling of the Pleuroxus aduncus problem . Hydrobiologia , 262 : 145 – 188 .
- Frey , DG . 1993b . Subdivision of the genus Pleuroxus (Anomopoda, Chydoridae) into subgenera worldwide . Hydrobiologia , 262 : 133 – 144 .
- Frey , DG . 1995 . Changing attitudes toward Chydorid anomopods since 1769 AD . Hydrobiologia , 307 : 43 – 55 .
- Fryer , G . 1968 . Evolution and adaptive radiation in the Chydoridae (Crustacea: Cladocera). A study in comparative functional morphology and ecology . Philosophical Transactions of the Royal Society of London B , 254 : 221 – 385 .
- Fryer , G . 1974 . Evolution and adaptive radiation in the chydoridae (Crustacea: Cladocera). A study in comparative functional morphology and ecology . Philosophical Transactions of the Royal Society of London B , 254 : 226 – 385 .
- Fryer , G . 1995 . Phylogeny and adaptive radiation within the Anomopoda: a preliminary exploration . Hydrobiologia , 307 : 5768 – 5768 .
- Glew JR , Last WM 2001 . Chrysophytes . In : Last WM , Smol JP , Tracking environmental change using lake sediments , 1 . Basin analysis, coring, and chronological techniques . Dordrecht , Kluwer Academic . 73 105 .
- Green , J . 1966 . Seasonal variation in egg production by Cladocera . Journal of Animal Ecology , 35 : 77 – 104 .
- Greenwood , TL , Green , JD , Hicks , BJ and Chapman , MA . 1999 . Seasonal abundance of small cladocerans in Lake Mangakaware, Waikato, New Zealand . New Zealand Journal of Marine and Freshwater Research , 33 : 399 – 415 .
- Hall , CJ and Burns , C . 2003 . Responses of crustacean zooplankton to seasonal and tidal salinity changes in the coastal Lake Waihola, New Zealand . New Zealand Journal of Marine and Freshwater Research , 37 : 31 – 43 .
- Hann , BJ . 1995 . Invertebrate associations with submerged aquatic plants in prairie wetlands . UFS (Delta Marsh) Annual Report , 30 : 78 – 84 .
- Harmsworth , RV . 1968 . The developmental history of Belham Tarn (England) as shown by animal microfossils, with special reference to the Cladocera . Ecological Monographs , 38 : 223 – 241 .
- Hofmann , W . 2000 . Response of the chydorid faunas to the rapid climatic changes in four alpine lakes at different altitudes . Palaeogeography, Palaeoclimatology, Palaeoecology , 159 : 281 – 292 .
- James , MR and Forsyth , DJ . 1990 . Zooplankton-phytoplankton interactions in a eutrophic lake . Journal of Plankton Research , 12 : 455 – 472 .
- Jeppesen , E , Lauridsen , T , Mitchell , SF and Burns , CW . 1997 . Do planktivorous fish structure the zooplankton communities in New Zealand lakes? . New Zealand Journal of Marine and Freshwater Research , 31 : 163 – 173 .
- Jeppesen , E , Lauridsen , T , Mitchell , SF , Christoffersen , K and Burns , CW . 2000 . Trophic structure in the pelagial of 25 shallow New Zealand lakes: changes along nutrient and fish gradients . Journal of Plankton Research , 22 : 951 – 968 .
- Kattel GR , Augustinus P 2010 . Cladocera-inferred environmental change in an Auckland maar lake, Lake Oneopoto . New Zealand Journal of Geology and Geophysics 53 : 30 41 , doi: 10.1080/00288301003631772 .
- Kattel , GR , Mackay , AW and Battarbee , RW . 2006 . Variation in patterns of composition and abundance of cladocerans in littoral and open water habitats of Loch Coire Fionnaraich, Scotland . International Journal of Lakes and Rivers , 1 : 35 – 50 .
- Korhola , A . 1999 . Distribution patterns of Cladocera in subarctic Fennoscandian lakes and their potential in environmental reconstruction . Ecography , 22 : 357 – 373 .
- Korhola AA , Rautio M 2001 . Cladocera and other branchipod crustaceans In Smol JP , Birks HJB , Last WM Tracking environmental change using lake sediments 4 Zoological indicators , Dordrecht Kluwer Academic
- Korovchinsky , NM . 1996 . How many species of Cladocera are there? . Hydrobiologia , 321 : 191 – 204 .
- Korovchinsky , NM . 1997 . On the history of studies on cladoceran taxonomy and morphology, with emphasis on early work and causes of insufficient knowledge of the diversity of the group . Hydrobiologia , 360 : 1 – 11 .
- Korovchinsky , NM . 2006 . The Cladocera (Crustacea: Branchiopoda) as a relict group . Zoological Journal of the Linnean Society , 147 : 109 – 124 .
- Kotov , A . 2009 . A revision of Leydigia Kurz, 1875 (Anomopoda, Cladocera, Branchiopoda), and sub-generic differentiation within the genus . Zootaxa , 2082 : 1 – 84 .
- Lee , DE , McDowall , RM and Lindqvist , JK . 2007 . Galaxias fossils from Miocene lake deposits, Otago, New Zealand: the earliest records of the Southern Hemisphere family Galaxidae (Teleostei) . Journal of the Royal Society of New Zealand , 37 : 109 – 130 .
- Lotter , AF , Birks , HJB , Eicher , U , Hofmann , W and Marchetto , A . 1997 . Modern diatom, Cladocera, chironomid, and chrysophyte cyst assemblages as quantitative indicators for the reconstruction of past environmental conditions in the Alps. I. Climate . Journal of Paleolimnology , 18 : 395 – 420 .
- Maguire , BJ . 1963 . The passive dispersal of small aquatic organisms and their colonization of isolated bodies of water . Ecological Monographs , 33 : 161 – 185 .
- McDowall , RM . 1990 . New Zealand freshwater fishes: a natural history and guide , Auckland : Heinemann Reed MAF .
- Müller OF 1785 . Entomostraca seu Insecta Testacea quae in aquis Daniae et Norvegiae reperit discripsit et iconibus illustravit . Lipsiae Havniae , 1 135 .
- Nevalainen L , Luoto TP 2010 . Temperature sensitivity of gamogenesis in littoral cladocerans and its ecological implications . Journal of Limnology 69 : 120 125 , doi: 10.3274/JL10-69-11 .
- Sacherova , V and Hebert , PDN . 2003 . The evolutionary history of the Chydoridae (Crustacea: Cladocera) . Biological Journal of the Linnean Society , 79 : 629 – 643 .
- Santos-Wisniewski MJ , Rocha O , Matsura-Tundisi T 2001 . First record of Alona setigera Brehm (Cladocera: Chydoridae) in the neotropical region . Brazilian Journal of Biology 61 : 701 702 doi: 10.1590/S1519-698420010040022 .
- Sars , GO . 1993 . On the freshwater crustaceans occurring in the vicinity of Christiania , Bergen : University of Bergen Press .
- Schallenberg , M , Hall , CJ and Burns , C . 2003 . Consequences of climate-induced salinity increases on zooplankton abundance and diversity in coastal lakes . Marine Ecology Progress Series , 251 : 181 – 189 .
- Shiel , RJ and Dickson , JA . 1995 . Cladocera recorded from Australia . Transactions of the Royal Society of South Australia , 119 : 29 – 40 .
- Sinev 1997 . Review of the affinis group of Alona Baird, 1943, with the description of a new species from Australia (Anomopoda Chydoridae) . Arthropoda Selecta 6 : 47 58 .
- Sinev , AY . 1999a . Alona werestschagini sp.n., new species of genus Alona Baird, 1843, related to A. guttata Sars, 1862 (Anomopoda, Chydoridae) . Arthropoda Selecta , 10 : 5 – 18 .
- Sinev , AY . 1999b . Alona costata Sars, 1862 versus related palaeotropical species: the first example of close relations between species with a different number of main head pores among chydoridae (Crustacea: Anomopoda) . Arthropoda Selecta , 8 : 131 – 148 .
- Sinev , AY . 2001 . Distribution and polymorphism of Alona rectangula Sars, 1862 (Branchiopoda, Anomopoda, Chydoridae) in Russia and surrounding countries . Arthropoda Selecta , 10 : 83 – 86 .
- Sinev , AY . 2004 . Armatalona gen. n. A new genus of subfamily Aloninae (Anomopoda: Chydoridae) separated from genus Alona Baird 1840 . Hydrobiologia , 520 : 29 – 47 .
- Sinev , AY . 2007 . Occurrence of the Australian cladoceran Armatalona macrocopa (Sars, 1894) (Cladocera: Anomopoda: Chydoridae) in Thailand . Invertebrate Zoology , 4 : 25 – 29 .
- Smirnov NN 1971 Chydoridae of world fauna, USSR. Rakoobrasnie. T.I 2 . Leningrad in Russian
- Smirnov NN 1989 . Tropicheckiye Cladocera. 1. Novyye vidyrodov Alona ei Biapertura (Aloninae, Chydoridae) tropicheskoy Avastralii . Zool Zhurnal 68 : 135 140 in Rusian
- Smirnov , NN . 1998 . A revision of the genus Camptocercus (Anomopoda, Chydoridae, Aloninane) . Hydrobiologia , 386 : 63 – 83 .
- Smirnov NN 2007 . Pleuroxus-like Chydorids (Crustacea: Anomopoda) from South Africa, with the description of Dumontiellus africanus gen. n., sp.n . Hydrobiologia 575 : 433 439 , doi: 10.1007/s10750-006-0275-0 .
- Smirnov , NN and Timms , BV . 1983 . A revision of the Australian Cladocera (Crustacea) . Records of the Australian Museum Suppl. , 1 : 1 – 132 .
- Smirnov NN , Kotov AA , Coronel JS 2006 . Partial revision of the aduncus-like species of Pleuroxus Baird, 1843 (Chydoridae, Cladocera) from the southern hemisphere with comments on subgeneric differentiation within the genus . Journal of Natural History 40 : 1617 1639 , doi: 10.1080/000222930600958870 .
- Swar , DB and Fernando , CH . 1979 . Cladocera on Pokhara Valley, Nepal with notes on distribution . Hydrobiologia , 66 : 113 – 128 .
- Van Damme , K , Kotov , AA and Dumon , HJ . 2010 . A checklist of names in Alona Baird 1843 (Crustacea: Cladocera: Chydoridae) and their current status: an analysis of the taxonomy of a lump genus . Zootaxa , 2330 : 1 – 63 .
- Whiteside , MC . 1974 . Chydorid (Cladocera) ecology: seasonal patterns and abundance of populations in Elk Lake, Minnesota . Ecology , 55 : 538 – 550 .
- Whiteside , MC and Swindoll , MR . 1988 . Guidelines and limitations to cladoceran paleoecological interpretations. Palaeogeography, Palaeoclimatology . Palaeoecology , 62 : 405 – 412 .
- Whiteside , MC , Williams , JB and White , CP . 1978 . Seasonal abundance and pattern of chydorid, Cladocera in mud and vegetative habitats . Ecology , 59 : 1177 – 1188 .