Abstract
Seabirds deposit large quantities of marine detritus on land, but little is known of the soil arthropods processing this material. Burrow-nesting seabirds concentrate their activities within their burrows, so we tested the hypothesis that burrow arthropod fauna is more marine-like in its isotopic enrichment (13C/12C, 15N/14N; expressed as δ13C and δ15N) than the arthropods on the adjacent forest floor. Results from a Westland petrel (Procellaria westlandica) colony on the South Island of New Zealand did not support the hypothesis. Instead, δ15N was universally marine (13–22‰). While δ13C separated into two clusters, the distribution was not according to arthropod provenance. Most taxa had a terrestrial δ13C; only two taxa (a leiodid beetle and the mesostigmatic mite Ayersacarus woodi) incorporated marine C. The leiodid beetle occurs both in burrows and on the forest floor; beetles from both habitats had a marine δ13C. Ayersacarus woodi is found only in burrows. We conclude that, in this system, marine and terrestrial detrital C is processed separately, and that marine detrital C enters the terrestrial ecosystem through a very few arthropod taxa.
Introduction
Seabirds can profoundly influence terrestrial systems (Polis et al. Citation2004; Kolb et al. Citation2010), but surprisingly little is known of the C sources and trophic relationships of soil fauna associated with seabird colonies. Stable isotope ratios, especially 13C/12C and 15N/14N, are increasingly used to identify food resources and trophic niches of soil invertebrates (Oelbermann & Scheu Citation2010; Maraun et al. Citation2011) because soil organisms are inherently difficult to study directly. The technology is especially suited to the study of seabird colony soil fauna, because the enrichment of both 13C/12C and 15N/14N in marine systems provides a natural tracer of marine C and N flow into terrestrial systems (Wainwright et al. Citation1998; Hawke & Clark Citation2010a).
Studies using stable isotopes have shown that marine N pervades the seabird colony environment, being incorporated in plants, soil litter invertebrates, frugivorous birds, and predators (Hawke & Holdaway Citation2005; Major et al. Citation2007). However, vascular plant C shows no sign of significant marine origin (Hawke & Newman Citation2007). Instead, marine C appears only in animals with food chains based directly on seabird material, whether predators consuming live seabirds (Major et al. Citation2007), necrovores consuming seabird corpses (Hawke & Clark Citation2010a), or detritivores (and their predators) consuming seabird discards (Hawke & Clark Citation2010b). Consequently, the transfer of marine C to the wider terrestrial environment appears constricted and the oft-noted ‘marine nutrient subsidy’ derived from seabirds may only apply to N and P and certain trace elements (Xu et al. Citation2011).
Many studies have demonstrated transfer of marine C to terrestrial food webs via predation of seabirds, often by introduced predators (Major et al. Citation2007; Harper Citation2007). However, more marine C is likely to enter the terrestrial environment through seabird detritus than through predation, and few studies have investigated uptake of marine C from detritus by invertebrates. The obvious place to look is where nutrient cycling is most intense. For some alcids (Alcidae) and most petrels (Procellariidae) that come ashore only to breed, burrows are used to shelter incubating birds and then the vulnerable young. Consequently, burrows during the breeding season potentially receive more seabird detritus than surrounding areas, which the birds use only when moving to and from take-off and landing points. In this study, we hypothesized that the soil arthropod fauna of Westland petrel (Procellaria westlandica (Falla, 1946)) burrows is more marine-like in its isotopic enrichment (13C/12C, 15N/14N) than is the fauna on the adjacent forest floor.
Methods
Westland petrels breed colonially in the austral winter, in forest near Punakaiki on the western coast of New Zealand's South Island (Best & Owen Citation1976). Sampling was carried out in July 2010 at 42°08.8′S, 171°20.5′E on a steep, SSE-facing slope within the Westland Petrel Specially Protected Area of Paparoa National Park; photographs of the study area are given in Hawke (Citation2004). Burrow density is c. 0.2 m 2 (Waugh et al. Citation2003), although burrows tend to be concentrated up slope (pers. obs.). The soil is an Orthic Brown Soil (New Zealand Soil Classification), equivalent to a Dystrudept in USDA Soil Taxonomy; a complete soil description is given in Hawke (Citation2010). Vegetation is dominated by tree ferns (Cyathea spp.) and kamahi (Weinmannia racemosa), with scattered podocarps (especially matai Prumnopitys taxifolia and rimu Dacrydium cupressinum).
Soil δ13C can vary systematically between non-adjacent burrows in a seabird colony, complicating interpretation of arthropod isotopic data (Hawke & Clark Citation2010b). Furthermore, sites downslope of seabird burrows typically have lower δ15N (Hawke & Clark Citation2010a). We therefore minimized the area sampled and did not attempt to sample the entire breeding colony. A feature of Westland petrels is their low level of burrow nesting occupancy within a given breeding season, although many burrows are actively maintained by non-breeding birds (Waugh et al. Citation2003). Certain seabird burrow invertebrates consume both terrestrial and marine C (Hawke & Clark Citation2010b), and this may conceivably be affected by the presence or absence of a steady supply of detritus. We therefore decided to include both occupied and unoccupied burrows in our comparison. Samples came from a strip c. 50 m down slope × 10 m across through an altitude range of 118–141 m, as measured by calibrated barometric altimeter. Fourteen burrows were sampled, seven burrows with and seven without an incubating petrel. Most burrows were in the upper c. 20 m of the study site. On the forest floor, five points were located 5 m apart along a transect running down slope through the area containing most of the burrows. Burrows with multiple entrances, or otherwise complex geometries, were avoided; all study burrows had had 20 cm diameter inspection hatches installed near the nest chamber by other researchers.
A single surface (1–2 cm depth; c. 0.5 L) soil sample was collected from each burrow or transect point into plastic boxes using a small garden trowel. Burrow samples were collected from 30 cm inside the burrow entrance. Six petrel guano deposits were collected from the forest floor, by scraping into a plastic jar. The deposits had entrained vegetation and soil fragments, which needed to be removed. To do this, the pooled guano sample was dried (60 oC), lightly ground with a pestle and mortar, and fragments of vegetation and soil were removed by hand. Subsequent fine grinding (0.25 mm) was part of the stable isotope analysis. After transport in a dark insulated container to the laboratory, arthropods were collected from the soil samples by Tullgren extraction into water. Arthropods were identified to genus or species level where possible. Once collected, arthropods were starved for 24 h to empty their gut, killed by freezing at 18 oC, and dried at 50 oC. All taxa with sufficient sample mass (either as individuals or pooled) were analysed isotopically. Voucher specimens are deposited with the Entomology Research Museum, Lincoln University (New Zealand).
Stable isotope ratios were measured on finely ground material using a Thermo-Finnigan DeltaPlus (Bremen, Germany) continuous-flow isotope ratio mass spectrometer at the NIWA Stable Isotope Laboratory (Wellington, New Zealand). Larger arthropods (≥0.5 mg) were analysed individually. Small arthropods were pooled for analysis, replicated where possible (). Isotope ratios were calculated as a per mil (‰) deviation from the international limestone standard VPDB (13C/12C) or atmospheric N (15N/14N) and reported as δ13C and δ15N. The standard deviations of repeated analyses of L-glutamic acid (NIST 8573) were 0.09‰ (δ13C) and 0.07‰ (δ15N).
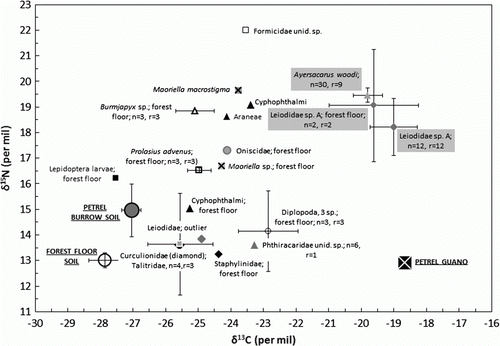
Figure 1 Isotopic enrichment (mean ± SD) in soil arthropods from a Westland petrel colony. Animals were collected from within burrows unless noted. Burrow and forest floor data for the Talitridae, as known leaf litter detritivores, are shown combined. The number of animals and analysis replicates for each taxon is given by n and r; unreplicated taxa have no values shown. One soil sample was analysed per burrow; guano data came from a single pooled sample. The error bars for burrow Leiodidae do not include the outlier. Key: Acari, grey triangles; Arachnida, black triangles; Chilopoda, crosses; Diplopoda, open circles; Diplura, open triangle; Isopoda, grey circle; Talitridae, grey square; Coleoptera (various), diamonds; Leiodidae (Coleoptera), grey diamonds; Lepidoptera, black square; Formicidae, open squares. Filled labels indicate marine C consumers; soil and guano isotopic end-members are shown by the large symbols.
Some seabird burrow arthropods may consume soil derived from terrestrial C (Hawke & Clark Citation2010b). Statistical analysis therefore began by comparing the soil from occupied burrows, unoccupied burrows, and the forest floor. Non-parametric statistics were used where possible, in recognition of the small sample sizes. Each isotope was compared separately, using non-parametric one-way ANOVA (Kruskal-Wallis test) and a Dunn's Multiple Comparisons post-hoc test. Having shown that burrow occupation did not affect either isotope in soil, we then compared the isotopic signatures of the most common burrow arthropods (the mesostigmatic mite Ayersacarus woodi Clark, 2011 and a leiodid beetle) between occupied and unoccupied burrows using a Mann-Whitney U-test. The absence of a significant difference in either isotope across both taxa, combined with the absence of a significant difference in soil isotopic signature led us to combine all burrow arthropod data.
We tested the hypothesis that the burrow arthropod fauna has an isotopic enrichment distinct from the fauna of the adjacent forest floor in two ways. First, two-step cluster analysis identified the optimal number of clusters for all taxa according to provenance, data from occupied and unoccupied burrows being combined as previously noted. Isotopes were evaluated both together and separately, as high δ13C reflects marine input whereas high δ15N can result from marine input, an open N cycle, or a high trophic level. Secondly, the four taxa found both in burrows and on the forest floor were compared for each isotope using paired t-tests, there being insufficient taxa for a non-parametric test. To evaluate the isotopic niches provided by petrel detritus, we compared δ13C and δ15N between the two taxa dependent on marine C (the leiodid beetle and A. woodi) by Mann-Whitney U-test. Pairing across burrows was explored, but only four burrow samples contained both taxa in sufficient quantities for isotopic analysis and the results differed little from the unpaired analysis. Statistical software was IBM SPSS 19 (IBM Corporation, Armonk, NY, USA; cluster analysis) or GraphPad InStat (GraphPad Software Inc., La Jolla, CA, USA; remaining tests).
Results
Burrow and forest floor soils both had much lower δ13C than petrel guano (). Occupied burrow soil (n = 7), unoccupied burrow soil (n=7), and forest floor soil (n=5) had different mean isotopic values (one-way ANOVA; δ13C, Kruskal-Wallis statistic=8.3, P=0.004; δ15N, Kruskal-Wallis statistic=10.6, P=0.0007). For both isotopes, Dunn's Multiple Comparisons post-tests showed insignificant differences between occupied and unoccupied burrows; similar results were obtained using parametric statistics (results not shown). Significant differences were found between forest soil and occupied burrow soil (δ13C, P<0.05; δ15N, P<0.01), but differences were less clear between unoccupied burrows and forest soil (δ13C, not significant; δ15N, P<0.05). Measurements of soil microbial C concentration showed a similar pattern, of insignificant differences between burrow types but a significant difference between each type of burrow and the forest floor (JRV, unpub. results).
Ayersacarus woodi and the leiodid beetle were found in sufficient numbers across occupied and unoccupied burrows for a comparison of the effect of burrow occupancy on isotopic signature. For both taxa and both isotopes, burrow occupancy effects were not significant (A. woodi; δ13C, U=8.0, P = 0.90; δ15N, U = 7.0, P = 0.71, r=3 replicates from occupied burrows, r=6 from unoccupied burrows; leiodid beetle; δ13C, U=14.0, P=0.93; δ15N, U=11.0, P=0.54, n=5 individuals from occupied burrows, n=6 from unoccupied burrows).
Seventeen taxa had sufficient numbers for pooled isotopic analysis or were large enough as individuals (). These taxa came from groups of known detritivores (Talitridae, Diplopoda) or predators (Chilopoda, Arachnida), as well as taxa with less well defined trophic levels. Ayersacarus woodi occurred in all burrows (both occupied and unoccupied), but nowhere on the forest floor. Four taxa were found both in burrows and on the forest floor. The leiodid beetle was found in 12 (86%) of the 14 burrows and one (17%) of the six forest floor transect points; a centipede (Maoriella sp. Attems, 1903) was found in one (14%) occupied burrow and one (17%) forest floor transect point; an amphipod (Talitridae) was found in one (14%) each of occupied and unoccupied burrows, and three (50%) forest floor transect points; and a harvestman (Cyphophthalmi) was found in two (29%) occupied burrows and one (17%) forest floor transect point.
Using δ13C to identify marine input, the optimal number of clusters was two, regardless of distance measure or clustering criterion. Contrary to our hypothesis, membership of the clusters was not governed by whether the animals came from petrel burrows or from the forest floor. The marine-like cluster comprised the leiodid beetle (whether from the forest floor or from the combined burrow types), and A. woodi. Incorporating both δ13C and δ15N also yielded two clusters, an unidentified ant (Formicidae; high δ15N) from a petrel burrow being added to the marine cluster already identified using δ13C. The four taxa found both in burrows (occupied or unoccupied) and on the forest floor did not differ by provenance, in either isotope (δ13C, P = 0.56, paired t = 0.66, d.f. = 3; δ15N, P = 0.37, paired t = 1.1). Compared with A. woodi, the median δ13C of leiodid beetles from the combined burrow types was significantly higher (U = 27.0, P = 0.03, A. woodi r = 9, leiodid beetle n = 14, difference between medians 0.7‰) and the median δ15N was significantly lower (U = 23.0, P = 0.01, difference between medians 1.1‰).
Taxa with a terrestrial δ13C enclosed a much larger isotopic space (range of δ13C, 14‰; δ15N, 9‰) than the two taxa with a marine δ13C (δ13C, 1‰; δ15N, 2‰). As expected, predators (Chilopoda, Arachnida) consuming terrestrial C had higher δ15N than detritivores (Diplopoda, Talitridae). Of the replicated taxa, the dipluran Burmjapyx Silvestri, 1931 had δ15N comparable to the predators, implying that the species we sampled is a predator. The ant Prolasius advenus (Smith, 1862) also had a moderately high δ15N. Among the unreplicated taxa, the single oniscid isopod had a much higher δ15N than expected for a detritivore, and the unidentified ant species had the highest δ15N of all individual animals collected. One leiodid beetle from a petrel burrow had a ‘terrestrial’ δ13C and could have been a different species, many leiodid species being found in New Zealand forest (Leschen Citation2000). In contrast to the terrestrial C consumers, there was a c. 5‰ gap in δ15N between the putative marine C source (guano) and marine C consumers ().
Discussion
Many researchers have argued that food web structure and dynamics are affected by the size of any nutrient subsidy (Polis et al. Citation1997; Marczak et al. Citation2007). Our study, over a small spatial scale, did not support the hypothesis that Westland petrel colony soil arthropods have an isotopic signature defined by their provenance in burrows or on the forest floor. Instead, there were two groups of soil arthropods based on their δ13C. Most taxa, whether from burrows or from the forest floor, had a low δ13C indicative of terrestrial C consumption. The large range of δ13C values displayed by the terrestrial C group is consistent with food webs based on leaf litter fractions having a wide range of δ13C values (Bowling et al. Citation2008), the different fractions providing ecological niches for a diversity of soil arthropods (Pollierer et al. Citation2009). Terrestrial C burrow invertebrates may be feeding within the burrow, or merely using the burrow for shelter. Although replication within the terrestrial C taxa was limited, δ15N was distributed along predictable trophic lines. All δ15N values were marine, implying that marine N dominates N cycling whether inside or outside burrows.
The high δ13C group, which comprised only two of the 17 taxa, was either found exclusively (A. woodi) or predominantly (leiodid beetle) in petrel burrows. Ayersacarus woodi depends on petrel guano, most likely predating soil mesofauna or their eggs (Clark & Hawke Citation2011). The most commonly observed marine detritus (guano, feathers, egg membrane) vary in their δ13C, with guano being least enriched in 13C (Hawke & Clark Citation2010b; Clark & Hawke Citation2011). The small but significant differences in both δ13C and δ15N between the two imply complementary ecological niches, with both taxa ultimately depending on petrel guano. We have only ever found A. woodi in burrows, but the isotopic similarity between samples of leiodid beetle from burrows and the forest floor implies that forest floor leiodids may be supported by petrel guano deposited outside the burrow. The role of petrels in leiodid beetle nutrition identified here is supported by the higher abundance of leiodid beetles in areas with burrowing petrels (Gardner-Gee & Beggs Citation2009). The commensalism between A. woodi and Westland petrels implied by the δ13C results is interesting in the context of other relationships between mites and seabirds. Relationships are often parasitic (Gómez-Díaz & González-Solís Citation2010), but mutualism is also important and possibly under-reported (Blanco et al. Citation1997).
The separation of arthropod δ13C into two clusters contrasted with results from blue penguin Eudyptula minor (Forster, 1781) burrows (Hawke & Clark Citation2010b). Here, δ13C of soil arthropods was a continuum between marine and terrestrial, consistent with co-metabolism of various combinations of feathers, guano and soil. Unlike Westland petrels, however, blue penguins occupy their burrows year-round. As well as using their burrows for breeding, blue penguins come ashore each day to roost, and use their their burrows for the annual moult (Marchant & Higgins Citation1990). Alternatively, the distribution we found with Westland petrels may be a wintertime sampling artefact, and sampling at warmer times of the year when soil arthropods are more abundant may reveal taxa between the marine and terrestrial extremes of δ13C.
The small (but significant) isotopic differences between the two marine C consumers (A. woodi, leiodid beetle) implies that petrel detritus provides a simpler range of feeding opportunities than does terrestrial C from forest litter. The proportion of petrel C that passes through A. woodi and the leiodid beetle is unknown. Even though many invertebrates graze microbes rather than feeding directly on a particular substrate, a significant proportion of petrel C may conceivably bypass arthropods entirely to be remineralized microbially ( in Gessner et al. Citation2010).
We did not identify any arthropod consumers of either A. woodi or the leiodid beetle, despite the diversity of predatory fauna in our samples. The role of non-arthropod fauna is unclear. Among vertebrates, weka (Gallirallus australis) (Sparrman, 1786), an endemic flightless rail, may consume petrel-dependent invertebrates (Hawke & Holdaway Citation2005). Although lizards prey on seabird burrow arthropods on predator-free islands around New Zealand (Markwell & Daugherty Citation2002; Hawke & Clark Citation2010a), introduced mammalian predators have greatly decreased lizard abundance at mainland sites such as ours (Worthy & Holdaway Citation2002). Similarly, introduced predators have led to behavioural changes in still-common avian insectivores such as bellbirds (Anthornis melanura) (Sparrman, 1786); bellbirds feed on the ground in the absence of introduced predators (Hawke & Holdaway Citation2009) but are otherwise arboreal. Tuatara (Sphenodon spp.) consume both seabird material (eggs and chicks) and burrow invertebrates on Stephens I. (Cree et al. Citation1999), and may also have inhabited mainland seabird sites.
We conclude that marine C from petrels at our study site is conveyed into the terrestrial ecosystem through only a few arthropod taxa, although some caution is needed because we sampled only in winter. From a conservation perspective, the leiodid beetle and mesostigmatic mite that process petrel C must be regarded as an integral part of the Westland petrel system. We recommend investigation of other sites with burrowing petrels, to see if a similar biogeochemical bottleneck is present. Ideally, such investigations would include sites less affected by introduced predators, given that mice and rats adversely affect both abundance and faunal composition of terrestrial invertebrates (Marris Citation2000; Gibbs Citation2009). Investigation of summer breeding petrels would be particularly interesting, as increased summertime invertebrate abundance would coincide with the pulse of detritus from the seabirds.
Acknowledgements
P Johns (Canterbury Museum), W Don, and R Emberson and J Marris (Lincoln University) helped with arthropod identifications. GC Wood (New Zealand Department of Conservation; DOC) guided us through the petrel colony. R Holdaway commented on a draft manuscript, and the journal editor and referees contributed helpful insights. The interest of DOC West Coast Conservancy staff in Westland petrel research is also gratefully acknowledged; the study was carried out under DOC Permit WC-27794-RES.
References
- Best , HA and Owen , KL . 1976 . Distribution of breeding sites of the Westland black petrel (Procellaria westlandica) . Notornis , 23 : 232 – 242 .
- Blanco , G , Tella , JL and Potti , J . 1997 . Feather mites on group-living red-billed choughs: a non-parasitic interaction? . Journal of Avian Biology , 28 : 197 – 206 . doi: 10.2307/3676970
- Bowling , DR , Pataki , DE and Randerson , JT . 2008 . Carbon isotopes in terrestrial ecosystem pools and CO2 fluxes . New Phytologist , 178 : 24 – 40 . doi: 10.1111/j.1469-8137.2007.02342.x
- Clark , JM and Hawke , DJ . 2011 . The sub-Antarctic mite Ayersacarus: a new species from mainland New Zealand, and its isotopic ecology (Acari: Mesostigmata: Leptolaelapidae) . New Zealand Journal of Zoology , 38 : 43 – 54 . doi: 10.1080/03014223.2010.525746
- Cree , A , Lyon , GL , Cartland-Shaw , L and Tyrrell , C . 1999 . Stable carbon isotope ratios as indicators of marine versus terrestrial inputs to the diets of wild and captive tuatara (Sphenodon punctatus) . New Zealand Journal of Zoology , 26 : 243 – 253 . doi: 10.1080/03014223.1999.9518193
- Gardner-Gee , R and Beggs , JR . 2009 . Does the presence of burrowing seabirds increase local invertebrate abundance? . New Zealand Entomologist , 32 : 41 – 47 . doi: 10.1080/00779962.2009.9722174
- Gessner , MO , Swan , CM , Dang , CK , McKie , BG , Bardgett , RD , Wall , DH and Hättenschwiler , S . 2010 . Diversity meets decomposition . Trends in Ecology and Evolution , 25 : 372 – 380 . doi: 10.1016/j.tree.2010.01.010
- Gibbs , GW . 2009 . The end of an 80 million year experiment: a review of evidence describing the impact of introduced rodents on New Zealand's ‘mammal-free’ invertebrate fauna . Biological Invasions , 11 : 1587 – 1593 . doi: 10.1007/s10530-008-9408-x
- Gómez-Díaz E , González-Solís J 2010 . Trophic structure in a seabird-host-parasite food web: insights from stable isotope analysis . PLoS ONE 5: e10454 .
- Harper , GA . 2007 . Detecting predation of a burrow-nesting seabird by two introduced predators, using stable isotopes, dietary analysis and experimental removals . Wildlife Research , 34 : 443 – 453 . doi: 10.1071/WR07037
- Hawke , DJ . 2004 . Maximum possible age of a petrel breeding colony near Punakaiki (South Island, New Zealand) from radiocarbon and stable isotope analysis of soil . Journal of the Royal Society of New Zealand , 34 : 1 – 7 . doi: 10.1080/03014223.2004.9517760
- Hawke , DJ . 2010 . Using 137Cs and 210Pb to characterise soil mixing by burrowing petrels: an exploratory study . New Zealand Journal of Zoology , 37 : 53 – 57 . doi: 10.1080/03014221003692829
- Hawke , DJ and Clark , JM . 2010a . Incorporation of the invasive mallow Lavatera arborea into the food web of an active seabird island . Biological Invasions , 12 : 1805 – 1814 . doi: 10.1007/s10530-009-9591-4
- Hawke , DJ and Clark , JM . 2010b . Isotopic signatures (13C/12C; 15N/14N) of blue penguin burrow soil invertebrates: carbon sources and trophic relationships . New Zealand Journal of Zoology , 37 : 313 – 321 . doi: 10.1080/03014223.2010.519036
- Hawke , DJ and Holdaway , RN . 2005 . Avian assimilation and dispersal of carbon and nitrogen brought ashore by breeding Westland petrels Procellaria westlandica: a stable isotope study . Journal of Zoology , 266 : 419 – 426 . doi: 10.1017/S0952836905007065
- Hawke , DJ and Holdaway , RN . 2009 . Nutrient sources for forest birds captured within an undisturbed petrel colony, and management implications . Emu , 109 : 163 – 169 . doi: 10.1071/MU08035
- Hawke , DJ and Newman , J . 2007 . Carbon-13 and nitrogen-15 enrichment in coastal forest foliage from nutrient-poor and seabird-enriched sites in southern New Zealand . New Zealand Journal of Botany , 45 : 309 – 315 . doi: 10.1080/00288250709509719
- Kolb , GS , Jerling , L and Hambäck , PA . 2010 . The impact of cormorants on plant-arthropod food webs on their nesting islands . Ecosystems , 13 : 353 – 366 . doi: 10.1007/s10021-010-9323-8
- Leschen , RAB . 2000 . Pseudoliodini (Coleopteran: Leiodidae: Leiodinae) of New Zealand . New Zealand Entomologist , 22 : 33 – 44 . doi: 10.1080/00779962.1999.9722053
- Major , HL , Jones , IL , Charette , MR and Diamond , AW . 2007 . Variations in the diet of introduced Norway rats (Rattus norvegicus) inferred using stable isotope analysis . Journal of Zoology , 271 : 463 – 468 . doi: 10.1111/j.1469-7998.2006.00230.x
- Maraun , M , Erdmann , G , Fischer , BM , Pollierer , MM , Norton , RA , Schneider , K and Scheu , S . 2011 . Stable isotopes revisited: their use and limits for oribatid mite trophic ecology . Soil Biology and Biochemistry , 43 : 877 – 882 . doi: 10.1016/j.soilbio.2011.01.003
- Marchant S , Higgins PJ 1990 . Handbook of Australian, New Zealand and Antarctic birds . 1A . Ratites to petrels . Melbourne : Oxford University Press . 1408
- Marczak , LB , Thompson , RM and Richardson , JS . 2007 . Meta-analysis: trophic level, habitat, and productivity shape the foodweb effects of resource subsidies . Ecology , 88 : 140 – 148 . doi: 10.1890/0012-9658(2007)88[140:MTLHAP]2.0.CO;2
- Markwell , TJ and Daugherty , CH . 2002 . Invertebrate and lizard abundance is greater on seabird-inhabited islands than on seabird-free islands in the Marlborough Sounds, New Zealand . EcoScience , 9 : 293 – 299 .
- Marris , JWM . 2000 . The beetle (Coleoptera) fauna of the Antipodes Islands, with comments on the impact of mice; and an annotated checklist of the insect and arachnid fauna . Journal of the Royal Society of New Zealand , 30 : 169 – 195 . doi: 10.1080/03014223.2000.9517616
- Oelbermann , K and Scheu , S . 2010 . Trophic guilds of generalist feeders in soil animal communities as indicated by stable isotope analysis (15N/14N) . Bulletin of Entomological Research , 100 : 511 – 520 . doi: 10.1017/S0007485309990587
- Polis , GA , Anderson , WB and Holt , RD . 1997 . Toward an integration of landscape and food web ecology: the dynamics of spatially subsidized food webs . Annual Reviews of Ecology and Systematics , 28 : 289 – 316 . doi: 10.1146/annurev.ecolsys.28.1.289
- Polis , GA , Sánchez-Piñero , F , Stapp , PT , Anderson , WB and Rose , MD . 2004 . “ Trophic flows from water to land: marine input affects food webs of islands and coastal ecosystems worldwide ” . In Food webs at the landscape level , Edited by: Polis , GA , Power , ME and Huxel , GR . 200 – 216 . Chicago : Chicago University Press .
- Pollierer , MM , Langel , R , Scheu , S and Maraun , M . 2009 . Compartmentalization of the soil animal food web as indicated by dual analysis of stable isotope ratios (15N/14N and 13C/12C) . Soil Biology and Biochemistry , 41 : 1221 – 1226 . doi: 10.1016/j.soilbio.2009.03.002
- Wainwright , SC , Haney , JC , Kerr , C , Golovkin , AN and Flint , MV . 1998 . Utilization of nitrogen derived from seabird guano by terrestrial and marine plants at St. Paul, Pribilof Islands, Bering Sea, Alaska . Marine Biology , 131 : 63 – 71 . doi: 10.1007/s002270050297
- Waugh , SM , Cabrera , H , Wood , GC and Davis , LS . 2003 . Burrow occupancy in Westland petrels (Procellaria westlandica) . Notornis , 50 : 123 – 127 .
- Worthy , TH and Holdaway , RN . 2002 . The lost world of the moa , 718 Bloomington : Indiana University Press .
- Xu L-Q , Liu X-D , Sun L-G , Yan H , Liu Y , Luo Y-H , Huang J 2011 . Geochemical evidence for the development of coral island ecosystem in the Xisha Archipelago of South China Sea from four ornithogenic sediment profiles . Chemical Geology 286: 135 145