Abstract
The first pre-Quaternary anurans from New Zealand are reported from the Early Miocene (19–16 Ma) St Bathans Fauna based on 10 fossil bones. Four bones representing two new species differing in size are described in Leiopelma: Leiopelmatidae, and are the first Tertiary records for the family. Six indeterminate frog fossils are morphologically similar to leiopelmatids and represent two species consistent in size with those known from diagnostic material. These records are highly significant, as minimally, they reduce the duration of the leiopelmatid ‘ghost lineage’ by c.20 million years and demonstrate that a diversity of leiopelmatids has long been present on New Zealand, supporting the ancient dichotomy of the extant species based on molecular data.
Leiopelma miocaenale Worthy, Tennyson, Scofield & Hand in pressurn:lsid:zoobank.org:act:83F24868-A212-435B-B892-AD510796B5BB
Leiopelma acricarina Worthy, Tennyson, Scofield & Hand in pressurn:lsid:zoobank.org:act:D3955F53-37C2-4EAD-B0BC-CE8D23FCD51B
Introduction
The genus Leiopelma Fitzinger, 1861 includes the only anurans naturally known from New Zealand, with four extant and three extinct Holocene species (Bell et al. Citation1998; King et al. Citation2009). In contrast, on the nearest landmass of Australia, 227 species are listed in a recent checklist (Tyler & Knight Citation2009) in five families Myobatrachidae, Limnodynastidae, Hylidae, Microhylidae and Ranidae, which are widely distributed on the neobatrachian phylogeny (Pyron & Wiens Citation2011). The extant Leiopelma species include three terrestrial and morphologically very similar allopatric taxa of which Leiopelma archeyi is the smallest species and Leiopelma hamiltoni and Leiopelma pakeka are larger, attaining 46 and 50 mm snout–vent length respectively (Bell Citation2010). The species status of L. pakeka is debated: it was distinguished from the nearby population of L. hamiltoni using multivariate analyses of 19 external measurements taken from relatively few living animals and some allozyme differences (Bell et al. Citation1998), but its specific distinction is not supported by DNA analyses (Holyoake et al. Citation2001). The few available bones of L. hamiltoni—as restricted to Stephens Island, sensu Bell et al. (1998), and whose rarity has precluded the availability of skeletons—do not differ from those of L. pakeka (observations herein). Given that bones of L. archeyi differ only in size from those of L. pakeka (see Worthy Citation1987, where bones of this species were studied under the name L. hamiltoni) it is likely that most isolated bones of L. hamiltoni will be morphologically indistinguishable from L. pakeka. The fourth extant species is the more robust aquatic taxon Leiopelma hochstetteri, which attains 44 mm snout–vent length (Bell Citation2010). The dichotomy of the terrestrial versus the aquatic taxa is supported by numerous other data (e.g. Green et al. Citation1989; Bell Citation2010) and is maintained with the addition of the three extinct and much larger taxa. Of these, Leiopelma markhami and Leiopelma auroraensis are osteologically more similar to L. hochstetteri, and Leiopelma waitomoensis is most similar to L. hamiltoni (see Worthy Citation1987).
Leiopelma species together with North America's two Ascaphus species have long been considered to be the sister group to remaining crown group anurans and have together been placed in Leiopelmatidae (e.g. Duellman & Trueb Citation1994; Frost et al. Citation2006). Characters shared by Leiopelma and Ascaphus include the presumed plesiomorphic states of nine presacral amphicoelous and ectochordal vertebrae in which the notochord is persistent in the adult, intervertebral cartilages that are undivided, and the presence of the tail-wagging muscle m. caudalipuboischiotibialis (Noble Citation1924; Ritland Citation1955; Frost et al. Citation2006). Synapomorphies of Leiopelmatidae (sensu Frost et al. Citation2006; Ascaphus and Leiopelma) include molecular evidence and loss of the columella (Stephenson NG Citation1951; Frost et al. Citation2006). Ritland (Citation1955) also suggested that the presence of the tail-wagging muscle m. caudalipuboischiotibialis might be an apomorphy for the Leiopelma +Ascaphus clade. In addition, both genera have an epipubis, which is otherwise only seen in Xenopus among Anura (Stephenson EM Citation1952) and an absence of vocal sacs (Green & Cannatella Citation1993). Synapomorphies of Leiopelma include the presence of ventral inscriptional ribs, low diploid chromosome numbers (18–22), absence of horny beaks in the larvae, and reduction of opercular folds during development resulting in the lack of a closed branchial chamber and spiracle (Noble Citation1924, Citation1931; Ritland Citation1955; Stephenson EM Citation1952, Citation1960; Green & Cannatella Citation1993; Bell Citation2010).
Analyses of morphological and molecular data have indicated that Ascaphus and Leiopelma are deeply divergent sister taxa of the order of 180–200 million years ago (Ma) (Green et al. Citation1989; Hay et al. Citation1995; Roelants and Bossuyt Citation2005; San Mauro et al. Citation2005; Frost et al. Citation2006; Roelants et al. Citation2007; San Mauro Citation2010). A dissenting opinion was given by Ford & Cannatella (Citation1993) in their revision of the anuran classification based on existing evidence, wherein they advocated a non-sister-group relationship, with Ascaphus sister to a clade of Leiopelma and remaining anurans. Most recently, the sister relationship of Ascaphus and Leiopelma was supported by analyses of complete mitochondrial genomes (Irisarri et al. Citation2010) and comprehensive analyses of molecular data (2871 species including 86% genera) by Pyron & Wiens (2011), who advocated that Leiopelma and Ascaphus be placed in Leiopelmatidae and Ascaphidae, respectively.
The oldest fossil anuran is Prosalirus bitis Shubin and Jenkins, 1995, which is known from the Early Jurassic in Arizona from partly articulated three-dimensional bones (Roček Citation1994). This taxon shares several plesiomorphic features with Leiopelma, including notochordal amphicoelous vertebrae with free ribs, an ilium with a very low tuber superior that is relatively anteriorly placed, but differs in many ways including retention of the columella, possessing a more elongate premaxilla with more teeth, and humerus with a small unossified eminentia capitata (Roček Citation1994; Jenkins & Shubin Citation1998). Gondwanan taxa such as the Early Jurassic Vieraella Reig, 1961 from southern Patagonia in Argentina, and especially the well-known Middle to Late Jurassic Notobatrachus Reig, 1955 (in Stipanicic & Reig Citation1955), also from Patagonia, share many morphological features with Leiopelma and were placed in Ascaphidae, now Leiopelmatidae, by Estes & Reig (1973). More recently, Roček (Citation2000) considered Vieraella to be a structural ancestor to Leiopelmatidae and confirmed the leiopelmatid relationships of Notobatrachus; however, Gao & Wang (Citation2001) provided weak support for Notobatrachus also being basal to Leiopelmatidae. The perceived relationship between Notobatrachus and Leiopelma led to the hypothesis that Leiopelma in New Zealand had a vicariant origin (e.g. Duellman & Trueb Citation1994). The Early to mid-Jurassic separation of Leiopelma and Ascaphus based on molecular divergence estimates supports the ancient history of the Leiopelma lineage in Gondwana and that Vieraella and Notobatrachus could be stem-group leiopelmatids. A vicariant origin for Leiopelma in New Zealand would necessarily date to between 82 and 60 Ma, during which interval Zealandia, the continental fragment on which New Zealand is now emergent, separated from East Gondwana (Gaina et al. Citation1998; Schellart et al. Citation2006). That New Zealand is now separated from Australia by about 2000 km of ocean and Leiopelma is intolerant of salt water adds support for its hypothesized vicariant origin in New Zealand.
Until now the fossil record of Leiopelma has been restricted to Late Pleistocene and Holocene sediments in New Zealand, wherein abundant remains include three species (L. markhami, L. auroraensis and L. waitomoensis), which are much larger than extant taxa (Worthy Citation1987). This combined with the existence of leiopelmatid-grade frogs in the Middle Jurassic and the even earlier calculated divergence of Ascaphus from Leiopelma at about 200 Ma based on molecular data (Roelants et al. Citation2007), requires the existence of a ghost lineage extending from the Early Jurassic to the Late Pleistocene. We report here the discovery of leiopelmatid remains from the St Bathans Fauna (Worthy et al. Citation2007) from Early Miocene deposits in New Zealand. Although these remains are few and fragmentary they result from 10 years of excavations during which more than 30 m3 of fossiliferous beds have been sorted for microvertebrates, producing several thousand avian bones and hundreds of squamate bones, so frog bones are rare in these lacustrine deposits. This rarity is on a par with the remains of other terrestrial taxa such as Sphenodon (Jones et al. Citation2009) and many of the terrestrial birds, e.g. acanthisittid wrens (Worthy et al. Citation2010a). This rarity is therefore real and not related to limited search effort and justifies presentation of these data at this time. These records are highly significant, as at the very least, they significantly reduce the duration of the leiopelmatid ‘ghost lineage’ in New Zealand and demonstrate a diversity of leiopelmatids in New Zealand in the Early Miocene.
Materials and methods
Abbreviations
Ma, million years; NMNZ, Museum of New Zealand Te Papa Tongarewa, Wellington, New Zealand; SAM, South Australia Museum, Adelaide, South Australia.
Comparisons
The frog bones were compared to the detailed descriptions and images given by Stephenson EM (Citation1952, Citation1960) and Worthy (Citation1987) and to the following specimens: Leiopelmatidae: L. waitomoensis NMNZ S.23415 holotype, NMNZ S. 39702; L. markhami NMNZ S.23120 (holotype) and the following paratypes NMNZ S.23121, 23140, 23152, 23414; L. auroraensis NMNZ S.23413, holotype; L. hochstetteri NMNZ AM.201, 206, and referred Holocene fossils NMNZ S.35163; L. pakeka, NMNZ AM.198; L. hamiltoni, NMNZ AM.293, and referred Holocene fossils NMNZ S.27939, S.39263. Scaphiopodidae: Scaphiopus couchii, SAM R66850. In addition, modern skeletons of representatives of families known from the Australasian region were examined as follows: Hylidae: Cyclorana platycephalus, SAM R66915; Cyclorana australis, SAM R66914; Litoria adelaidensis, SAM R66348; Litoria flavipunctata, SAM R66910; Litoria cyclorhyncha, SAM R66911; Litoria fallax, SAM R66349; Litoria dahlia, SAM R66351, SAM R66352; Litoria nannotis, SAM R66909; Nyctimystes pulchra, SAM R66912. Microhylidae: Phrynomantis lateralis, SAM R66354; Hylophorbus rufescens, SAM R66913. Myobatrachidae: Rheobatrachus silus, SAM R66350, R67304; Mixophyes schevilli, SAM R67306; Uperoleia crassa, SAM R67305. Ranidae: Platymantis vitianus, NMNZ AM272, AM273. Bufonidae: Rhinella marina (formerly Bufo marinus), NMNZ AM294.
Sites and excavation
The fossils described here all derive from the St Bathans Fauna of the lower Bannockburn Formation, Manuherikia Group, Early Miocene (Altonian), 19–16 Ma, which is the only fossil fauna from the Tertiary of New Zealand preserving a terrestrial vertebrate biota (Worthy et al. Citation2007). This fauna was deposited in littoral zone lacustrine sediments in a palaeolake some 5600 km2 in area (Pole et al. Citation2003), now widely exposed in the Central Otago region. This fauna is dominated by fish, but includes sphenodontines, crocodilians, turtles, lizards, many birds, bats and a non-therian mammal (Molnar & Pole Citation1997; Lee MSY et al. Citation2009; Jones et al. Citation2009; Scofield et al. Citation2010; Worthy et al. Citation2006, Citation2007, Citation2008, Citation2009, Citation2010a, Citation2010b, Citation2011a, Citation2011b). The fossils derive from three specific locations as follows and described by Worthy et al. (Citation2007) and Schwarzhans et al. (Citation2012):
Bed HH1a, Manuherikia River Site, a c.5–10 cm thick sand and mud cobble layer with abundant bone and stromatolite fragments, 6.88–7.0 m above the base of the Bannockburn Formation; true left side Manuherikia River, Home Hills Station, St Bathans, Otago, New Zealand; 44.90794°S, 169.85822°E; New Zealand Fossil Record File Number H41/f88.
Bed HH1b Trench excavation, Manuherikia River Site, at foot of hill 50 m across terrace from river bank, at 44.90780°S, 169.85844°E; c.10 cm thick sand and coarse cobble layer, c.9.5–9.58 m above the base of the Bannockburn Formation; Home Hills Station, Otago; New Zealand Fossil Record File Number H41/f0103.
Site 2, Vinegar Hill, St Bathans, Otago, outcrop c.1.8 m above base of the Bannockburn Formation; 44.87808°S, 169.74975°E; New Zealand Fossil Record File Number H41/f0111.
Fossiliferous layers were excavated by hand with trowels following removal of overburden where necessary. Sediment was washed through a 1-mm mesh sieve, dried, and the concentrate was sorted under magnification with a binocular microscope. Measurements were made with Tesa® dial callipers and rounded to 0.1 mm.
Nomenclature
The fossils were compared within the phylogenetic framework offered by Frost et al. (Citation2006) and Pyron & Wiens (Citation2011), and we follow the clade names therein advocated. We restrict Leiopelmatidae to Leiopelma following Pyron & Wiens (Citation2011). Anatomical terminology follows Sanchiz (Citation1998).
Systematic palaeontology
SUBCLASS: Batrachia Latreille, 1800
ORDER: Anura Fischer von Waldheim, 1813
FAMILY: Leiopelmatidae Mivart, 1869
Leiopelma Fitzinger, 1861
The fossils are assigned to Leiopelma because they share the following unique combination of characters (although it is acknowledged that in this plesiomorphic taxon no single osteological feature is autapomorphic): ilium with a low, elongate and relatively anteriorly placed tuber superior without evidence of a secondary elevation, an uncompressed elliptical pars cylindriformis (ilial shaft) without a crista dorsalis, a low angled pars ascendens, a simple pars descendens without development medially of an enlarged flange in the preacetabular region, and no interiliac tubercle (Tyler Citation1976; Worthy Citation1987); humeri with a relatively narrow ossified eminentia capitata and a relatively large epicondylus radialis; and vertebrae that are amphicoelous and ectochordal, with a relatively broad foramen vertebrale, a low carina neuralis, and which lack a significant mid-ventral ridge. Prosalirus shares several of these ilial features with Leiopelma, indicating their plesiomorphic nature, but differs in lacking a supracetabular fossa, the presence of a shaft with a low crista dorsalis, and a pair of short parallel ridges extending dorsoposteriorly–ventroanteriorly along the lateral surface of the base of the shaft (Jenkins & Shubin Citation1998; Gardner et al. Citation2010).
Leiopelma miocaenale sp. nov.
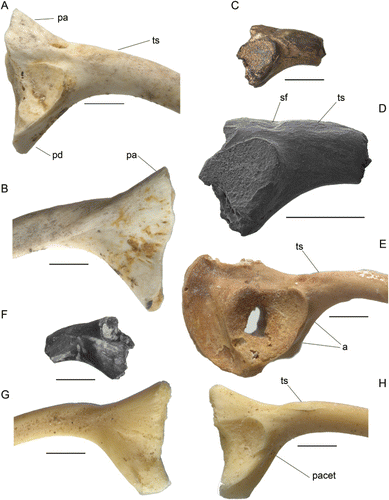
Holotype. NMNZ S.52919, a partial right ilium (C, D, F).
Differential diagnosis. A frog similar in size to smaller individuals of L. waitomoensis and distinguished from all leiopelmatids by the following unique combination of features: a low, elongate tuber superior whose posterior end slightly overlaps the anterior extent of the acetabular margin, the depth of the ilial shaft at the posterior end of the tuber superior is much greater than at its anterior end, the supracetabular fossa is located posterior to the anterior rim of the acetabulum, and, in lateral view, the dorsal and ventral profiles are weakly divergent (c.47°) posteriorly of the tuber superior.
Ilia of the extant leiopelmatids L. hamiltoni (including L. pakeka), L. hochstetteri and L. archeyi are very much smaller than the fossil, with the diameter of the ilial shaft less than half that in the fossil. All three extinct Holocene leiopelmatids have ilia approximating the size of the fossil, but all differ in the more divergent planes in lateral view of the dorsal profile posterior to the tuber superior and the ventral profile of the pars descendens (see Worthy Citation1987: figure 28), which in each species forms an angle of c.80° (compared with a markedly less divergent 47° in the fossil). In addition, the pars ascendens projects dorsally at a distinct angle of about 25° to the plane over the base of the tuber superior in all other Leiopelma species. In the fossil, although only a length of about 2 mm is preserved posterior of the tuber superior, this includes the supracetabular fossa and the base of the pars ascendens and over its length the dorsal profile remains parallel to the alignment of the tuber superior, not rising markedly as other Recent leiopelmatids (). In addition, ilia of L. waitomoensis, L. pakeka (bones described under the name L. hamiltoni) and L. archeyi were described as lacking a dorsal prominence (= tuber superior) by Worthy (Citation1987). However, while it is true that a prominence is lacking, the ligamental scar for musculus gluteus maximus (Tyler Citation1976) that attaches to the tuber superior is present and is confluent with the shaft surface (e.g. L. waitomoensis; A). The distinct slightly elevated tuber superior of the fossil is more similar to that in the remaining leiopelmatid taxa (D, E, H).
The large extinct Leiopelma species each differ from the fossil in their ilial morphology. Ilia of L. waitomoensis (A, B) share with NMNZ S.52919 a similar rapid attenuation of shaft depth between the posterior and anterior ends of the tuber superior, but differ, in addition to the lower tuber superior, with the posterior margin of the tuber superior level with the anterior rim of the acetabulum (not overlapping) and by a larger supracetabular fossa, which is more separated dorsally from the acetabulum and extends farther posteriorly. NMNZ S.52919 is smaller than ilia of L. waitomoensis except for those from the northern parts of the range of this species, which displays a marked latitudinal cline with northern individuals smallest (Worthy Citation1987). Ilia of L. markhami (G, H) are about the same size as the fossil but differ as follows: the tuber superior is more anteriorly placed and is distinctly separated from the acetabular rim; the supracetabular fossa extends well anterior to the anterior margin of the acetabulum; and the depths of the shaft at the posterior and anterior ends of the tuber superior are roughly similar, so that the dorsal and ventral shaft margins are subparallel in this region (not markedly convergent anteriorly). The ilium of L. auroraensis, known only from the holotype (E), is similar in size to that of the fossil and shares with it an anteriorly attenuating shaft depth along the length of the tuber superior, but differs, as do all Recent leiopelmatids, in the more divergent planes in lateral view of the dorsal profile posterior to the tuber superior and the ventral profile of the pars descendens. The fossil differs further from ilia of L. auroraensis and L. markhami by the presence of a ridge that increases in height posteriorly and is located above the dorsal rim of the acetabulum (breakage posteriorly reveals this ridge in partial section) (D). In these Holocene taxa, the laterodorsal facies above the rim is flat to slightly concave rather than convex.
Ilia of Ascaphus, as figured by Gardner et al. (Citation2010), differ markedly from those of Leiopelma and the fossil by the following features: the pars descendens is relatively larger and descends from the shaft at a near right angle, the tuber superior extensively overlaps the acetabulum posteriorly, and the supracetabular fossa is located further posteriorly and adjacent to the ischial margin. Ascaphus also has a small pars ascendens that barely projects dorsally of the alignment of the tuber superior and the shaft.
Etymology. Gender neuter; named for its Miocene age, from the suffix -alis, belonging or pertaining to.
Type locality. Bed HH1b Trench excavation, Manuherikia River Site, collected 3 March 2010.
Stratigraphy/Age/Fauna. Bannockburn Formation, Manuherikia Group, Early Miocene (Altonian); 19–16 Ma; St Bathans Fauna.
Measurements (mm). Maximum preserved height over acetabulum 3.20 mm; height at anterior end of tuber superior 1.78 mm.
Description and comparison. NMNZ S.52919 is part of a worn ilium that has lost most of the pars ascendens and the pars cylindriformis (ilial shaft) from just anterior to the tuber superior (= dorsal prominence). The ischial margin adjacent to the acetabulum is eroded and the acetabulum retains some sediment in it contributing to a shallow appearance. NMNZ S.52919 has the following features: 1, the tuber superior is about 1.8 mm long, and assuming the tuber base is horizontal, slightly overlaps posteriorly with the acetabular margin; 2, depth of the pars cylindriformis at the posterior end of the tuber superior is much greater than at its anterior end because of rapid attenuation of shaft depth anterior to the acetabulum; 3, the posterior side of the tuber superior abuts a shallow sulcus (supracetabular fossa), which is separated from the acetabular rim by a rounded ridge and lies well posterior to the anterior rim of the acetabulum; 4, the pars descendens (ventral acetabular expansion) is very poorly developed as typifies leiopelmatids and presents a flattened facies anteriorly that is bound medioventrally by a low ridge that projects only slightly anteroventrally of the ventral acetabular rim (in lateral view) (not developed into a distinct flange as in e.g. Litoria sp.); 5, the preserved dorsal profile of the base of the pars ascendens suggests a low angle of divergence from the axis of the tuber superior; and 6, the ilial shaft just anterior to the tuber superior is elliptical with no evidence of a crista dorsalis.
Ilia of other taxa differ from Leiopelma most notably, but not only, by the following features: discoglossoids, e.g. Latonia, have a well-developed prominent tuber superior and a prominent interiliac tubercle (Roček Citation1994; Holman Citation1998; Folie et al. Citationin press); pipids have a very well-developed tuber superior located posterior to the anterior margin of the acetabulum, and markedly reduced pars ascendens and pars descendens (Báez et al. Citation2012); rhinophrynids have a well-developed and relatively posteriorly located tuber superior (Roček & Rage Citation2000); scaphiopodids have a large tuber superior located posterior to the anterior rim of the acetabulum and a large pars descendens (e.g. Scaphiopus couchii; Roček & Rage Citation2000); pelodytids either lack a tuber superior, or if present it is low and located posterior to the anterior margin of acetabulum (Holman Citation1998; Roček & Rage Citation2000); pelobatids lack a tuber superior and may have an interiliac tubercle (Holman Citation1998; Folie et al. Citationin press); palaeobatrachids have an interiliac tubercle and a well-developed tuber superior (Roček & Rage Citation2000); all neobatrachians have a distinct and prominent tuber superior that varies in its location relative to the acetabulum (which except in some myobatrachids is usually strongly overlapping with or entirely posterior to the anterior rim of the acetabulum), in its orientation dorsally (e.g. myobatrachids) or laterally (e.g. hylids), and in the distinction of a dorsal protuberance from the dorsal prominence, and many neobatrachians have a prominent dorsal crest, e.g. Limnodynastidae and all ranoids (Tyler Citation1976, Citation1982; Holman Citation1998; Roček & Rage Citation2000; Folie et al. Citationin press). Among Australasian taxa, myobatrachids, hylids, microhylids including the fossil genus Australobatrachus, and ranids differ further from leiopelmatids by a well-developed pars descendens or preacetabular medial flange (Tyler Citation1976).
Tentatively referred specimen. NMNZ S.50861, distal left humerus (F, L).
Locality. Bed HH1a, Manuherikia River Site, Otago, New Zealand, collected 10 January 2008: details as above.
Stratigraphy/Age/Fauna. As for holotype.
Measurements (mm). Preserved length 6.5, maximum distal width 5.3, width eminentia capitata 2.4, proximodistal length eminentia capitata 2.9, maximum depth eminentia capitata 3.0, proximodistal length epicondylus ulnaris 2.5.
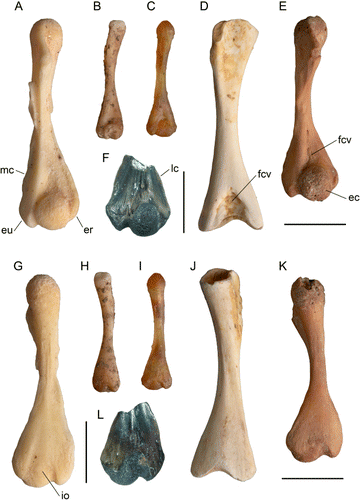
Description and comparison. NMNZ S.50861 is a distal fragment (F, L) preserving less than half the original length of the humerus. The eminentia capitata (capitulum) is well ossified, slightly elongate proximodistally, and occupies 45% of distal width. It has a shallow fossa cubitalis ventralis, well-formed lateral and medial crests, the latter enclosing ventrally a shallow fossa proximal to a large prominent medial epicondylus ulnaris (=entepicondyle). Relative to the eminentia capitata, the epicondylus ulnaris has equal proximal extent, but does not extend so far distally. The lateral epicondylus radialis (ectepicondyle) is prominent, and about 50% of the width of the epicondylus ulnaris, is evenly convex and does not extend as far proximally as the eminentia capitata. The dorsal facies is evenly convex with a low central ridge defined by shallow sulci located towards its proximal end. The epicondylus radialis in dorsal aspect is triangular in shape and merges with the dorsal facies of the eminentia capitata. The impressio olecraneana (olecranon scar) is centrally located and longer than wide. Both facies reveal several nutrient foramina penetrating the bone.
An ossified eminentia capitata defines the clade Leiopelmatidae and all more derived frogs (Gao & Wang Citation2001), although in the Triassic stem-group frog Czatkobatrachus polonicus it was ossified (Evans & Borsuk-Bialynicka Citation1998). In contrast, while members of Caudata have a subspherical eminentia capitata, it usually remains cartilaginous (Sigurdsen & Bolt Citation2009). Compared to distal width, the eminentia capitata is relatively small in leiopelmatids, Notobatrachus, and pipoids (Estes & Reig Citation1973). A small eminentia capitata is probably a primitive feature, because in the temnospondyl Doleserpeton annectens, perhaps the ancestor of lissamphibians, the eminentia capitata was 51.9% of distal width (Sigurdsen & Bolt Citation2009), and in discoglossids and all other taxa more derived than pipoids, it is relatively larger. Leiopelmatids are also characterized by near equal development of the epicondyles, or have a relatively large epicondylus radialis (Estes & Reig Citation1973; Worthy Citation1987). This feature may be an apomorphy, because in the fossil taxa Czatkobatrachus from the Early Triassic, Vieraella herbstii from the Lower Jurassic, and in discoglossids such as Latonia, and all more derived frogs, the epicondylus radialis is relatively reduced (Evans & Borsuk-Bialynicka Citation1998; Roček Citation1994, Citation2000; T.H. Worthy pers. obs.).
NMNZ S.50861 is very similar to humeri of Leiopelma, particularly in the relatively narrow width of the eminentia capitata compared with distal width and relatively large epicondylus radialis and so is referred to Leiopelma. NMNZ S.50861 is tentatively referred to L. miocaenale as, assuming similar proportions to other Leiopelma species, it represents a similar-sized frog. Relative size of the frog can be estimated because the holotype ilium of L. miocaenale is, comparing depths at the posterior side of the tuber superior, noticeably larger than that of the L. markhami paratype NMNZ S.23121 (G, H). The distal width of the humerus in NMNZ S.23121 is 4.4 mm and in NMNZ S.23120 (A, G) is 4.9 mm, that is, slightly smaller than the fossil humerus at 5.3 mm distal width. Therefore, the fossil humerus probably derives from a similar-sized frog to that from which the ilium was derived. The fossil humerus shares with L. markhami (A, G), L. auroraensis (E, K) and the much smaller L. hochstetteri (C, I) well-developed lateral and medial crests (F, L). Humeri of L. waitomoensis markedly differ from the fossil in being relatively neotenic with the eminentia capitata never fully ossified into a sphere and they lack development of the lateral and medial crests, e.g. as shown in the large adult (D, J). A similar neotenic condition typifies the much smaller L. hamiltoni and L. pakeka, wherein maximum observed ossification results only in a lenticular eminentia capitata (e.g. B, H). The very much smaller L. archeyi is even more neotenic than L. pakeka. NMNZ S.50861 differs from all extant and recently extinct leiopelmatids in its aspherical and relatively narrower eminentia capitata, 45% of distal width compared with about half to slightly more than half of distal width in Recent leiopelmatids (Worthy Citation1987).
Leiopelma acricarina sp. nov. (Fig. 3A–I)
Holotype. NMNZ S.51263, vertebra 3 or 4, collected 15 January 2008 ().
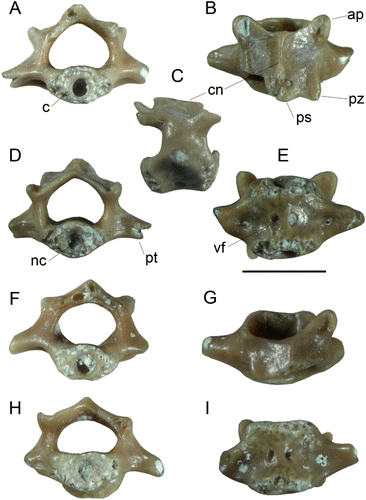
Differential diagnosis. Frog about the size of L. hamiltoni (snout–vent length c.45 mm) with amphicoelous and ectochordal vertebra on which processus transversi originate from the mid-length of the centrum, the neural arch is slightly longer than wide in dorsal view and slightly imbricate, and which have a distinct and acute carina neuralis that has a planar dorsal surface.
Leiopelma acricarina differs from all Holocene and extant leiopelmatid taxa in having a strong, sharp and dorsally flat carina neuralis, which has equal elevation both anteriorly and posteriorly. All other Leiopelma species lack a crest anteriorly and at best have a low rounded one posteriorly (Worthy Citation1987: figures 14, 16, 17; ). In L. hochstetteri vertebrae (P–T), the neural arch is low with no median crest; in L. pakeka (U–Y), L. hamiltoni and L. archeyi, the arch is higher, but is also rounded both in lateral profile and in sagittal section. In these Recent taxa, the posterior part of the neural arch is not ossified as extensively as in the fossil, resulting in the non-imbricate nature of their vertebral column, as noted by Trueb (1973). Vertebrae of L. markhami (A–E), L. auroraensis (F–J) and L. waitomoensis (K–O) are also much larger than L. acricarina. Leiopelma waitomoensis further differs with slightly dorsoventrally compressed centra.
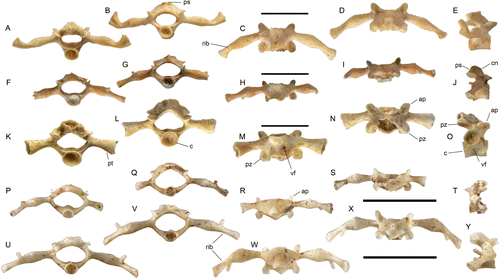
Ascaphus vertebrae differ by: 1, the processus transversi on vertebrae 2 to 5 are distinctly angled posteriorly (rather than at right angles to centrum); 2, they appear to lack vascular foramina penetrating the centrum; and 3, the neural arch is markedly shorter than the minimum inter-zygapophyseal width (Ritland Citation1955; Stephenson EM Citation1952, Citation1960).
Etymology. from acer, Latin for sharp, and carina, for its sharp carina neuralis; gender neuter.
Type locality. Bed HH1a, Manuherikia River Site, Otago, New Zealand: details as above.
Stratigraphy/Age/Fauna. Bannockburn Formation, Manuherikia Group, Early Miocene (Altonian); 19–16 Ma; St Bathans Fauna.
Measurements of holotype (mm). Length centrum 2.0, anterior depth centrum 1.0, posterior depth centrum 1.0, diameter of notochordal fossa 0.5, preserved width across processus transversi 3.7, width across prezygapophyses 2.5, preserved length of neural arch on midline 1.8, minimum width neural arch between pre- and postzygapophyses 2.0, neural canal at anterior end 1.2 deep at midline and 1.6 wide.
Paratype. NMNZ S.50023, vertebra 3, collected 6–16 January 2007 ( F–I).
Locality. Bed HH1a, Manuherikia River Site.
Stratigraphy/Age/Fauna. As for holotype.
Measurements of paratype (mm). Length centrum 1.8, preserved width across processus transversi 3.4.
Description. NMNZ S.51263 and NMNZ S.50023 have very similar morphology and size, however the former is more complete with both prezygapophyses and some of the right postzygapophysis preserved (). Both have a circular amphicoelous, ectochordal (sensu Griffiths Citation1963) and perichordal (sensu Kluge & Farris Citation1969) centrum with deep circular fossae for the notochordal canal visible at either end. The term perichordal applies to a greater range of taxa than those that are ectochordal and relates to chondrification and ossification of the sclerotomic cells around the notochord leading to a circular section-shape of the centrum: as defined by Kluge & Farris (Citation1969), it does not relate to persistence of the notochord, and so describes any centrum with a circular section. The centrum is shorter than prezygapophysal width and is dorsoventrally penetrated by two vascular foramina at mid-length, one either side of the midline. The neural arch is little elevated above the zygapophyses, has a sharp median carina neuralis that is planar in lateral view and increases slightly in elevation posteriorly to a poorly developed processus spinosus. The prezygapophyses are about 0.5 mm long and oval. The postzygapophyses, partially preserved only on the right side of S.51263, have similar posterior extent to the neural arch and are not separated from the arch by a marked notch, which together with the anterior profile of the arch (which is level with the mid-length of the prezygapophyses rather than entirely posterior to them) indicates that the vertebra is at least partly imbricate with the spinal canal completely covered. The processus transversi originate at mid-length of the centrum: in S.50023, they are directed cranially and ventrally with a distinct sulcus at the junction with the centrum ventrally and attenuate in width laterally and in NMNZ S.51263 are directed laterally and ventrally. The short preserved length of the processus transversi in NMNZ S.51263 precludes determining whether they attenuate in width laterally or expand and hence whether the vertebra was rib-bearing. NMNZ S.51263 further differs from S.50023 with foramina entering the centrum from the sulci at the base of the processus transversi.
Comparisons. The fossils are superficially similar to sacral and post-sacral vertebrae of Sphenodon, which are also amphicoelous and have lateral processes. However, they differ markedly from Sphenodon vertebrae, in addition to markedly smaller size, by 1, a considerably larger foramen vertebrale that is about twice the diameter of the centrum rather than of similar diameter; 2, relatively shorter prezygapophyses that are more widely separated than in Sphenodon, wherein they are more elongate and more centrally located; 3, a much lower crista neuralis; and 4, the ventral surface lacks a distinct crest unlike the obvious one in Sphenodon that supports a considerably better-developed subvertebral musculature. The fossils are very similar to leiopelmatid vertebrae. The only extant frogs known to have amphicoelous (intervertebral discs that are not ossified resulting in concave anterior and posterior ends of the centrum) and ectochordal (centrum cylindrical with a persistent notochord) vertebrae are Leiopelma and Ascaphus (Nicholls Citation1916; Stephenson EM Citation1952, Citation1960; Ritland Citation1955; Griffiths Citation1963; Trueb Citation1973). The St Bathans fossils and Leiopelma vertebrae differ significantly from those of Ascaphus from North America as follows: 1, in dorsal view, processus transversi originate from a point at mid-length on the vertebrae, whereas in Ascaphus they originate more anteriorly (Ritland Citation1955); 2, on all vertebrae, the neural arch is relatively longer and the processus transversi extend at right angles to the axis, whereas in Ascaphus the neural arch is clearly wider than long and the processus transversi on vertebrae 3–5 are distinctly angled posteriorly (Ritland Citation1955; Stephenson EM Citation1952, Citation1960; Worthy Citation1987); and 3, they have a pair of large ventrally placed vascular foramina on the centrum, which Ascaphus vertebrae appear to lack: none are depicted in the ventral view of the vertebral column given by Ritland (Citation1955).
The fossils indicate that the vertebral column was slightly imbricate, that is, the posterior section of the neural arch overlaps the anterior section of the arch on the adjacent vertebra. While Leiopelma is said to lack imbrication (Trueb Citation1973) this is not true for all species in the genus. The neural arch of presacral vertebrae in L. archeyi, L. hamiltoni, L. hochstetteri and L. waitomoensis are/were not imbricate, but they were in the more heavily ossified L. auroraensis and L. markhami with no exposure of the neural canal dorsally, as shown by Worthy (Citation1987).
The size of the fossil vertebra, as measured by centrum diameter, is similar to that of vertebra of both L. hamiltoni and L. hochstetteri, but considerably larger than L. archeyi (e.g. Stephenson EM Citation1952, Citation1960; Worthy Citation1987). They are therefore considerably smaller than those of the Holocene taxa L. markhami and L. auroraensis and therefore also much smaller than those expected for L. miocaenale, which was similar in size to these large Holocene taxa. Leiopelma waitomoensis, as the largest leiopelmatid known, has much larger vertebrae than these new fossils (Worthy Citation1987).
Both fossil vertebrae have a pair of vascular foramina penetrating the centrum ventrally whose presence contributes to the perception of a mid-ventral ridge, although no ridge similar to that seen in for example skinks is present. Similar foramina are seen in the vertebra of the large adult specimen of L. waitomoensis NMNZ S.23415 (M). However, in another specimen of L. waitomoensis NMNZ S.24465, foramina were only present in vertebrae 3 and 4 and were absent in more caudal vertebrae. Similar foramina were not observed in adults of other Leiopelma species. However, a more detailed survey to assess the presence of such foramina across an ontogenetic series of all species is required to assess the taxonomic significance of this feature.
The form of the processus transversi is useful in identifying where in the vertebral column a vertebra lies (Worthy Citation1987). In L. hamiltoni and L. hochstetteri, vertebra 2 is characterized by gracile anteriorly directed processus transversi. Vertebrae 3 and 4 have the most robust processus transversi, whose diameter increases laterally at first, with those on vertebra 3 having a slight anterior inclination, but those on vertebrae 4 is only directed laterally. In L. hamiltoni, these anterior processus transversi are distinctly inclined ventrally, whereas they are horizontal in L. hochstetteri (see Worthy Citation1987). In both species, the processus transversi on more posterior vertebrae are increasingly gracile and generally attenuate in diameter laterally, with those on vertebrae 5–9 angled somewhat dorsally. In L. hamiltoni, the processus transversi of vertebra 5–9 are laterally directed, rather than angled anteriorly or posteriorly (Stephenson EM Citation1960), whereas in L. hochstetteri those on vertebrae 7–9 are anteriorly directed, more so on vertebra 9 (Stephenson EM Citation1952). The fossil NMNZ S.50023 is most similar to vertebra 3 of L. hamiltoni in having the processus transversi directed anteriorly and somewhat ventrally, whereas NMNZ S.51263 could be vertebra 4 as its processus transversi are not inclined anteriorly.
Other possible leiopelmatid fossils
Six additional fossils (fragments of femora, tibiofibulae and radioulnae), are not diagnosable as, but are consistent in form with, leiopelmatids. They are much more robust than most neobatrachians, for example Litoria and Platymantis, and represent two species, a small one the size of L. hamiltoni and a larger one the size of L. markhami, and therefore could be from the same leiopelmatid taxa described above. All specimens are from the St Bathans Fauna, Bannockburn Formation, Manuherikia Group, Early Miocene, 19–16 Ma.
Indeterminate specimens consistent in size with Leiopelma miocaenale sp. nov.
Specimen 1. NMNZ S. 50504, a distal half of a right tibiofibula.
Locality. Site 2, Vinegar Hill, St Bathans, Otago; 44°52.685'S, 169°44.985'E; collected 10 January 2007 from 1.8 m above base of Bannockburn Formation.
Measurements. Preserved length 15.5 mm, maximum distal width 4.8 mm, minimum width 2.4 mm.
Description. This tibiofibula fragment includes part of an ossified epiphyseal cap. The fibular side of original mid-section of the shaft has a marked crest, whereas the opposing tibial side is flattened. It has similar robust proportions and size as tibiofibulae of L. markhami.
Specimen 2. NMNZ S.52130, a fragment of a distal femur.
Locality. Bed HH1b Trench excavation, Manuherikia River Site, 9 January 2008.
Measurements. Preserved maximum width 4.0 mm.
Description. Fragment has a well ossified epiphyseal cap.
Specimen 3. NMNZ S.53124, a distal half radioulna.
Locality. Bed HH1b Trench excavation, Manuherikia River Site, 3 March 2010.
Measurements. Preserved maximum width 3.85 mm, shaft width<1.7 mm.
Description. This fragment has an ossified epiphyseal cap and its distal width is markedly wider than shaft width.
Indeterminate specimens consistent in size with Leiopelma acricarina sp. nov.
Specimen 1. NMNZ S.50829, slightly less than the distal half of a right tibiofibula.
Locality. Bed HH1b Trench excavation, Manuherikia River Site, 6–16 January 2007.
Measurements. Preserved length 8.0 mm, maximum distal width 2.6 mm.
Description. Fragment of tibiofibula with typical anuran form. Marked asymmetry with the fibular portion of lesser diameter than the tibial portion identifies it as the distal half of this element. Along the lateral or fibular side of what was the mid-section of the shaft is a crest. This fossil is similar in size and form to tibiofibulae of L. hamiltoni.
Specimen 2. NMNZ S.53106, a proximal tibiale of a tibiale-fibulare.
Locality. Bed HH1b Trench excavation, Manuherikia River Site, 3 March 2010.
Description. Fragment 2.3 mm diameter with a well ossified epiphyseal cap.
Specimen 3. NMNZ S.52756, a fragment of a proximal femur.
Locality. Bed HH1b Trench excavation, Manuherikia River Site, 9 January 2008.
Description. Fragment 2.6 mm diameter with a well ossified epiphyseal cap.
Discussion
Here we describe 11 anuran fossils from the Early Miocene 19–16 Ma St Bathans Fauna of New Zealand. These records are highly significant, as they represent the first pre-Pleistocene record of anurans for New Zealand and demonstrate that a diversity of leiopelmatids has long been on New Zealand. Four specimens are referred to the extant genus Leiopelma in Leiopelmatidae and described as two new species. Leiopelma miocaenale sp. nov. is based on a right ilium and a tentatively referred distal humerus from a species a little larger than L. markhami. Leiopelma acricarina sp. nov., based on two vertebrae, represents a small species about the size of L. hamiltoni, perhaps 40–45 mm snout–vent length. Six fossil limb fragments are possible leiopelmatids whose size does not preclude them belonging to the new taxa: three (parts of a femur, a tibiofibula and a radioulna) being large enough to derive from L. miocaenale sp. nov. and three (parts of a femur, a tibiofibula and a tibiale-fibulare) conforming in size to L. acricarina sp. nov.
Leiopelma miocaenale sp. nov. is similar in size and humeral morphology to the extinct L. markhami and L. auroraensis, two Recent taxa that are most closely related to L. hochstetteri among extant taxa (Worthy Citation1987). Extant taxa comprise a dichotomy, with L. hochstetteri differing greatly from L. archeyi, L. hamiltoni and L. pakeka (Green et al. Citation1989; Bell Citation2010). The divergence of two of the four extant species of Leiopelma (L. archeyi and L. hochstetteri) was estimated to have occurred between about 50 and 40 Ma (Roelants et al. Citation2007), which would imply long ghost lineages for both clades. It is possible that L. miocaenale sp. nov. is a member of the L. hochstetteri lineage, but given the marked differences of L. acricarina from all Recent taxa, the latter probably does not belong to either Recent lineage. The discovery of additional fossil material will be required to assess the phylogenetic relationships of these Miocene taxa, but they do partially fill what would otherwise be minimally a 60-million-year ghost lineage of leiopelmatids on Zealandia.
These anuran taxa add considerably to the described diversity of the St Bathans Fauna. Already this Early Miocene fauna has a diverse described herpetofauna with sphenodontines, crocodilians, turtles and squamates (Molnar & Pole Citation1997; Jones et al. Citation2009; Lee MSY et al. Citation2009; Worthy et al. Citation2011a), but the addition of anurans to it is significant. Leiopelma is one of the quintessentially New Zealand taxa and is deeply divergent from its sister taxon Ascaphus (Ascaphidae) which together form the sister group of all other anurans (Frost et al. Citation2006; Roelants et al. Citation2007; Pyron & Wiens Citation2011). Its addition to the St Bathans Fauna shows that all the iconic Recent endemic vertebrate taxa were present in Zealandia (the continental fragment whose emergent part is now New Zealand) during the Early Miocene: sphenodontines, leiopelmatids, moas (Dinornithiformes), kiwi (Apterygidae), New Zealand wrens (Acanthisittidae), adzebills (Aptornithidae) and burrowing bats (Mystacinidae) (Molnar & Pole Citation1997; Hand et al. Citation2007; Jones et al. Citation2009; Lee MSY et al. Citation2009; Tennyson et al. Citation2010; Worthy et al. Citation2010a, Citation2011b, in press). To this list can be added New Zealand's first terrestrial mammals, from an as yet unidentified group and of similar high biogeographical interest and apparent ancient origins, reported recently from the St Bathans Fauna (Worthy et al. Citation2006). The widely cited early report of a snake from this fauna (Worthy et al. Citation2002) was based on teeth now known to derive from the glossohyal of a galaxiid fish.
Therefore, the Zealandian terrestrial fauna, at a time perhaps just 4 million years after maximum inundation during the Oligocene marine transgression, not only had all the key elements of the modern New Zealand biota, but several other probably endemic family-group taxa, including a bat (Hand et al. Citation2007) and a terrestrial mammal (Worthy et al. Citation2006). In the subsequent 16 million years, probably not one of the extant iconic family-group taxa dispersed to New Zealand. There is also no evidence that any member of the diverse neobatrachian frog lineage, presently represented by five families in Australia (Myobatrachidae, Limnodynastidae, Hylidae, Microhylidae and Ranidae, see Tyler & Knight Citation2009), have dispersed naturally to New Zealand, although a few species have been introduced (King et al. Citation2009). A procoelous vertebra formerly considered to be from a probable neobatrachian (e.g. Worthy et al. Citation2011c) is now recognized as probably a juvenile first sacral vertebra of a skink. These data do not support the hypothesis of total inundation of Zealandia during the late Oligocene (Campbell & Hutching Citation2007; Landis et al. Citation2008), which would require all fauna in Recent New Zealand to have arrived by dispersal since that time (e.g. Goldberg et al. Citation2008). Rather, they favour the long-held views of biogeographers (e.g. Fleming Citation1979) that part of the Recent biota is of ancient origin, long preceding the Oligocene inundation and most parsimoniously of vicariant origin (Gibbs Citation2006; Worthy et al. Citation2006; Jones et al. Citation2009; Tennyson et al. Citation2010). Such an understanding is also supported by recent investigations of the palaeofloras from the Oligocene to Miocene interval, which show no evidence for any loss of diversity or species turnover during the period of maximum marine transgression (Lee DE et al. Citation2012).
In conclusion, the fossil frogs described here are highly significant, as minimally they reduce the duration of the leiopelmatid ‘ghost lineage’ on New Zealand by c.20 million years. Further, the Early Miocene leiopelmatid diversity we reveal supports the proposed ancient dichotomy of the extant species in New Zealand based on molecular data.
Acknowledgements
The authors are indebted to the unstinting efforts of many field assistants who helped in excavations for the St Bathans Fauna project in the period 2001–2009 and especially to Jennifer Worthy for tireless hours sorting the microvertebrates from the sediment. We are particularly thankful for the continued generous support to this project by the land owners Ann and Euan Johnstone (Home Hills Station) and Jack Enright (Dunstanburn Station). This research is part of Australian Research Council project DP0770660 (to the authors and M. Archer) and DP120100486, and is also supported by the authors' institutions. RPS is supported by the Brian Mason Technical Fund. We thank Mark Hutchinson (SAM) for access to skeletal material and discussions on frog osteology. We thank Jean-Claude Stahl and Raymond Coory (NMNZ) for providing the comparative images of Leiopelma species. We thank Annelise Folie for a constructive review of an earlier draft and the comments of two reviewers that have further improved the text.
References
- Báez AM, Gómez RO, Taglioretti ML 2012. The archaic ilial morphology of an enigmatic pipid frog from the upper Pleistocene of the South American pampas. Journal of Vertebrate Paleontology 32: 304–314.
- Bell BD 2010. The threatened leiopelmatid frogs of New Zealand: natural history integrates with conservation. Herpetological Conservation and Biology 5: 515–528.
- Bell BD, Daugherty CH, Hay JM 1998. Leiopelma pakeka, n. sp. Anura: Leiopelmatidae, a cryptic species of frog from Maud Island, New Zealand, and a reassessment of the conservation status of Leiopelma hamiltoni from Stephens Island. Journal of the Royal Society of New Zealand 28: 39–54.
- Campbell H, Hutching G 2007. In Search of Ancient New Zealand. Wellington, New Zealand, Penguin and GNS Sciences.
- Duellman WE, Trueb L 1994. Biology of Amphibians. Baltimore, MD, The Johns Hopkins University Press.
- Estes R, Reig OA 1973. The early fossil record of frogs: a review of the evidence; In: Vial JL ed. Evolutionary Biology of the Anurans: Contemporary Research on Major Problems. Columbia, University of Missouri Press. Pp. 11–63.
- Evans SE, Borsuk-Bialynicka M 1998. A stem-group frog from the Early Triassic of Poland. Acta Palaeontologica Polonica 43: 573–580.
- Fleming CA 1979. The geological history of New Zealand and its life. Auckland, Auckland University Press and Oxford University Press.
- Folie A, Rana RS, Rose KD, Sahni A, Kumar K, Singh L, Smith T In press 2013. Early Eocene frogs from Vastan Lignite Mine, Gujarat, India. Acta Palaeontologica Polonica 58 (3): 511–524.
- Ford LS, Cannatella DC 1993. The major clades of frogs. Herpetological Monographs 7: 94–117.
- Frost DR, Grant T, Faivovich J, Bain RH, Haas A, Haddad CFB, et al. 2006. The amphibian tree of life. Bulletin of the American Museum of Natural History 297: 1–370.
- Gaina C, Roest WR, Müller RD, Symonds P 1998. The opening of the Tasman Sea: a gravity anomaly animation. Earth Interactions 2-004: 1–23.
- Gao K-Q, Wang Y 2001. Mesozoic anurans from Liaoning Province, China, and phylogenetic relationships of archaebatrachian anuran clades. Journal of Vertebrate Paleontology 21: 460–476.
- Gardner JD, Roček Z, Přikryl T, Eaton JG, Blob RW Sankey JT 2010. Comparative morphology of the ilium of anurans and urodeles (Lissamphibia) and a re-assessment of the anuran affinities of Nezpercius dodsoni Blob et al., 2001. Journal of Vertebrate Paleontology 30: 1684–1696.
- Gibbs G 2006. Ghosts of Gondwana. The history of life in New Zealand. Nelson, New Zealand, Craig Potton Publishing.
- Goldberg J, Trewick SA, Paterson AM 2008. Evolution of New Zealand's terrestrial fauna: a review of molecular evidence. Philosophical Transactions of the Royal Society B 363: 3319–3334.
- Green DM, Cannatella DC 1993. Phylogenetic significance of the amphicoelous frogs, Ascaphidae and Leiopelmatidae. Ethology, Ecology and Evolution 5: 233–245.
- Green DM, Sharbel TF, Hitchmough RA, Daugherty CH 1989. Genetic variation on the genus Leiopelma and relationships to other primitive frogs. Zeitschrift fur Zoologische Svstematik und Evolutionsforschung 27: 65–79.
- Griffiths I 1963. The phylogeny of the Salientia. Biological Reviews 38: 241–292.
- Hand S, Beck R, Worthy T, Archer M, Sigé B 2007. Australian and New Zealand bats: the origin, evolution, and extinction of bat lineages in Australasia. Journal of Vertebrate Paleontology 27(3, supplement) Abstracts: 86A.
- Hay JM, Ruvinsky I, Hedges SB, Maxson LR 1995. Phylogenetic relationships of amphibian families inferred from DNA sequences of mitochondrial 12S and 16S ribosomal RNA genes. Molecular Biology and Evolution 12: 928–937.
- Holman AJ 1998. Pleistocene amphibians and reptiles in Britain and Europe. Oxford Monographs on Geology and Geophysics 38. New York, Oxford, Oxford University Press.
- Holyoake A, Waldman B, Gemmell NJ 2001. Determining the species status of one of the world's rarest frogs: a conservation dilemma. Animal Conservation 4: 29–36.
- Irisarri I, Mauro DS, Green DM, Zardoya R 2010. The complete mitochondrial genome of the relict frog Leiopelma archeyi: Insights into the root of the frog Tree of Life. Mitochondrial DNA 21: 173–182.
- Jenkins FA, Shubin NH 1998. Prosalirus bitis and the anuran caudopelvic mechanism. Journal of Vertebrate Paleontology 18: 495–510.
- Jones MEH, Tennyson AJD, Worthy JP, Evans SE, Worthy TH 2009. A sphenodontine (Rhynchocephalia) from the Miocene of New Zealand and palaeobiogeography of the tuatara (Sphenodon). Proceedings of the Royal Society B 276: 1385–1390.
- King CM, Roberts CD, Bell BD, Fordyce RE, Nicoll RS, Worthy TH, et al. 2009. Phylum Chordata: lancelets, fishes, amphibians, reptiles, birds, and mammals. In Gordon DP ed. New Zealand Inventory of Biodiversity: Volume One: Kingdom Animalia. Christchurch, Canterbury University Press. Pp 431–527.
- Kluge AG, Farris JS 1969. Quantitative phyletics and the evolution of anurans. Systematic Zoology 18: 1–32.
- Landis CA., Campbell HJ, Begg JG, Mildenhall DC, Paterson AM, Trewick SA 2008. The Waipounamu Erosion Surface: questioning the antiquity of the New Zealand land surface and terrestrial fauna and flora. Geological Magazine 145: 173–197.
- Lee DE, Conran JG, Lindqvist JK, Bannister JM, Mildenhall DC 2012. New Zealand Eocene, Oligocene and Miocene macrofossil and pollen records and modern plant distributions in the Southern Hemisphere. Botanical Reviews 78: 235–260.
- Lee MSY, Hutchinson MN, Worthy TH, Archer M, Tennyson AJD, Worthy JP, et al. 2009. Miocene skinks and geckos reveal long-term conservatism of New Zealand's lizard fauna. Biology Letters 5: 833–837.
- Molnar RE, Pole M 1997. A Miocene crocodilian from New Zealand. Alcheringa 21: 65–70.
- Nicholls GE 1916. The structure of the vertebral column in the Anura Phaneroglossa and its importance as a basis of classification. Proceedings of the Linnean Society of London 128: 80–92.
- Noble GK 1924. A new spadefoot toad from the Oligocene of Mongolia with a summary of the evolution of the Pelobatidae. American Museum Novitates 132: 1–15.
- Noble GK 1931. The biology of the Amphibia. New York and London, McGraw-Hill.
- Pole M, Douglas B, Mason G 2003. The terrestrial Miocene biota of southern New Zealand. Journal of the Royal Society of New Zealand 33: 415–426.
- Pyron RA, Wiens JJ 2011. A large-scale phylogeny of Amphibia including over 2800 species, and a revised classification of extant frogs, salamanders, and caecilians. Molecular Phylogenetics and Evolution 61: 543–583.
- Ritland RM 1955. Studies on the post-cranial morphology of Ascaphus truei. I. Skeleton and spinal nerves. Journal of Morphology 97: 119–178.
- Roček Z 1994. Taxonomy and distribution of Tertiary discoglossids (Anura) of the genus Latonia V. Meyer, 1843. Geobios 27: 717–751.
- Roček Z 2000. Mesozoic anurans, Chapter 14. In: Heatwole H, Carroll PL eds, Amphibian Biology, Volume 4: Paleontology, The evolutionary history of amphibians. Chipping Norton, NSW, Australia, Surrey Beatty and Sons. Pp 1295–1331.
- Roček Z, Rage J-C 2000. Tertiary Anura of Europe, Africa, Asia, North America, and Australia, Chapter 15. In: Heatwole H, Carroll PL eds, Amphibian Biology, Volume 4: Paleontology, The evolutionary history of amphibians. Chipping Norton, NSW, Australia, Surrey Beatty and Sons. Pp 1332–1387.
- Roelants K, Bossuyt F 2005. Archaeobatrachian paraphyly and Pangaean diversification of crown-group frogs. Systematic Biology 54: 111–126.
- Roelants K, Gower DJ, Wilkinson M, Loader SP, Biju SD, Guillaume K, et al. 2007. Global patterns of diversification in the history of modern amphibians. Proceedings of the National Academy of Sciences of the United States of America 104: 887–892.
- San Mauro D 2010. A multilocus timescale for the origin of extant amphibians. Molecular Phylogenetics and Evolution 56: 554–561.
- San Mauro D, Vences M, Alcobendas M, Zardoya R, Meyer A 2005. Initial diversification of living amphibians predated the breakup of Pangaea. American Naturalist 165: 590–599.
- Sanchiz B 1998. Salientia. Part 4. In: Wellnhofer P ed. Handbuch der Paläoherpetogie. Munich, Verlag Dr. Friedrich Pfeil. Pp. 11–275.
- Schellart WP, Lister GS, Toy VG. 2006. A Late Cretaceous and Cenozoic reconstruction of the Southwest Pacific region: tectonics controlled by subduction and slab rollback processes. Earth Science Reviews 76: 191–233.
- Schwarzhans W, Scofield RP, Tennyson AJD, Worthy JP, Worthy TH 2012. Fish remains, mostly otoliths, from the non-marine Early Miocene of Otago, New Zealand. Acta Palaeontologica Polonica 57: 319–350.
- Scofield RP, Worthy TH, Tennyson AJD 2010. A heron (Aves: Ardeidae) from the Early Miocene St Bathans Fauna of southern New Zealand. Records of the Australian Museum 62: 89–104.
- Sigurdsen T, Bolt JR 2009. The Lissamphibian humerus and elbow joint, and the origins of modern amphibians. Journal of Morphology 270: 1443–1453.
- Stephenson EM 1952. The vertebral column and appendicular skeleton of Leiopelma hochstetteri Fitzinger. Transactions of the Royal Society of New Zealand 79: 601–613.
- Stephenson EM 1960. The skeletal characters of Leiopelma hamiltoni McCulloch, with particular reference to the effects of heterochrony on the genus. Transactions of the Royal Society of New Zealand 88: 473–488.
- Stephenson NG 1951. Observations on the development of the amphicoelous frogs, Leiopelma and Ascaphus. Journal of the Linnean Society of London 42: 18–28.
- Stephenson NG, Stephenson EM 1956. The osteology of the New Zealand geckos and its bearing on their morphological status. Transactions of the Royal Society of New Zealand 84: 341–358.
- Stipanicic PN, Reig OA 1955. Breve noticia sobre el hallazgo de anuros en el denominado “Complejo porfirico de la Patagonia extraandina”, con consideraciones acerca de la composicion geologica del mismo. Revista de la Asociacion Geologica Argentina 10: 215–233.
- Tennyson AJD, Worthy TH, Jones CM, Scofield RP, Hand SJ 2010. Moa's Ark: Miocene fossils reveal the great antiquity of moa (Aves: Dinornithiformes) in Zealandia. Records of the Australian Museum 62: 105–114.
- Trueb L 1973. Bones, frogs and evolution. In Vial JL ed. Evolutionary Biology of the Anurans: contemporary research on major problems. Columbia, University of Missouri Press. Pp. 65–132
- Tyler MJ 1976. Comparative osteology of the pelvic girdle of Australian frogs and description of a new fossil genus. Transactions of the Royal Society of South Australia 100: 3–14.
- Tyler MJ 1982. Tertiary frogs from South Australia. Alcheringa 6: 101–103.
- Tyler MJ, Knight F 2009. Field guide to the frogs of Australia. Canberra, CSIRO Publishing.
- Worthy TH 1987. Osteology of Leiopelma (Amphibia: Leiopelmatidae) and descriptions of three new subfossil (Leiopelma) species. Journal of the Royal Society of New Zealand 17: 201–251.
- Worthy TH, Tennyson AJD, Archer M, Musser AM, Hand SJ, Jones C, Douglas BJ, McNamara JA, Beck RMD 2006. Miocene mammal reveals a Mesozoic ghost lineage on insular New Zealand, southwest Pacific. Proceeding of the National Academy of Sciences United States of America 103: 19419–19423.
- Worthy TH, Tennyson AJD, Jones C, McNamara JA, Douglas BJ 2007. Miocene waterfowl and other birds from Central Otago, New Zealand. Journal of Systematic Palaeontology 5: 1–39.
- Worthy TH, Tennyson AJD, Hand SJ, Scofield RP 2008. A new species of the diving duck Manuherikia and evidence for geese (Aves: Anatidae: Anserinae) in the St Bathans Fauna (Early Miocene), New Zealand. Journal of the Royal Society of New Zealand 38: 97–114.
- Worthy TH, Hand SJ, Worthy JP, Tennyson AJD, Scofield RP 2009. A large fruit pigeon (Columbidae) from the Early Miocene of New Zealand. The Auk 126: 649–656.
- Worthy TH, Hand SJ, Nguyen JMT, Tennyson AJD, Worthy JP, Scofield RP, et al. 2010a. Biogeographical and phylogenetic implications of an Early Miocene wren (Aves: Passeriformes: Acanthisittidae) from New Zealand. Journal of Vertebrate Paleontology 30: 479–498.
- Worthy TH, Tennyson AJD, Archer M, Scofield RP 2010b. First record of Palaelodus (Aves: Phoenicopteriformes) from New Zealand. Records of the Australian Museum 62: 77–88.
- Worthy TH, Tennyson AJD, Hand SJ, Godthelp H, Scofield RP 2011a. Terrestrial turtle fossils from New Zealand refloat Moa's Ark. Copeia 2011: 72–76.
- Worthy TH, Tennyson AJD, Jones C, McNamara JA 2002. A diverse early-Miocene terrestrial fauna from New Zealand reveals snakes and mammals. IPC2002, Geological Society of Australia, Abstracts68: 174–175.
- Worthy TH, Tennyson AJD, Scofield RP 2011b. Fossils reveal an early Miocene presence of the aberrant gruiform Aves: Aptornithidae in New Zealand. Journal of Ornithology 152: 669–680.
- Worthy TH, Worthy JP, Tennyson AJD, Salisbury SW, Hand SJ, Scofield RP. in press. Miocene fossils show that kiwi (Apteryx, Apterygidae) are not phyletic dwarves. Proceedings volume of the 8th International Meeting of the Society of Avian Palaeontology and Evolution at the Natural History Museum of Vienna. Annalen des Naturhistorischen Museums in Wien, Serie A.
- Worthy TH, Worthy JP, Archer M, Hand SJ, Scofield RP, Marshall BA, et al. 2011c. A decade on, what the St Bathans Fauna reveals about the Early Miocene terrestrial biota of Zealandia. In: Litchfield NJ and Clark K eds. Abstract volume, Geosciences 2011 Conference, Nelson, New Zealand. Geoscience Society of New Zealand Miscellaneous Publication 130A: p. 120.