Abstract
Cyanoramphus parakeets are a key biogeographic element of the Pacific. Many of these parakeets are, however, endangered, with ongoing conservation management hampered by the unresolved taxonomic status of some populations. We used modern and ancient DNA (mitochondrial DNA control region) to assess the taxonomy of the Auckland Islands populations of red-crowned (Cyanoramphus novaezelandiae novaezelandiae) and yellow-crowned (Cyanoramphus auriceps) parakeets. Our analyses show that both red-crowned and yellow-crowned parakeets on the Auckland Islands are nested within the mainland New Zealand diversity of the two species. However, we also found an orange-fronted parakeet (Cyanoramphus malherbi) mitochondrial DNA lineage within the genome of both of these species in the Auckland Islands population. Further sampling of historic orange-fronted parakeet museum skins showed that the orange-fronted morphotype is paraphyletic with respect to mitochondrial haplotype, which is probably caused by hybridisation or incomplete lineage sorting. In light of this, we review and critically assess the taxonomic history of the orange-fronted parakeet, and address whether the species was historically present on the Auckland Islands.
Introduction
Cyanoramphus parakeets are a key biogeographic element of the South Pacific. The lineage is thought to have originated in New Caledonia 500–625 thousand years ago when Cyanoramphus split from Eunymphicus (Boon et al. Citation2008). Their known distribution extends from New Caledonia to Tahiti and Rapa (Austral Islands), and to New Zealand and its sub-Antarctic islands (Boon et al. Citation2001b; Tennyson & Anderson Citation2012) (A) but excludes the Australian mainland. There are currently ten species and five subspecies recognised, including several historically extinct sub-Antarctic and Pacific taxa, based on a combination of morphological, ecological and genetic data (Boon et al. Citation2001a, Citation2001b; Kearvell et al. Citation2003; Gill et al. Citation2010; ).
Figure 1 A, Distribution of Cyanoramphus parakeets in New Zealand and the sub-Antarctic based on modern and historical records. See key for distributions of: yellow-crowned parakeet (Cyanoramphus auriceps); orange-fronted parakeet (Cyanoramphus malherbi); red-crowned parakeet (Cyanoramphus novaezelandiae novaezelandiae); Chatham Islands red-crowned parakeet (Cyanoramphus n. chathamensis); Antipodes Island parakeet (Cyanoramphus unicolor); Reischek's parakeet (Cyanoramphus hochstetteri); and Forbes parakeet (Cyanoramphus forbesi). The red-crowned parakeet (C. n. novaezelandiae) at Campbell Island and the Macquarie Island parakeet Cyanoramphus n. erythrotis (Wagler, 1832) are extinct (Chambers & Boon Citation2005; Scofield Citation2005; Holdaway et al. Citation2010). B, Distribution of red-crowned, yellow-crowned and hybrid (the hybrids are red-crowned morph/orange-fronted haplotype and yellow-crowned morph/orange-fronted haplotype) parakeet DNA samples from the Auckland Islands. Numbers indicate sample sizes. (For a colour version of this figure, the reader is referred to the online version of this article.)
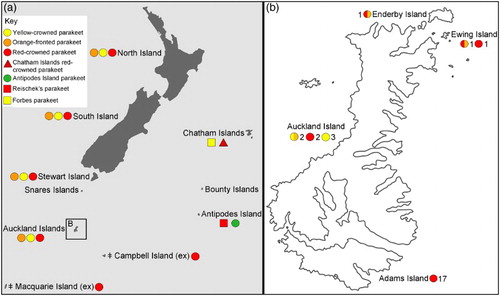
Boon et al. (Citation2001b) conducted a taxonomic review of the genus, but could not fully resolve issues relating to taxonomy and biogeography because of a lack of quality specimens for several extinct and extant populations (Chambers & Boon Citation2005; Scofield Citation2005; Gill et al. Citation2010). These poorly sampled populations included the Auckland Islands populations of red-crowned parakeet Cyanoramphus novaezelandiae novaezelandiae (Sparrman, 1787) and yellow-crowned parakeet Cyanoramphus auriceps (Kuhl, 1820).
The Auckland Islands () archipelago is dominated by two 12-million-year-old Miocene volcanoes, subsequently eroded and dissected. These islands rest on older volcanic rocks 15–25 million years old, plus some older granites and fossil-bearing sedimentary rocks from around 100 million years ago (Denison & Coombs Citation1977). Located 465 km south of Bluff, New Zealand, the Auckland Islands may have been partially glaciated during the early Pleistocene (Fraser et al. Citation2009). Consequently, the biota of the islands is thought to have been established via a period of long-distance dispersal followed by differentiation, and now shows high levels of endemism (Fleming Citation1976).
The origins and taxonomic status of red-crowned and yellow-crowned parakeets on the Auckland Islands remain unresolved and it is considered that the population may consist of a ‘hybrid swarm’ (Buckingham et al. Citation1991). Habitat modification (Johnstone Citation1985), predation (Doole Citation1998) and subsequent loss of ecological pre-zygotic isolating barriers have resulted in hybridisation between the two morphotypes. The red-crowned morphotype dominates following the near extinction of the yellow-crowned morphotype (Graeme Elliott, NJR, AJDT, GKC pers. obs.). Hybridisation of parakeets as a result of habitat modification has also occurred on Mangere Island (part of the Chatham Islands group located c. 680 km east of New Zealand; A) between the Chatham Islands red-crowned parakeet (Cyanoramphus n. chathamensis Oliver, 1930) and Forbes parakeet (Cyanoramphus forbesi Rothschild, 1893) (Taylor Citation1985; Chan et al. Citation2006a). There is also one doubtful report of an orange-fronted parakeet (Cyanoramphus malherbi Souance, Citation1857) from the Auckland Islands (Gray Citation1859, Citation1870; Salvadori Citation1891; Harrison Citation1970; Kearvell et al. Citation2003). Assessing the taxonomic status of the Auckland Island forms is vital to their future conservation management. In this paper, we used modern DNA and ancient DNA (aDNA) to address the origin and taxonomic status of Cyanoramphus parakeets on the Auckland Islands.
Material and methods
Source of specimens
Blood samples (n = 12) and historical skins (n = 10) of red-crowned parakeets (exhibiting red-crowned or hybrid morphotype) from the Auckland Islands were obtained from the New Zealand Department of Conservation (DoC) and museum collections (B; Appendix S1). Due to the rarity of yellow-crowned parakeets on the Auckland Islands, no fresh samples and only five historical skins were available for analysis (B; Appendix S1). Additional historical skins of red-crowned (n = 3), yellow-crowned (n = 12) and orange-fronted (n = 18) parakeets (including the Metz Museum, France, syntype of the orange-fronted parakeet) from mainland New Zealand were sampled from across the historical geographic range of each species (Appendix S1).
Modern DNA extraction, polymerase chain reaction amplification and DNA sequencing
Whole genomic DNA was extracted from blood samples using a Roche High Pure PCR Template Preparation Kit (Roche, Basel, Switzerland) according to the manufacturer's instructions with an overnight incubation at 55 °C. A 2.5 kb mitochondrial DNA (mtDNA) segment containing tRNAPhe, tRNAGlu and the control region (CR) located between the flanking ND6 and 12S rRNA genes was amplified using the primer pair L16518/H1800 (Boon et al. Citation2001b). Each polymerase chain reaction (PCR) (12.5 µL) consisted of: Expand High Fidelity PCR buffer (Roche), 1.5 mm MgCl2, 100 µm dNTPs (Amersham, Chalfont St Giles, UK), 0.5 µm each primer, 0.65 U Expand High Fidelity Enzyme Mix (Roche) and 2 µL DNA. The PCR thermocycling conditions consisted of 95 °C for 3 min, 35 cycles of 95 °C for 15 s, 55 °C for 30 s, 72 °C for 2 min, with a cumulative increase in extension time of 20 s/cycle for the last 25 cycles.
The PCR products were run on a 1% 1×TBE agarose gel. PCR products were purified using ExoSap (1.5 U ExoI, 1 U SAP; Fermentas, Waltham, MA, USA) by incubation at 37 °C for 30 min and 80 °C for 15 min and sequenced bi-directionally using the primers above and internal primers developed by Boon et al. (Citation2001b) using Big Dye Terminator technology and an ABI 3130xl.
Ancient DNA analysis
All aDNA extractions and PCR set up were carried out at Victoria University of Wellington and the Allan Wilson Centre (Massey University, Palmerston North) in purpose-built aDNA laboratories physically isolated from other molecular laboratories as advocated by Knapp et al. (Citation2012). Strict aDNA procedures were followed to minimise contamination of samples with exogenous DNA and to authenticate DNA sequences, including the use of negative extraction and PCR controls (Cooper & Poinar Citation2000).
DNA was extracted from toe pads using the Qiagen QIAmp DNA MicroKit (Qiagen, Hilden, Germany) following the manufacturer's instructions with an overnight incubation at 55 °C. A highly variable and diagnostic 458 bp fragment of the mtDNA CR was amplified using the primers L90-110/H519 (Boon et al. Citation2001b) or in three overlapping fragments using the primer pairs CyanohistF1 (5′-GCCATAAACCGCCCTCGGAT-3′)/ CyanohistR1 (5′-GACCATCTCAAAACCAGGGA-3′) (187 bp including primers); CyanohistF2 (5′-TAAACCAGGTCCCAGGATTC-3′)/ CyanohistR2 (5′-GGTGGAGCAGATTGTGTACG-3′) (184 bp including primers); and CyanohistF3 (5′-TCCACGAAGACGTGTCAACC-3′)/ CyanohistR3 (5′-CTGACCGAGGAACCAGAGGC-3′) (156 bp including primers). Each PCR contained: TaKaRa PCR buffer (TaKaRa, Shiga, Japan), 2.5 mm MgCl2, 1 m Betaine (Sigma, St Louis, MO, USA), 100 µm dNTPs (Amersham), 0.5 µm each primer, 1 U TaKaRa Ex Taq (TaKaRa) and 2 µL DNA. Unsuccessful PCRs were repeated with 2 U TaKaRa Ex Taq and 4 µL DNA or 2 µL 1 : 10 DNA. PCR thermocycling conditions consisted of 95 °C for 4 min, 55 cycles of 95 °C for 30s, 55 °C for 20s, 74 °C for 2 min, with a final extension of 74 °C for 10 min.
PCR products were run on a 2% 1×TBE agarose gel and were purified and sequenced as above, except that bi-directional sequencing was conducted from independent PCR products. When an inconsistency between sequences from an individual was observed due to DNA damage (exclusively C-T and G-A transitions) or discordant species presence and geographic location, additional PCRs and bi-directional sequencing were conducted, and a majority rule consensus was applied to the independent replicates (after Brotherton et al. Citation2007).
Phylogenetic analysis
Contiguous sequences were constructed using Sequencher (Genecodes, MI, USA) and aligned in MEGA 4.0 (Kumar et al. Citation2004) against published Cyanoramphus sequences (Boon et al. Citation2000, Citation2001a, Citation2001b), and checked by eye to correct for sequence misalignments (giving a data set including 62 new sequences and 73 published sequences). The New Caledonian red-crowned parakeet Cyanoramphus saisetti Verreaux & Des Murs, 1860, Forbes parakeet (Chatham Islands), Norfolk parakeet Cyanoramphus cooki (G. R. Gray, Citation1859), Reschek's parakeet Cyanoramphus hochstetteri (Reischek, 1889) and Antipodes Island parakeet Cyanoramphus unicolor (Lear, 1831) were included as out-groups. ModelTest was used to determine the most appropriate model of nucleotide substitution under the Akaike Information Criterion (GTR + I + G was selected). Maximum likelihood analysis was performed using PhyML (Guindon et al. Citation2010) with nearest-neighbour interchange branch-swapping and the model parameters were estimated and optimised. The level of support for the tree topology was evaluated using 1000 bootstrap (BS) replicates for maximum likelihood and maximum parsimony (with equal weights and 10 random addition sequence replicates) performed in PAUP* (Swofford Citation2002), and with Bayesian posterior probabilities (PP) from MrBayes. Bayesian analyses were performed using MrBayes v3.2.4 with the maximum likelihood model employing six substitution types (‘nst = 6') and rate variation across sites was modelled using a gamma distribution, and a proportion of sites being invariant (‘rates = invgamma’). The Markov-chain Monte-Carlo searches were run twice with four chains for 20,000,000 generations, with trees being sampled every 1000 generations. Convergence of the duplicate runs was assessed both in Tracer v1.6 (Rambaut et al. Citation2014), and via the average standard deviation of split frequencies. The first 25% of trees, i.e. 5,000,000 generations, were discarded as ‘burn-in’ in each of the analyses. Results were analysed in FigTree. DNA sequences are deposited in GenBank (#KT596958-KT597050).
To evaluate how the level of missing sequence data for the historic specimens affected tree topology, the analyses were repeated using the smaller fragment common to all specimens, and the GTR + I + G model of nucleotide substitution (as determined by ModelTest).
Results
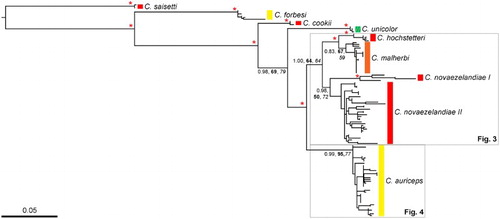
The specimens gave a 1605 bp alignment for the mtDNA CR sequences. The smaller fragment common to all 118 specimens was a 331 bp subset of that full length alignment (i.e. the historic specimens sequenced for the 331 bp aligned fragment, whereas the modern specimens sequenced for the full length 1605 bp of the aligned fragment). The historic specimens were sequenced for 43 out of 52 historical skins (see Appendix S1). Our phylogenetic analysis (; Appendix S2) produced a tree where each major clade was well supported, with varying levels of support for internal nodes in the phylogeny. While Bayesian analysis firmly placed C. novaezelandiae I within red-crowned parakeets (PP = 0.98), the bootstrap support for the red-crowned parakeet clade (C. novaezelandiae I and II) is relatively weak (BS = 50–72%). The 1605 bp data set, containing missing sequence data, produced a more resolved phylogeny than the smaller 331 bp data set. While the tree topology varied between the 1605 and 331 bp versions of the data set, each species still formed a monophyletic clade with varying support (results not shown). Maximum likelihood analyses perform relatively well with missing data, hence the better resolved phylogeny produced using the 1605 bp data set is preferred over the smaller data set with less resolution.
Figure 2 Phylogeny of Cyanoramphus parakeets based on 1605 bp mitochondrial DNA CR alignment. Branch lengths are proportional to the number of substitutions/site. The maximum likelihood tree was generated in PhyML using the GTR + I + G model of nucleotide substitution. For clarity, only Bayesian posterior probability (PP) and bootstrap support (BS; bold = maximum likelihood; italics = maximum parsimony) values for major clades are shown. The asterisks indicate strong support from all of these measures, i.e. Bayesian posterior probability of 0.95 and above, and bootstrap values of 80% and above. Coloured bars represent the dominant crown colouration of each species as per . Clades in grey-outlined boxes are shown in detail in (Cyanoramphus novaezelandiae and Cyanoramphus malherbi) and (Cyanoramphus auriceps). (For a colour version of this figure, the reader is referred to the online version of this article.)
Auckland Islands red-crowned parakeet
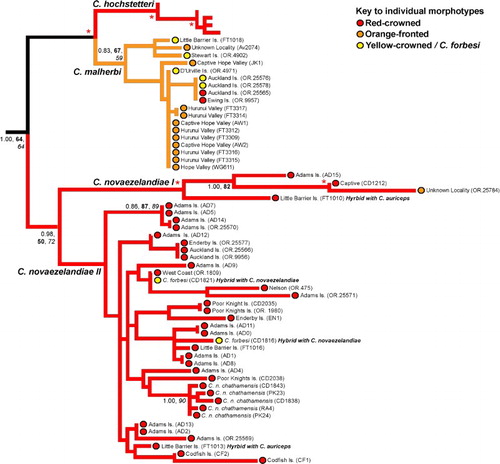
Our phylogenetic analysis showed that the majority of haplotypes within red-crowned parakeets from the Auckland Islands (n = 14) nested within the mainland New Zealand red-crowned parakeet clades (, 3). However, two historical red-crowned parakeet skins (National Museum of New Zealand Te Papa Tongarewa (NMNZ) OR.9957, OR.25565) had a mtDNA CR haplotype characteristic of orange-fronted parakeets (, 3). We found no yellow-crowned parakeet morphotypes with red-crowned mitochondrial haplotypes (see ).
Auckland Islands yellow-crowned parakeet
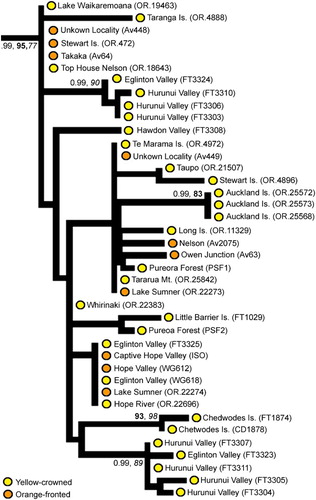
There are two haplotypes within yellow-crowned parakeets from the Auckland Islands. The first haplotype (NMNZ OR.25568, OR.25572, OR.25573) clusters within the mainland New Zealand yellow-crowned parakeet clade (, 4). The second haplotype (NMNZ OR.25576, OR.25578) is identical to the extinct orange-fronted parakeet haplotype seen by us in red-crowned parakeets from the Auckland Islands (NMNZ OR.9957, OR.25565) (, 3). Two yellow-crowned parakeet historical skins (NMNZ OR.4902 Stewart Island, OR.4971 D'Urville Island) and one modern specimen (FT1018 Little Barrier Island; reported by Boon et al. Citation2000) also had mtDNA CR haplotypes that clustered within the orange-fronted parakeet clade ().
Figure 3 Sub-phylogeny of red-crowned (Cyanoramphus novaezelandiae) and orange-fronted (Cyanoramphus malherbi) parakeets based on 1605 bp mitochondrial DNA CR alignment, showing the phylogenetic position of red-crowned and orange-fronted parakeet lineages from the Auckland Islands (from Adams, Enderby, Ewing and Auckland Islands), and orange-fronted parakeets from mainland New Zealand. For clarity, for internal nodes only Bayesian posterior probability (PP, > 0.95) and bootstrap support (bold = maximum likelihood; italics = maximum parsimony, > 80%) values for major clades are shown where the node has strong support from at least two different methods. The asterisks indicate strong support from all of these measures, i.e. Bayesian posterior probability of 0.95 and above, and bootstrap values of 80% and above. Lineage colours represent the dominant crown colouration of each species, while coloured circles represent the morphotype of individual specimens. (For a colour version of this figure, the reader is referred to the online version of this article.)
Orange-fronted parakeet
Our additional sampling of historical skins to determine the historical genetic diversity of orange-fronted parakeets showed that there were multiple paraphyletic lineages (with respect to morphotype) within this taxon, nested within the orange-fronted (), red-crowned () and yellow-crowned () parakeet clades. Of the 13 orange-fronted parakeet historical skins sampled, only one (unknown locality CM Av2074; see ) had a haplotype that clustered within the orange-fronted parakeet clade, one (unknown locality NMNZ OR.25784) contained red-crowned mtDNA (see ) and ten contained yellow-crowned mtDNA (see ). The sample from the Metz Museum syntype of C. malherbi did not contain amplifiable DNA.
Figure 4 Sub-phylogeny of yellow-crowned parakeets (Cyanoramphus auriceps) based on the 1605 bp mitochondrial DNA CR alignment showing the position of yellow-crowned parakeets from the Auckland Islands. For clarity, for internal nodes only Bayesian posterior probability (PP, > 0.95) and bootstrap support (bold = maximum likelihood; italics = maximum parsimony, > 80%) values for major clades are shown where the node has strong support from at least two different methods. Yellow-crowned and orange-fronted morphotypes have been indicated on the phylogeny. (For a colour version of this figure, the reader is referred to the online version of this article.)
Discussion
Our phylogenetic analyses show that red-crowned and yellow-crowned parakeets from the Auckland Islands are nested within the mainland New Zealand genetic diversity of those species. Additionally, Taylor (Citation1985) was correct in noting that many Auckland Islands birds are hybrids between red-crowned and yellow-crowned parakeets. Surprisingly, however, there is an orange-fronted parakeet haplotype within historical skins from the Auckland Islands, raising the possibility that in the past, orange-fronted parakeets may have colonised this archipelago also or alternatively, and equally likely, that this mtDNA lineage arrived in birds that were already hybrids. Additional sampling of historical orange-fronted parakeet skins from New Zealand shows that multiple lineages were present within this taxon.
Auckland Islands parakeets
Multiple red-crowned parakeet haplotypes (n = 13) are present within the Auckland Islands population (, 3), supporting preliminary findings by Boon et al. (Citation2001b). Our phylogenetic analysis supports the retention of the Auckland Island red-crowned parakeet as the same as the mainland New Zealand subspecies of red-crowned parakeet (C. n. novaezelandiae) as advocated by Gill et al. (Citation2010).
In contrast to red-crowned parakeets, we found only one yellow-crowned haplotype within the Auckland Islands population (in all three individuals sampled; , 4). Although unique, this haplotype falls within the broader clade of mainland yellow-crowned parakeets. Until further samples are available, we consider the Auckland Islands yellow-crowned parakeet to be a geographically restricted population of C. auriceps—in agreement with Gill et al. (Citation2010). The Auckland Islands populations of red-crowned and yellow-crowned parakeets do not need separate conservation management as they are not unique, and could potentially be restocked from mainland populations if needed, pending resolution of conservation management concerns regarding hybridisation, which should ease with re-vegetation (see Aikman & Miskelly Citation2004; Chan et al. Citation2006b).
The apparent absence of yellow-crowned parakeet morphotypes with red-crowned mtDNA haplotypes (see , 4) suggests that gender-biased hybridisation is occurring between these two species (female red-crowned parakeet, male yellow-crowned parakeet). Given the small number of samples analysed in this study (especially of the yellow-crowned morphotype), additional fast evolving hyper-variable nuclear loci (e.g. microsatellite data; Andrews et al. Citation2013; Knafler et al. Citation2014) or genotyping by sequencing (Elshire et al. Citation2011) is needed to clarify the Cyanoramphus phylogeny, and the extent and direction of hybridisation. Fast evolving loci are crucial as the radiation of Cyanoramphus is within the past 500–625 thousand years (Boon et al. Citation2008) and more conserved loci will fail to detect differences between species. While Bayesian analyses firmly place C. novaezelandiae I within red-crowned parakeets, the bootstrap support for the red-crowned parakeet node (I and II) is relatively weak. Based on the tree topology and branch lengths alone, C. novaezelandiae I is suggestive of a ‘cryptic species’ (e.g. Murphy et al. Citation2011) or an accelerated evolutionary rate explained by nuclear mitochondrial sequences or duplicated control regions (Eberhard et al. Citation2001; Schirtzinger et al. Citation2012), of which the latter is something that Boon et al. (Citation2000) suggested, and requires further investigation.
The presence of an orange-fronted parakeet haplotype within the Auckland Islands population of both red-crowned and yellow-crowned parakeets (, 3) was unexpected and raises some interesting possibilities. The historical evidence for orange-fronted parakeets on the Auckland Islands is not reliable (e.g. Kearvell et al. Citation2003; see section on Orange-fronted parakeet taxonomy below). Regardless of the interpretations of the historical record and taxonomic status, there are two hypotheses that can explain the presence of orange-fronted parakeet mtDNA haplotypes on the Auckland Islands. The first hypothesis is that orange-fronted parakeets recently colonised the archipelago, contrary to the conclusions of Harrison (Citation1970), Taylor (Citation1998) and Kearvell et al. (Citation2003). When historical records of parakeet population booms and eruptions on mainland New Zealand (Potts Citation1882; Hutton & Drummond Citation1905) are taken into account, it is unsurprising that red-crowned, yellow-crowned and orange-fronted parakeets could have colonised the Auckland Islands. Exotic passerines managed to colonise the sub-Antarctic islands within 40 years of their introduction to New Zealand in the 19th century (Williams Citation1953). Subsequent hybridisation between morphotypes due to low population size and habitat modification (Taylor Citation1971; Johnstone Citation1985) may have led to the introgression of the orange-fronted parakeet haplotype into the Auckland Islands population. Historical records suggest that by the early 20th century, parakeets were very scarce at the Auckland Islands (Waite Citation1909; Wilson Citation1966; Taylor Citation1971). We speculate that habitat modification (Johnstone Citation1985) resulted in an increase in red-crowned parakeet numbers because of increased open grassland habitat, which is favoured by red-crowned parakeets, with gender-biased hybridisation with yellow-crowned and orange-fronted parakeets possibly resulting in the near or complete extinction of these two morphotypes.
The second hypothesis is that when red-crowned and yellow-crowned parakeets colonised the Auckland Islands, they already carried the orange-fronted parakeet haplotype due to hybridisation on mainland New Zealand or incomplete lineage sorting (see Orange-fronted parakeet ancient DNA below). What is clear is that the orange-fronted parakeet haplotype at the Auckland Islands potentially represents lost genetic diversity from extant mainland New Zealand populations.
Orange-fronted parakeet taxonomy and historical presence on the Auckland Islands
The validity of historical records of orange-fronted parakeets on the Auckland Islands is linked with the 157 years of taxonomic debate surrounding this species. In Gray's (Citation1859) List of the specimens of birds in the collection of the British Museum, he considered there to be three specimens of Platycercus [=Cyanoramphus] malherbi in the Natural History Museum (NHMUK) collections. Two were collected by the ‘Antarctic Expedition’ of James Clarke Ross, one supposedly from the Auckland Islands and one from the Bay of Islands, and the third was presented by Sir George Grey from an unspecified location. In Reichenow's monograph Conspectus Psittacorum (Reichenow Citation1882) C. malherbi is considered only to occur on the Auckland Islands, perhaps based on Gray (Citation1870) Hand-list of genera and species of birds distinguishing those contained in the British Museum.
The orange-fronted parakeet is represented by two syntypes (Voisin & Voisin Citation2008), held in the Metz Museum and the Muséum National d'Histoire Naturelle, Paris (MNHN). The term ‘co-type’ used by Harrison (Citation1970), Kearvell et al. (Citation2003) and Kearvell et al. (Citation2014) to describe the MNHN specimen is no longer used in zoological nomenclature and technically refers to either syntypes or paratypes (see Tennyson & Bartle Citation2008). Although Kearvell et al. (Citation2003) stated that the Metz syntype ‘cannot now be found’, it was examined there by Sandy Bartle in 2005 (pers. comm. 2014) and again as part of this study. Based on photographs (Appendix S3), the specimen does have ‘le front d'un jaune orange’ (=yellow-orange front) but it is not as extensive or fully developed as in most examples of this species (Scofield & Stephenson Citation2013). This suggests that the Metz specimen is not fully grown (Higgins Citation1999). Kearvell et al. (Citation2014) stated that the description of C. malherbi by Souance (Citation1857) reads ‘orange frons and rump patch, with blue-green shading of the contour feathers and a pale yellow crown (Souance Citation1857)’. Based upon examination of digital photographs of the Metz syntype, Kearvell et al. (Citation2014) stated that ‘both frons and rump patch were a distinct orange (pers. comm. Francoise Clemang, Metz Museum)’. With regards to the rump patch, the translation is incorrect as it reads ‘Une tache d'un rouge écarlate de chaque cote du croupion’ (= a bright red spot on each side of the rump). We can confirm Kearvell's statements about the Metz syntype, however the rump patch is only on the right hand side (under the wing), and is composed of a single feather. Kearvell et al. (Citation2014) also stated ‘Analysis of mitochondrial DNA (mtDNA) by Boon et al. (Citation2001b) confirmed the species identity of presumed orange-fronted parakeets held in museum collections and descriptions of the type specimens’. Again, Kearvell is incorrect, as Boon et al. (Citation2001b) did not produce amplifiable DNA from orange-fronted parakeet museum skins, and did not examine the C. malherbi syntypes. The original label on the base of the Metz specimen mount reads:
Cyanoramphus malherbi
de Malherbe
Iconographie des Perroquets pl. 79
l'Australie?
Revue et Mag de Zool
p 98
pl 79
adulte decrit et f. our
Mr Souance
ex plus jeune est au
de Paris
Cyanoramphus auriceps
par une tache plus grande
The label gives the type locality as ‘de l'Australie?’ with a capital letter meaning Australia and is apparently an error (as opposed to ‘de l'australie?’, meaning austral or southern). The original description by Souance (Citation1857) does not designate a type locality (‘Habitat inconnu’). Mathews & Iredale (Citation1913) designated the type locality as South Island, perhaps based on the knowledge of the likely collection location. It is possible that the specimen was collected at the same time as the MNHN syntype, which presumably Souance (Citation1857) knew had been collected in Tasman Bay (South Island) based on its label (Voisin & Voisin Citation2008).
The second syntype (Appendix S4) is held in the collections of the MNHN and is not ‘misplaced’, as stated by Kearvell et al. (Citation2003), though he subsequently examined the digital photographs of the specimen before 2014 (Kearvell et al. Citation2014). It was examined by Sandy Bartle in 1980 (pers. comm. 2014), again as part of this study, and is discussed by Voisin & Voisin (Citation2008). The description by Souance (Citation1857) states ‘Un autre individu du muse de Paris, beaucoup plus jeune, n'en differe que par la band frontale a peine distincte’ (= Another individual in Paris museum, much younger, only differs by its less pronounced frontal band). Our direct examination of the MNHN syntype indicates that the specimen is a juvenile, based on its plumage. The specimen is very faded, with a discoloured green-yellow forehead, yellow crown and no trace of a red or orange frons. The rump is light green and contrasts with the green tail. There is a small, orange-tipped feather on the left rump only (under the wing). The orange rump spot and the absence of a red frons (which is present in juvenile red-crowned and yellow-crowned parakeets) indicate that the MNHN specimen is an orange-fronted parakeet. This description is in contrast to the statement by Kearvell et al. (Citation2014) ‘that both the frons and rump patch … is a distinct orange (pers. obs.)’. Kearvell's description of the frons is incorrect (see Appendix S4). The oldest inscription (on the base of the MNHN specimen mount) reads:
Nelle Zelande Baie-Tasman
Astrolabe + Zelee
1841—No 188
Platycercus auriceps Kuhl
1406
‘1406' refers to the old MNHN catalogue number. The newest label for the Paris syntype reads:
Cyanoramphus malherbi Souance Citation1857
C. G. 2004–78
N. C. 652
C. malherbi Souance Citation1857
Rev. et. Mag. Zool. 9: 98
C. 1406 Baie Tasman
Despite this, no collection locality was mentioned for C. G. 2004–278 by Souance (Citation1857) and this lack of information appears to have caused some subsequent confusion over the geographic range and type location of the orange-fronted parakeet. Gray (Citation1859, Citation1870) in his hand-list of birds in the NHMUK stated that the species occurred on the Auckland Islands. This was based on a specimen, collected on the Antarctic Expedition of Erebus and Terror, that Gray considered to be distinct based on its smaller dimensions. Finsch (Citation1868) considered ‘Platycercus malherbi’ to occur on the Auckland Islands but could not distinguish the Antarctic Expedition specimen (in the NHMUK) from C. auriceps. Salvadori (Citation1891) in his catalogue of parrots in the NHMUK, reclassified the Antarctic Expedition ‘Cyanoramphus malherbi’ specimen as C. auriceps, stating it was ‘exactly like C. auriceps, only smaller … equally small birds are found in New Zealand; and I doubt whether this smaller form, on which a new name would have to be bestowed, can really be separated from C. auriceps … ’. Based on Salvadori (Citation1891), there seems little doubt that this specimen is a juvenile yellow-crowned parakeet. Harrison (Citation1970) was uncertain about whether orange-fronted parakeets had been collected on the Auckland Islands concluding ‘it is possible but unlikely that the species ever occurred there’. Kearvell et al. (Citation2003) confused the issue by stating mistakenly that the Antarctic Expedition NHMUK specimen was the MNHN P. malherbi syntype. Voisin & Voisin (Citation2008) added further to the confusion by stating ‘GC2004–278 … was collected during the expedition to the South Pole and Oceania headed by Durmont D'Urville on the corvettes Astrolabe and Zelee in the years 1837 to 1840', and considered it probable (but erroneously) that it was collected in 1841 in Tasman Bay. There were two Astrolabe and Zelee expeditions to New Zealand. The first visited New Zealand in 1827 and was based in Tasman Bay for several days, making significant collections of bird specimens (Bartle Citation1993). The second expedition, on which Voisin & Voisin (Citation2008) implied the MNHN syntype was collected, did not visit Tasman Bay but did visit the Auckland Islands in 1840 (Kearvell et al. Citation2003). Voisin & Voisin (Citation2008) apparently took the date of ‘1841—No. 188' on the MNHN specimen label to mistakenly mean the specimen was collected from Tasman Bay on the second expedition. Quoy & Gaimard (Citation1830) and Jacquinot & Pucheran (Citation1853) describe many (but not all) of the birds collected on the first and second expeditions, respectively, but make no reference to parakeets. However, the original catalogue of the MNHN clearly shows that the MNHN syntype was collected from Tasman Bay in January 1827 on the first Astrolabe expedition, stating ‘1406 (652) Cyanoramphus malherbi Baie de Tasman Expedition d'Astrolabe No. 188'. Therefore the conclusion that it was collected on the second expedition (Voisin & Voisin Citation2008) is incorrect. Although parakeets may have been collected at the Auckland Islands in March 1840 by the second expedition (D'Urville Citation1846), the MNHN syntype is clearly labelled ‘Baie Tasman’ and was originally catalogued as such.
Orange-fronted parakeet ancient DNA
Additional sampling of historical orange-fronted parakeet skins showed that the taxon is paraphyletic with respect to the mtDNA haplotype (, 4), with morphotypes of this species containing red-crowned, yellow-crowned and orange-fronted parakeet mitochondrial control region haplotypes. Previous genetic, morphological and ecological research has shown that the orange-fronted parakeet is a biological, phylogenetic and diagnostic species (Young & Keavell Citation2001; Boon et al. Citation2001b; Kearvell et al. Citation2003; Gill et al. Citation2010; Kearvell et al. Citation2014; Kearvell & Steeves Citation2015). Given the aDNA data presented in this study, hybridisation and incomplete lineage sorting are the most probable explanatory hypotheses for this paraphyly.
Evidence for historical hybridisation in New Zealand parakeets has some support. In captivity, modified habitats or reduced populations, the pre-zygotic mating barriers that prevent hybridisation, break down (Chan et al. Citation2006a). The majority of historical orange-fronted parakeet specimens were collected during the 19th and 20th centuries at sites where predation and habitat modification were widespread. As this taxon was once common and widespread throughout the South Island, and present in the North Island, it had presumably undergone a severe population bottleneck as a result of range contraction by the late 19th century; this was probably followed by subsequent hybridisation in severely depleted populations (Kearvell et al. Citation2003), though new ecological evidence suggests orange-fronted and yellow-crowned parakeets mate assortatively, even when the former is outnumbered by the latter (Kearvell & Steeves Citation2015). However, hybridisation can explain some of the discordant morphotype haplotype observations. Individuals from Hope Valley () and others sourced from depleted populations could be hybrids (Boon et al. Citation2000, Citation2001b; Kearvell et al. Citation2003) or the descendants of hybrids (Taylor et al. Citation1986). In addition, historical specimens now in collections could be from caged birds, rather than being wild caught, as keeping native parrots in captivity was common practice in the 19th century (Buller Citation1888).
Incomplete lineage sorting can also explain the paraphyletic nature of orange-fronted parakeets with respect to mtDNA haplotype. This is not unexpected given the recent radiation of Cyanoramphus during the mid- to late Pleistocene (Boon et al. Citation2001b). Recently, however, there has been a philosophical change towards species concepts in birds, with reciprocal monophyly no longer a strict prerequisite for recognising full species status. Joseph & Omland (Citation2009) showed that 44% of Australo-Papuan terrestrial birds that are biological species are paraphyletic.
Due to the single locus analysed in this study, additional hyper-variable nuclear loci (e.g. microsatellite data or genotyping by sequencing) are needed to determine whether hybridisation, incomplete lineage sorting, or both, can explain the paraphyletic nature of orange-fronted parakeets with respect to morphotype. Microsatellite loci developed for orange-fronted parakeets, which cross-amplify with yellow-crowned parakeets, offer this opportunity (Andrews et al. Citation2013). Preliminary evidence (Andrews Citation2013; Andrews et al. Citation2013; Kearvell et al. Citation2014; Kearvell & Steeves Citation2015) supports a lack of nuclear introgression in the orange-fronted and yellow-crowned parakeets, suggesting an ancient introgression event and retention of mtDNA haplotypes may explain the paraphyly of the orange-fronted parakeet morphotype. In conclusion, regardless of the history of the orange-fronted parakeet, we advocate that it be retained as a full species based on morphological, genetic and behavioural data (Boon et al. Citation2001b; Kearvell et al. Citation2003; Gill et al. Citation2010; Kearvell et al. Citation2014; Kearvell & Steeves Citation2015).
Supplementary data
Appendix S1. Cyanoramphus specimens used for sequences generated in this study.
Appendix S2. Phylogeny of Cyanoramphus parakeets based on 1605 bp mtDNA CR alignment.
Appendix S3. The orange-fronted parakeet (Cyanoramphus malherbi Souance, 1857) Metz Museum syntype.
Appendix S4. The orange-fronted parakeet (Cyanoramphus malherbi Souance, 1857) Musèum National d’Historie Naturelle, Paris, syntype.
Appendix S4 The orange-fronted parakeet (Cyanoramphus malherbi Souance, 1857) Musèum National d'Historie Naturelle, Paris, syntype.
Download PDF (679.8 KB)Appendix S3. The orange-fronted parakeet (Cyanoramphus malherbi Souance, 1857) Metz Museum syntype.
Download PDF (368.1 KB)Appendix S2. Phylogeny of Cyanoramphus parakeets based on 1605 bp mtDNA CR alignment.
Download PDF (297 KB)Appendix S1. Cyanoramphus specimens used for sequences generated in this study.
Download PDF (251.5 KB)Acknowledgements
We thank the following people and institutions for assistance sourcing and supplying samples for genetic analysis: Auckland Museum (Brian Gill), Canterbury Museum, Graeme Elliott (DoC), Kath Walker (DoC), Metz Museum (Francoise Clemang), Museum of New Zealand Te Papa Tongarewa (JA [Sandy] Bartle, Gillian Stone), and Otago Museum (Sue Heath, Ilka Soehle). We also thank Elodie Urlacher (University of Otago) and Jean-Claude Stahl (Museum of New Zealand Te Papa Tongarewa) for assistance with French translation; Jamie Wood (Landcare Research) for help with phylogenetic analyses; and Sandy Bartle for helpful discussion and obtaining a sample of the Metz Museum orange-fronted parakeet syntype. Photographs of the Metz Museum syntype were provided by Francoise Clemang, Laurianne Kieffer and Sandy Bartle; and the Paris syntype by Eric Pasquet.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
RP Scofield http://orcid.org/0000-0002-7510-6980
Additional information
Funding
References
- Aikman H, Miskelly C 2004. Birds of the Chatham Islands. Wellington, Department of Conservation. 116 p.
- Andrews BJ 2013. Conservation genetics of the orange-fronted kākāriki (Cyanoramphus malherbi). Unpublished MSc thesis. Christchurch, University of Canterbury.
- Andrews BJ, Hale ML, Steeves TE 2013. Characterisation of microsatellite loci in the critically endangered orange-fronted kakariki (Cyanoramphus malherbi) isolated using genomic next generation sequencing. Conservation Genetics Resources 5: 235–237.
- Bartle JA 1993. Differences between British and French organisation of zoological exploration in the Pacific 1793–1840. Tuatara 32: 75–81.
- Boon WM, Kearvell JC, Daugherty CH, Chambers GK 2000. Molecular systematics of New Zealand Cyanoramphus parakeets: conservation of orange-fronted and Forbes’ parakeets. Bird Conservation International 10: 211–239.
- Boon WM, Daugherty CH, Chambers GK 2001a. The Norfolk Island green parrot and New Caledonian red-crowned parakeet are distinct species. Emu 101: 113–121.
- Boon WM, Kearvell JC, Daugherty CH, Chambers GK 2001b. Molecular systematics and conservation of kakariki (Cyanoramphus spp.). Science for Conservation 176: 1–46.
- Boon WM, Robinet O, Rawlence N, Bretagnolle V, Norman JA, Christidis L, Chambers GK 2008. Morphological, behavioural and genetic differentiation within the Horned Parakeet (Eunymphicus cornutus) and its affinities to Cyanoramphus and Prosopeia. Emu 108: 251–260.
- Brotherton P, Endicott P, Sanchez JJ, Beaumont M, Barnett R, Austin JJ, Cooper A 2007. Novel high-resolution characterization of ancient DNA reveals C > U-type base modification events as the sole cause of post mortem miscoding lesions. Nucleic Acids Research 35: 5717–5728.
- Buckingham R, Elliott G, Walker K 1991. Bird and mammal observations on Adams Island and southern Auckland Island 1989. Department of Conservation Science and Research Internal Report No. 105. Wellington, Department of Conservation.
- Buller WL 1888. A history of the birds of New Zealand. 2nd edition. Volume 2. London, the Author.
- Chambers GK, Boon WM 2005. Molecular systematics of Macquarie Island and Reischeck's parakeets. Notornis 52: 249–250.
- Chan C, Ballantyne KN, Aikman H, Fastier D, Daugherty CH, Chambers GK 2006a. Genetic analysis of interspecific hybridisation in the world's only Forbes’ parakeet (Cyanoramphus forbesi) natural population. Conservation Genetics 7: 493–506.
- Chan C, Ballantyne KN, Aikman H, Daugherty CH, Chambers GK 2006b. Conservation genetics of the Forbes’ parakeet (Cyanoramphus forbesi) on Mangere Island, Chatham Islands. Department of Conservation Research and Development Series 254: 1–26.
- Cooper A, Poinar HN 2000. Ancient DNA: do it right or not at all. Science 289: 1139.
- Denison RE, Coombs DS 1977. Radiometric ages for some rocks from Snares and Auckland Islands, Campbell Plateau. Earth and Planetary Science Letters 34: 23–29.
- Doole P 1998. Conservation Management Strategy: subantarctic Islands 1998–2008. Southland Conservancy Conservation Management Planning Series No. 10. Wellington, New Zealand Department of Conservation.
- D'Urville D 1846. Voyage au pole sud et dans l'Oceanie sur les corvettes l'Astrolabe et la Zelee—Volume 9. Paris, Gide et C Editeurs.
- Eberhard JR, Wright TF, Bermingham E 2001. Duplication and concerted evolution of the mitochondrial control region in the parrot genus Amazona. Molecular Biology and Evolution 18: 1330–1342.
- Elshire RJ, Glaubitz JC, Sun Q, Poland JA, Kawamoto K, Buckler ES, Mitchell SE 2011. A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS One 6: e19379. doi:10.1371/journal.pone.0019379
- Finsch O 1868. Notes on Mr. Walter Buller's “Essay on the ornithology of New Zealand”. Transactions and Proceedings of the New Zealand Institute 1: 58–70.
- Fleming CA 1976. New Zealand as a minor source of terrestrial plants and animals in the Pacific. Tuatara 22: 30–37.
- Fraser CI, Nikula R, Spencer HG, Waters JM 2009. Kelp genes reveal effects of subantarctic sea ice during the Last Glacial Maximum. Proceedings of the National Academy of Science USA 106: 3249–3253.
- Gill BJ, Bell BD, Chambers GK, Medway DG, Palma RL, Scofield RP, Tennyson AJD, Worthy TH 2010. Checklist of the birds in New Zealand, Norfolk and Macquarie Islands, and the Ross Dependency Antarctica. 4th edition. Wellington, Te Papa Press in association with the Ornithological Society of New Zealand.
- Gray GR 1859. List of the specimens of birds in the collection of the British Museum. Part 3. London, The Trustees.
- Gray GR 1870. Hand-list of genera and species of birds distinguishing those contained in the British Museum. Part 2. London, The Trustees.
- Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Systematic Biology 59: 307–321.
- Harrison M 1970. The orange-fronted parakeet (Cyanoramphus malherbi). Notornis 17: 115–125.
- Higgins PJ 1999. Handbook of Australian, New Zealand and Antarctic birds—volume 4: parrots to dollarbird. Melbourne, Oxford University Press. 1248 p.
- Holdaway RN, Thorneycroft JM, McClelland P, Bunce M 2010. Former presence of a parakeet (Cyanoramphus sp.) on Campbell Island, New Zealand subantarctic, with notes on the island's fossil sites and fossil record. Notornis 57: 8–18.
- Hutton FW, Drummond J 1905. The animals of New Zealand: an account of the Dominion's air-breathing vertebrates. 2nd edition. Christchurch, Whitcombe and Tombs.
- Jacquinot H, Pucheran J 1853. Zoologie. Mammiferes et oiseaux. In: Voyage au Pôle Sud et dans l'Océanie sur les corvettes l'Astrolabe et la Zeelee—Exécuté par ordre du roi pendant les années 1837–1838–1839–1840. Volume 3. Paris, Gide and Baudry.
- Johnstone GW 1985. Threats to birds on sub Antarctic islands. In: Moors PJ ed. Conservation of island birds. ICBP Technical Publication 3. Cambridge, International Council of Bird Preservation. Pp. 101–121.
- Joseph L, Omland KE 2009. Phylogeography: its development and impact in Australo-Papuan ornithology with special reference to paraphylly in Australian birds. Emu 109: 1–23.
- Kearvell JC, Grant AD, Boon WM 2003. The orange-fronted parakeet (Cyanoramphus malherbi) is a distinct species: A review of recent research into its taxonomy and systematic relationship within the genus Cyanoramphus. Notornis 50: 27–35.
- Kearvell JC, Conner C, Farley M 2014. Field identification of the orange-fronted parakeet (Cyanoramphus malherbi): pitfalls for the unwary. Notornis 61: 200–204.
- Kearvell JC, Steeves TE 2015. Evidence for assortative mating in sympatric populations of orange-fronted (Cyanoramphus malherbi) and yellow-crowned (C. auriceps) kakariki. Notornis 62: 71–75.
- Knafler GJ, Jamierson IG, Robertson BC 2014. Microsatellite primers for the red-crowned parakeet (Cyanoramphus novaezelandiae). Conservation Genetics Resources: 7: 419–421.
- Knapp M, Clarke AC, Horsburgh KA, Matisoo-Smith EA 2012. Setting the stage—building and working in an ancient DNA laboratory. Annals of Anatomy - Anatomischer Anzeiger 194: 3–6.
- Kumar S, Tamura K, Nei M 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Briefings in Bioinformatics 5: 150–163.
- Mathews GM, Iredale T 1913. A reference list of the birds of New Zealand. Part 1. Ibis 2 (10th series): 201–263.
- Murphy SA, Joseph L, Burbidge AH, Austin J 2011. A cryptic and critically endangered species revealed by mitochondrial DNA analyses: the Western Ground Parrot. Conservation Genetics 12: 595–600.
- Potts TH 1882. Out in the open: a budget of scraps of natural history gathered in New Zealand. Christchurch, Lyttelton Times. 201 p.
- Quoy JRC, Gaimard JP 1830. In: Dumont-d'Urville J. Voyage de découvertes de l'Astrolabe exécuté par ordre du Roi, pendant les anneés 1826–1827–1828–1829, sous le commandement de M.J. Dumont-d'Urville. Zoologie—Volume 1. Paris, J. Tastu. Pp. 197.
- Rambaut A, Suchard MA, Xie D, Drummond AJ 2014. Tracer v1.6. http://beast.bio.ed.ac.uk/Tracer. (accessed 22 October 2015).
- Reichenow A 1882. Conspectus Psittacorum: systematische Uebersicht aller bekannten Papageienarten/ von Ant. Reichenow. Berlin, the Author.
- Salvadori T 1891. Catalogue of the Psittaci, or Parrots, in the Collection of the British Museum. Catalogue of the Birds of the British Museum 20: 590.
- Schirtzinger EE, Taveres ES, Gonzales LA, Eberhard JR, Miyaki CY, Sanchez JJ, Hernandez A, Mueller H, Graves GR, Fleischer RC, Wright TF 2012. Multiple independent origins of mitochondrial control region duplications in the order Psittaciformes. Molecular Phylogenetics and Evolution 64: 342–356.
- Scofield RP 2005. The supposed Macquarie Island parakeet in the collection of the Canterbury Museum. Notornis 52: 117–120.
- Scofield RP, Stephenson B 2013. Birds of New Zealand: a photographic guide. Auckland, Auckland University Press. 552 p.
- Souance C 1857. Descriptions de trois nouvelles especes de Perroquets. Revue et Magasin de Zoologie (Fevrier 1857): 98.
- Swofford DL 2002. PAUP*. Phylogenetic analysis using parsimony (*and other methods). Sunderland, Sinauer Associates.
- Taylor RH 1971. Influence of man on vegetation and wildlife of Enderby and Rose Islands, Auckland Islands. New Zealand Journal of Botany 9: 225–268.
- Taylor RH 1985. Status, habits and conservation of Cyanoramphus parakeets in the New Zealand region. In: Moors PJ ed. Conservation of island birds. ICBP Technical Publication 3. Cambridge, International Council of Bird Preservation. Pp. 195–211.
- Taylor RH 1998. A reappraisal of the orange-fronted parakeet (Cyanorarnphus sp.)—species or colour morph? Notornis 45: 49–63.
- Taylor RH, Heatherbell EG, Heatherbell EM 1986. The orange-fronted parakeet (Cyanoramphus malherbi) is a colour morph of the yellow-crowned parakeet (C. auriceps). Notornis 33: 17–22.
- Tennyson AJD, Anderson A 2012. Bird, reptile and mammal remains from archaeological sites on Rapa Island. In: Anderson A, Kennett DJ eds. Taking the high ground: the archaeology of Rapa, a fortified island in remote east Polynesia. Terra Australis 37. Canberra, Australian National University. Pp. 105–114.
- Tennyson AJD, Bartle JA 2008. Catalogue of type specimens of birds in the Museum of New Zealand Te Papa Tongarewa. Tuhinga—Records of the Museum of New Zealand Te Papa Tongarewa 19: 185–207.
- Voisin C, Voisin JF 2008. Liste des types d'oiseaux des collections du Muséum national d'Histoire naturelle de Paris. 16: Perroquets (Psittacidae) = List of the bird types in the collections of the Museum National d'Histoire Naturelle, Paris. 16: Parrots (Psittacidae). Zoosystema 30: 463–499.
- Waite ER 1909. Vertebrata of the subantarctic islands of New Zealand. In: Chilton C ed. The subantarctic islands of New Zealand—Volume 2. Wellington, Philosophical Institute of Canterbury. Pp. 542–600.
- Williams GR 1953. The dispersal from New Zealand and Australia of some introduced European passerines. Ibis 95: 676–692.
- Wilson E 1966. Diary of the ‘Discovery' expedition to the Antarctic 1901–1904. London, Blandford Press.
- Young J, Keavell JC 2001. Distinguishing between sexes and species: bill size in orange-fronted and yellow-crowned parakeets (Cyanoramphus auriceps). Emu 101: 137–142.
