ABSTRACT
The Chionoidea are a small, southern hemispheric shorebird clade that today includes the Magellanic Plover (Pluvianellidae) and two species of sheathbills (Chionidae). Here we describe the first fossil remains attributable to this group. The two newly described species, the early Miocene Neilus sansomae gen. et sp. nov. from New Zealand and the late Oligocene Chionoides australiensis gen. et sp. nov. from South Australia, are overall more similar to sheathbills, but the mosaic of characters shared with both Chionidae and Pluvianellidae preclude referral to either lineage. Attribution of fossils this age to these lineages also conflicts with divergence dates based on molecular data, as the split between the Magellanic Plover and sheathbills is hypothesised to be more recent. We therefore suggest that these Australasian, plover-size species represent the first record of stem-group taxa within Chionoidea.
http://zoobank.org/urn:lsid:zoobank.org:pub:2A5A2FD1-C3B5-4BAB-88D8-5862FE9E7976
Introduction
The survival of lineages that are relicts of ancestral diversification events reveals an ability of their members to adapt to environmental and geographical changes over geological time (e.g. Cassens et al. Citation2000; Jablonski Citation2002; De Pietri et al. Citation2015). Phylogenetic studies of shorebirds and allies (Aves: Charadriiformes) have demonstrated that several monotypic family-level taxa and distinctive, species-poor clades have long evolutionary branches (Paton et al. Citation2003; Baker et al. Citation2007), likely indicating the loss of taxa during the evolutionary history of these lineages (e.g. Wheeler Citation1992; Graybeal Citation1998; Wagner Citation2000; Wiens Citation2005; see also Rabosky & Lovette Citation2008). The fossil record of some of these lineages has also revealed that surviving members may differ in ecology from their ancestors (e.g. Mayr Citation2000; Mayr & Knopf Citation2007; De Pietri et al. Citation2011, Citation2015, Citation2016a; Smith & Clarke Citation2015), a disparity which probably reflects adaptations to new environmental conditions (De Pietri et al. Citation2016a).
Among shorebirds, members of the clade that encompasses plovers, oystercatchers, sheathbills and allies are grouped in the suborder Charadrii, one of the three major clades that make up the avian order Charadriiformes (Ericson et al. Citation2003; Paton et al. Citation2003; Baker et al. Citation2007). The Charadrii include one of the most species-rich radiations within shorebirds (i.e. plovers in the family-level taxon Charadriidae), as well as monotypic lineages (Magellanic Plover, Pluvianellidae; Egyptian Plover, Pluvianidae) and distinct, small clades such as sheathbills (Chionidae) and stone-curlews (Burhinidae). Interrelationships at the family level are well resolved owing to a robust phylogenetic framework based on molecular data (Ericson et al. Citation2003; Paton et al. Citation2003; Baker et al. Citation2007; see Mayr Citation2011).
The only family-level taxa within Charadrii to have an exclusive Gondwanan distribution are the sheathbills (Chionidae)—containing two living species—and their sister taxon the Magellanic Plover Pluvianellus socialis (Pluvianellidae). Sheathbills are all white, pigeon-sized, predominantly terrestrial birds with a breeding range restricted to the Antarctic Peninsula and subantarctic islands (Burger Citation1996); the Magellanic Plover inhabits southernmost South America, breeding inland and moving to coastal areas during the non-breeding season (Jehl Citation1975). Although the Pale-faced Sheathbill Chionis albus disperses to the southern parts of the South American and African continents in the winter, sheathbill vagrants have never been reported from New Zealand or Australia. The Black-faced Sheathbill Chionis minor was reported from the Pleistocene of South Australia (van Tets & Smith Citation1974), but the record was later invalidated (Olson Citation1976).
The clade including Chionis was first defined as a family-level taxon by Lesson in Citation1828 when he created his ‘Famille: Les Chionidées’ (Lesson Citation1828, p. 341), then containing only Chionis albus. In his description of Chionis minor, Hartlaub (Citation1841) included it within the Chionidae. Stejneger (Citation1885) was the first to coin the superfamily taxon Chionoideae, to describe a clade including Chionidae and Thinocoridae (seedsnipes), a relationship no longer advocated. Since its description by Gray (Citation1849), Pluvianellus socialis had been placed in the Charadriidae, but Jehl (Citation1975) considered that several characters indicated that it was not a ‘true’ plover, and coined the family-level taxon Pluvianellidae. It was demoted to being a subfamily within Charadriidae in Piersma (Citation1996). The possibility of a close relationship between the sheathbills and the Magellanic Plover was first hinted at by Jehl (Citation1975) based on their behaviour and general morphology (e.g. general body shape, presence of a crop, courtship displays, clutch size, chick-rearing behaviour), and was later substantiated by Strauch (Citation1978) based on some osteological features. Since then, analyses based on molecular data have strongly supported the sister taxon relationship between the Magellanic Plover and sheathbills (e.g. Paton et al. Citation2003; Paton & Baker Citation2006; Baker et al. Citation2007), which is recognised in the use of the superfamily Chionoidea.
The known pre-Quaternary fossil record of the Charadrii in the northern hemisphere (Mayr Citation2009; De Pietri et al. Citation2013; De Pietri & Scofield Citation2014; and references therein) is richer than that of the southern hemisphere, where it is currently limited to undescribed remains of burhinids from the Pliocene of South Africa (Manegold et al. Citation2013). Among the charadriiform birds from the early Miocene St Bathans Fauna (Central Otago, New Zealand) mentioned by Worthy et al. (Citation2007), there are at least three undescribed taxa that we are able to attribute to the Charadrii. Here we describe one of them, and refer the taxon to the clade encompassing sheathbills and the Magellanic Plover (Chionoidea). Additionally, we describe a further species of chionoidean affinities from the late Oligocene (c. 26–24 Ma) of South Australia. This Australasian material not only fills a significant gap in the regional fossil record of the Charadrii, but contributes to our understanding of the evolution of avian lineages on former Gondwanan land masses and the morphological and ecological adaptations that members of these lineages have undergone in response to geographical and environmental changes that took place during the Cenozoic.
Material and methods
Taxonomy and nomenclature follow del Hoyo et al. (Citation2016), and anatomical terminology is after Baumel and Witmer (Citation1993). All measurements are in mm and were rounded to the nearest 0.1. Commonly used abbreviations: proc, processus; R: right, L: left, d: distal, p: proximal, ML: maximum length, PW: proximal width, DW: distal width, Min.SW: minimal shaft width. The fossil specimens are deposited in Canterbury Museum (CM), Christchurch, New Zealand, in Museum of New Zealand Te Papa Tongarewa (NMNZ), Wellington, New Zealand, and in the South Australian Museum (SAM), Adelaide, South Australia, Australia.
Comparative material
Comparative material was sampled from CM; NMNZ; SAM; American Museum of Natural History (AMNH), New York, USA; Museum Victoria (NMV), Melbourne, Australia; Natural History Museum Basel (NMB), Switzerland; and Senckenberg Research Institute (SMF), Frankfurt, Germany.
Comparisons were made with the following representatives of extant species within Charadrii. Charadriidae: Anarhynchus frontalis, Pluvialis apricaria, Pluvialis fulva, Pluvialis squatarola, Pluvialis dominica, Thinornis cucullatus, Thinornis novaeseelandiae, Elseyornis melanops, Peltohyas australis, Charadrius bicinctus, Charadrius leschenaultii, Charadrius mongolus, Charadrius morinellus, Charadrius obscurus, Charadrius semipalmatus, Charadrius veredus, Vanellus miles, Vanellus spinosus, Vanellus tricolor. Burhinidae: Burhinus capensis, Burhinus grallarius, Burhinus oedicnemus, Esacus magnirostris. Recurvirostridae: Himantopus himantopus leucocephalus, Cladorhynchus leucocephalus, Recurvirostra avosetta, Recurvirostra novaehollandiae. Ibidorhynchidae: Ibidorhyncha struthersii (photos only). Haematopodidae: Haematopus ostralegus, Haematopus longirostris, Haematopus unicolor. Pluvianidae: Pluvianus aegyptius. Chionidae: Chionis albus, Chionis minor. Pluvianellidae: Pluvianellus socialis.
Systematic palaeontology
Aves Linnaeus, 1758
Charadriiformes Huxley, 1867
Charadrii Strauch, Citation1978; sensu Paton et al. Citation2003
Three major clades make up the order Charadriiformes (e.g. Ericson et al. Citation2003; Paton et al. Citation2003; Thomas et al. Citation2004): Lari (pratincoles, auks, gulls and relatives), Scolopaci (jacanas, seedsnipes, sandpipers and allies) and Charadrii. The material here described can be attributed to the suborder Charadrii based on the combination of the following postcranial features (only features relevant to the identification of the fossil material are noted): coracoid with (1) extremitas omalis with mediolaterally elongated tuberculum brachiale, i.e. projecting markedly medially from processus acrocoracoideus (A–E); (2) foramen nervi supracoracoidei present (F). Tarsometatarsus with (3) elongated trochlea metatarsi III (I; not in burhinids); (4) foramen vasculare distale relatively large (I). Character (1) distinguishes representatives of Charadrii from most Lari; (2) from all Scolopaci, buttonquails (Lari: Turnicidae), and some species in Glareolidae (Mayr Citation2011); (3) from some Lari (e.g. Laridae and Stercorariidae); and (4) from most Scolopaci (Mayr Citation2011; De Pietri & Mayr Citation2012; De Pietri et al. Citation2016a). For more details see Strauch (Citation1978), and Mayr (Citation2011).
Figure 1. Coracoids of the early Miocene Neilus sansomae gen. et sp. nov. (A, E–H), and the late Oligocene Chionoides australiensis gen. et sp. nov. (I, K, M) in comparison with the extant Black-faced Sheathbill Chionis minor (NMV B.30769) (B, J, L, N), and the Magellanic Plover Pluvianellus socialis (AMNH 17700) (C, D). Left coracoid of N. sansomae (holotype, NMNZ S.52628) in A, ventral, E, dorsomedial, F, dorsal, G, medial and H, lateral views. Right coracoid (reversed) of Chionis minor in B, N, ventral, J, medial and L, dorsal views. Left coracoid of Chionoides australiensis (holotype, SAM P41458) in I, medial, K, dorsal, M, ventral and O, lateral views. Right coracoid (reversed) of P. socialis in C, ventral and D, dorsomedial views. Abbreviations: cs, cotyla scapularis; da, dorsal area ventromedial of facies articularis humeralis; fac, facies articularis clavicularis; fah, facies articularis humeralis; ila, impressio ligamenti acrocoracohumeralis; ims, impressio m. sternocoracoidei; lgd1, ventromedial ligamental insertion area; lgd2, craniodorsal ligamental insertion area; pac, processus acrocoracoideus; pmm, projection on margo medialis; rid, ridge; ssc, sulcus m. supracoracoidei; tbr, tuberculum brachiale; tub, tuberosity; vfac, ventral facet of facies articularis clavicularis. Scale bars equal 2 mm. We note that C was supplied and is at a different angle compared to Figure 1A and B.
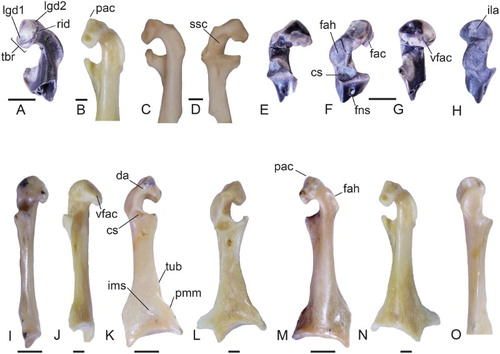
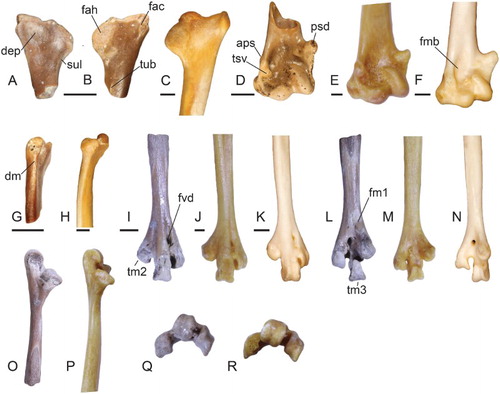
Figure 2. Postcranial elements tentatively attributed to Neilus sansomae gen. et sp. nov. (A, B, D, G, I, L, O, Q) in comparison to sheathbills (C, E, H, J, M, P, R) and the Magellanic Plover (AMNH 17700) ( F, K, N). Left scapulae of N. sansomae (NMNZ S.52888) (A, B, G) and Chionis albus (NMNZ OR.22149) (C, H) in A, medial, B, C, lateral and G, H, dorsal views. D, Left humerus (NMNZ S.44208) of N. sansomae in cranial view. Right humeri (reversed) of E, Chionis minor (NMV B.30769) and F, P. socialis in cranial view. Left tarsometatarsi of N. sansomae (CM 2013.18.684) (I, L, O, Q), C. minor (J, M, P, R), and P. socialis (K, N) in I, K, dorsal, L–N, plantar, O, P, medial and Q, R, distal views. Abbreviations: aps, attachment for m. pronator superficialis; dep, depression; dm, dorsal margin; fac, facies articularis clavicularis; fah, facies articularis humeralis; fm1, fossa metatarsi I; fmb, fossa m. brachialis; fvd, foramen vasculare distale; lig, ligamentum collaterale ventrale; psd, processus supracondylaris ventralis; sul, sulcus; tm2, trochlea metatarsi II; tm3, trochlea metatarsi III; tsv, tuberculum supracondylare ventrale; tub, tubercle. Scale bars equal 2 mm; O–R not to scale.
Superfamily Chionoidea Lesson, Citation1828
The following combination of features is present in living members of this group (Pluvianellus socialis and two species of Chionis), and distinguishes them from all other representatives of the Charadrii: Coracoid with: (1) tuberculum brachiale low (i.e. directed sternally rather than medially) and ventrally protruding relative to the shaft (A, G), (2) ventral portion of the facies articularis clavicularis convex medially (G, J); (3) processus acrocoracoideus high (omally projecting) relative to facies articularis clavicularis (G, I, J); (4) ventral area adjacent to the facies articularis humeralis deeply concave, separated cranially from the ligamental insertion area on the tuberculum brachiale by an angular and very prominent ridge (A–C). Tarsometatarsus with (5) a distinct fossa metatarsi I (L, M; weakly marked in P. socialis); (6) trochlea metatarsi II more proximally positioned than the trochlea metatarsi IV (I; only slightly more proximally situated in some other Charadrii); (7) trochlea metatarsi II flaring noticeably medially, i.e. less plantarly deflected (I).
Members of Chionoidea differ from Charadriidae in characters (1), (2), (5, but not from species of Vanellus and some species of Pluvialis) and (7); from Haematopodidae and Ibidorhynchidae in (1), (2), (3), (4), (5) and (6); and from Recurvirostridae in (1), (2), (3), (5) and (7). They differ from Pluvianus aegyptius in (1), (5) and (6), and from the closely related Burhinidae in (1), (5) and (6). We note that character (4), although present in most of the examined charadriid plovers and recurvirostrids, is less pronounced in these taxa.
Family indet.
Neilus sansomae gen. et sp. nov.
Holotype.
NMNZ S.52628, omal extremity of left coracoid (A, E–H).
Measurements.
See .
Table 1. Skeletal measurements of the material attributed to Neilus sansomae gen. et sp. nov. and Chionoides australiensis gen. et sp. nov., in comparison with the Black-faced Sheathbill Chionis minor (NMV B.30769) and the Magellanic Plover Pluvianellus socialis (AMNH 17700).
Type locality and age.
Bed HH1a, Manuherikia River, near St Bathans, Central Otago, South Island, New Zealand. Bannockburn Formation, Manuherikia Group; Altonian local stage, early Miocene, 19–16 Ma (Schwarzhans et al. Citation2012).
Etymology.
Generic name is after Neilus who in Greek mythology was the father of Chiona from whom the Greek word meaning ‘snow’ is derived. This name recognises our contention that this new taxon is closely related to the group that includes modern sheathbills (Chionidae). This name is also appropriate because Neilus derives from the Greek word meaning ‘river valley’ and so it describes the depositional environment in which the fossils were found. Gender is masculine. The specific name honours Olga Sansom (1900–1989), an ornithologist from Southland who, along with her daughter Maida Barlow, were pioneers in the study of shorebirds in southern New Zealand.
Tentatively referred material (fossil beds are shown in bold).
Coracoid: NMNZ S.52705 (R omal extremity; HH2b). Scapula: NMNZ S.52888 (L; HH1b). Humerus: NMNZ S.44208 (dL; HH1a). Tarsometatarsus: CM 2013.18.684 (dL, immature; Mata Creek 9); NMNZ S.43196 (dR; HH1a).
Localities.
For information on HH1a, HH1b and HH2b, see Schwarzhans et al. Citation2012. Mata Creek 9: This site lies on a westerly loop of the stream on the true right or western side of Mata Creek. A 30 m long bank up to 4 m high bounds the stream; 44.88048°S, 169.84027°E. New Zealand Fossil Record File Number H41/f0122.
Diagnosis.
Much smaller than extant sheathbills, smaller than the Magellanic Plover (), and about the size of the Oriental Plover Charadrius veredus. Other than the features already listed for Charadrii and Chionoidea, it is distinguished by the following combination of features: Coracoid with (1) ventral outline of facies articularis clavicularis facing markedly medially; G); (2) ventromedial surface of the tuberculum brachiale with two distinct ligamental insertion areas: a large and deep one ventromedially, and a smaller and shallower one craniodorsally, both separated by an area about the size of the larger depression (A); (3) dorsal area between the facies articularis humeralis and the facies articularis clavicularis craniosternally broad and equally deep mediolaterally and omo-sternally (F); (4) impressio ligamenti acrocoracohumeralis as wide as it is long (H); (5) area underneath the facies articularis clavicularis only very shallowly excavated (E); (6) foramen nervi supracoracoidei situated close to the cotyla scapularis, separated from the sternal margin of the cotyla scapularis by a distance less than the maximum diameter of the foramen (F). Humerus with: (7) prominent tuberculum supracondylare ventrale, adjacent to the ventral rim of the facet for the ligamentum collaterale ventrale (D). Tarsometatarsus with: (8) plantar surface of trochlea metatarsi III long proximodistally (L); (9) foramen vasculare distale situated close to the incisura intertrochlearis (area between the two smaller than the proximodistal width of foramen vasculare distale; L). [Note that the humerus and tarsometatarsus are only tentatively referred to N. sansomae].
Character (1) is more pronounced in N. sansomae than in other chionoideans. Neilus sansomae also differs from P. socialis and species of Chionis in (5), (6) and (8) (Character [8] is however present in the closely related burhinids). It agrees with species of Chionis, but not with P. socialis in (3), (4) and (9). It differs from Chionis albus in (2) and (7).
Remarks.
Neilus sansomae is a relatively rare wader in the St Bathans deposits, and some of the elements found belong to immature individuals. It is also the largest of the taxa of Charadrii found in St Bathans. The material is therefore referred to this taxon based on size, on the presence of features that are congruent with the taxonomic placement of this taxon as inferred from the type and referred material, and on the morphological differences with other small, undescribed waders from the St Bathans Fauna (see also Worthy et al. Citation2007).
Description and comparisons
Coracoid
The area medial to the processus acrocoracoideus (i.e. the tuberculum brachiale), which includes the ventral portion of the facies articularis clavicularis, and, ventrally, the attachment surfaces for the ligamentum acrocoraco-claviculare superficiale (see Baumel & Witmer Citation1993, p. 179) display a characteristic shape in chionoideans, especially in sheathbills. The ventral surface of the tuberculum brachiale (sensu Baumel & Witmer Citation1993) is more ventrally rotated; i.e. projecting markedly ventrally and sternally (A, B) than in other Charadrii, including burhinids. The ventral portion of the facies articularis clavicularis does not comprise a dorsoventrally flat and medially protruding surface for the articulation of the raised facies articularis acrocoracoidea of the furcula, as seen in most of the examined Charadrii. Instead, in chionoideans, it is an inconspicuous bulging (convex) surface (G, J), which articulates with a rather flat and strictly laterally placed facies articularis acrocoracoidea of the furcula; the furcula of N. sansomae has not been recovered however. We note that the Double-banded Plover Charadrius bicinctus also lacks a noticeably flat area in the ventral portion of facies articularis clavicularis, but the area is nevertheless considerably prominent medially, and the tuberculum brachiale does not project markedly ventrally off the shaft.
In ventral view, the omal extremity of the coracoid of chionoideans (Chionis albus being somewhat of an exception, see below) also shows important differences with other Charadrii. The ventral area adjacent to the facies articularis humeralis is deeply concave, and it is separated omally from the ligamental insertion area on the tuberculum brachiale by an angular and prominent ridge (A). A prominent ridge is present in some other taxa within Charadrii, such as Pluvianus aegyptius, some burhinids (e.g. Burhinus grallarius, Burhinus oedicnemus, and the fossil taxon Genucrassum bransatensis; see De Pietri & Scofield Citation2014), and some charadriid plovers (albeit less marked in most). It is absent in several taxa (e.g. some charadriid plovers, oystercatchers), which have a ventrally protruding tubercle instead (see also Roux Citation2002). It is weakly marked in Chionis albus, which also displays a prominent tubercle. This part of the tuberculum brachiale is ventromedially very prominent in chionoideans.
There are some differences in the ligamental insertion areas of the ventral surface of the tuberculum brachiale between Chionis albus on the one hand, and C. minor and P. socialis on the other. In C. albus, there are two distinct and adjacent impressions, one round, deep and wide situated near the ventrosternal tip of the acrocoracoid, and a shallower one more craniodorsally located. In C. minor, P. socialis and N. sansomae, both impressions are separated from each other by an area as wide as the diameter of the largest (ventrosternal) depression (A–C). In N. sansomae, the ventrosternal depression is deeper than in P. socialis, and occupies most of the entire width of the ventromedial surface of the tuberculum brachiale, as in C. minor (A, B). Contrary to species of Chionis, however, this depression is more dorsally situated, being entirely visible in medial view (G). This is an autapomorphy of N. sansomae. The smaller, more dorsally situated, impression is very shallow or nearly absent in the examined haematopodids, recurvirostrids and charadriid plovers.
In dorsal view, the area immediately medial to the cranial margin of the facies articularis humeralis (between the humeral facet and the facies articularis clavicularis) is relatively robust, broad and short (F), being similar to the condition observed in sheathbills (L). In P. socialis, this area is narrower omo-sternally, forming an elongated crest or ridge that separates the facies articularis humeralis from the facies articularis clavicularis (D). Although there is some variation in this area among species of Charadrii, N. sansomae and species of Chionis are closest in morphology (E, L). In N. sansomae, the impressio ligamenti acrocoracohumeralis is as wide as it is long (H), similar to species of Chionis, being more elongated in P. socialis. The cotyla scapularis is relatively large in comparison to the facies articularis humeralis and the impressio ligamenti acrocoracohumeralis, occupying over half the length of the facies articularis humeralis (F; see ).
In medial view, the area below the facies articularis clavicularis is more deeply excavated in species of Chionis than in N. sansomae, in which this recess is only very shallow. The sulcus m. supracoracoidei is excavated dorsally, a condition that is widely distributed within Charadrii. Unlike in burhinids, a depression on the ventral portion of the sulcus is absent (De Pietri & Scofield Citation2014).
A foramen nervi supracoracoidei is present, being relatively large as in C. minor (L) and situated in the middle of the shaft in dorsal view, medially below the cotyla scapularis (F). It is relatively closer to the cotyla scapularis than in species of Chionis and P. socialis (D, L).
Scapula.
This element is only tentatively attributed to N. sansomae. It differs from most of the examined taxa within Charadrii in lacking a pronounced mediolaterally constricted dorsal margin of the cranial end, caudal to the facies articularis clavicularis (G). In most Charadrii, this margin is blade-like, but in N. sansomae and species of Chionis, it is mediolaterally thicker (G, H).
It differs from chionids in having a deep and well-marked depression on the medial surface of the bone (A), ventral to the acromion. This depression is present in many representatives of the Charadrii, including P. socialis, but it is exceptionally well-developed in N. sansomae, occupying over half of the cranial medial surface. The acromion differs between species of Chionis and P. socialis, being more elongated cranially in the latter. Unfortunately the acromion is not entirely preserved in this specimen. Based on the internal structure of the bone where it is broken; however, it would not extend cranially past its current cranial margin, as only a dorsal portion is missing (B, G). The lateral, ridge-like margin of the facies articularis clavicularis is more craniocaudally elongated than in the examined charadriid plovers, in which it is relatively short and does not overlap with the craniocaudal length of the facies articularis humeralis in lateral view (B). As in burhinids, P. socialis and several of the examined charadriid plovers, there is a well-marked sulcus on the ventral margin of the scapula (A), adjacent to the processus glenoidalis scapulae. The facies articularis humeralis is not entirely preserved, but it does appear to have been round (i.e. as long as wide), similar to other chionoideans, and not craniocaudally elongated as in most other Charadrii. Most of the tuberculum coracoideum is missing.
Only part of the tubercle on the lateral surface of the corpus scapulae, caudal to the collum scapulae, is preserved in this specimen (B), so its relative development cannot be assessed (it is very prominent in some charadriid plovers and recurvirostrids for instance). The point of origin is slightly more cranial than in extant chionoideans.
Humerus
A distal fragment of a humerus is only tentatively attributed to N. sansomae. It matches the corresponding element of several species of Charadrii in many features, but it is especially close in morphology to chionoideans. The processus supracondylaris dorsalis (D), although slightly broken, is better developed than in recurvirostrids, haematopodids, and P. aegyptius, being well-developed in charadriid plovers and P. socialis (it is slightly better developed cranially in our specimens of C. minor compared with C. albus). The cranial face of the tuberculum supracondylare ventrale (D) is proportionally short proximodistally, and dorsoventrally expanded at its base. The tubercle for the attachment of m. pronator superficialis is very well marked, and nearly adjacent to the ventral rim of the tuberculum supracondylare ventrale. In C. albus however, this tubercle is more proximocaudally situated, and we did not find it to be particularly well-developed in the examined charadriid plovers. The fossa musculi brachialis is moderately deep, mostly restricted to the ventral half of the humerus, and well-delimited in its entirety, as in P. socialis (F).
Tarsometatarsus
As in chionoideans, the tarsometatarsus appears stouter compared with the same element in similar-sized charadriid plovers. Only the distal half of this bone is preserved in one specimen (CM 2013.18.684; I), and it belongs to an immature individual. The shaft of this specimen shows the characteristic porosity and striations present in subadult individuals (see also De Pietri et al. Citation2016a and references therein), as well as an incomplete ossification of the area distal to the foramen vasculare distale. The other specimen (NMNZ S.43196) is only slightly smaller, but so fragmentary that it is only tentatively assigned to N. sansomae. Based on skeletal proportions for charadriid plovers, especially compared with the coracoid, CM 2013.18.684 may appear too big to belong to the same taxon. However, proportions match those of C. minor (), and despite the immature status of the bone, we consider it likely to belong to N. sansomae.
In CM 2013.18.684, a fossa metatarsi I is present (L); it is better marked and slightly more plantarly situated than in species of Chionis. A fossa metatarsi I is absent in most other representatives of the Charadrii due to the loss or reduction of the hallux in several of its members. The dorsal fossa in which the foramen vasculare distale is situated is well-marked and short proximally. The foramen vasculare distale is only slightly larger than that of C. minor (J), but given the immaturity of the specimen it would probably be smaller in an adult. It is situated close to the incisura intertrochlearis lateralis, being wider proximodistally than the area separating the two. In P. socialis, the foramen is more proximally situated (N). Sheathbills and the Magellanic Plover have a dorsal opening of the canalis interosseous distalis, which is absent in CM 2013.18.684, although the area is not fully ossified in the specimen. This opening is absent in NMNZ S.43196 but, as noted, this specimen is only tentatively attributed to N. sansomae.
In CM 2013.18.684, the shaft of the tarsometatarsus widens in the proximal direction towards its mid-section, being distinctly narrow just proximal of the distal end (I). Although this condition is in all likelihood related to the immature status of this bone, a distinctly narrow shaft distally is also observed in C. minor (J).
As in most Charadrii, with the exception of members of Burhinidae, Haematopodidae and Ibydorhynchidae, the trochlea metatarsi II of CM 2013.18.684 is proximally elevated compared with the trochlea metatarsi IV. The trochlea metatarsi II ends distally past the origin of the incisura intertrochlearis lateralis, but proximal of the distal margin of trochlea metatarsi IV. Similar to chionoideans, trochlea metatarsi II is not as plantarly deflected as in charadriid plovers. In distal view, it is proportionally wider, mediolaterally, than in charadriid plovers. The plantar projection on trochlea metatarsi II is rather worn, as is the medial surface of this trochlea. The medial surface of the trochlea metatarsi III is also worn and some bone is missing, therefore the entire trochlea would have been mediolaterally wider than it appears (Q). As noted in the diagnosis, the shape of trochlea metatarsi III differs from that of extant chionoideans in having a proximodistally more elongated plantar surface (L). The poorly-marked furrow on this trochlea may be related to wear.
In recurvirostrids the tarsometatarsus is thin and very elongated. Trochlea metatarsi II is more plantarly orientated than in N. sansomae, trochlea metatarsi IV is more elongated, reaching past the distal half of trochlea metatarsi III, and in distal view, all trochleae are more plantarly elongated. In oystercatchers and the Ibisbill, the trochlea metatarsi III is distinctly inflated dorsally, and the trochlea metatarsi II is situated more distally, at nearly the same level as the trochlea metatarsi IV. Burhinids have a derived morphology of the tarsometatarsus, with a much reduced trochlea metatarsi II, proximodistally short trochlea metatarsi III, and a very small foramen vasculare distale situated in a deep groove.
Both sheathbills and the Magellanic Plover have proportionally short legs compared with most representatives of the Charadriidae (the Shore Plover Thinornis novaeseelandiae being one exception), but whether Neilus sansomae had similar proportions is unknown because CM 2013.18.684 is incomplete. The similarity in the proportions of the shaft of the tarsometatarsus to Chionis minor however, may indicate the possibility that this bone showed chionoidean proportions. We note that among members of the Charadrii, taxa with relatively short legs (e.g. Magellanic Plover, sheathbills, oystercatchers, Shore Plover) are better adapted to walking on rocky or irregular surfaces (Piersma Citation1996).
Family indet.
Chionoides australiensis gen. et sp. nov.
Holotype.
Left coracoid, SAM P41458 (I, K, M, O).
Measurements.
See .
Type locality and age.
Neville’s Nirvana, collection code VSQ 1978-74P of a joint Victoria, South Australia, and Queensland Museums expedition to Lake Palankarinna, South Australia; Etadunna Formation, Minkina LF, Zone A, late Oligocene, 26–24 Ma (Woodburne et al. Citation1994).
Etymology.
Generic name Chionoides means ‘Chionis-like’, alluding to the close similarity to members of this genus; gender is neutral. Specific name australiensis refers to the Australian continent.
Diagnosis.
Coracoid about the size of N. sansomae, displaying the following combination of features (other than those already listed for Chionoidea): (1) in dorsal view, area immediately medial of omal end of facies articularis humeralis narrow in omal–sternal direction (K); (2) impressio ligamenti acrocoracohumeralis as wide as it is long (O); (3) area below the facies articularis clavicularis only very shallowly excavated, being practically absent (K). Differs from P. socialis and agrees with species of Chionis in: (2), (4) prominent angle on the sternal medial surface more proximally situated (K).
Differs from species of Chionis in: (1), (5) small size and less robust (i.e. proportionally more elongate; K, and L). Differs from Chionis albus in: (6) the two distinct impressions on the ventral surface of the tuberculum brachiale separated from each other by an area as wide as the diameter of the largest (ventrosternal) depression. Differs from N. sansomae in: (1), (7) processus acrocoracoideus better developed (M); (8) ridge separating the ligamental insertion area and the ventral surface beside the facies articularis humeralis not as well-developed (M); (9) foramen nervi supracoracoidei more sternally positioned (K); (10) the ventral outline of facies articularis clavicularis does not face markedly medially.
Description and comparisons.
The coracoid closely matches that of Chionis minor, but it is much smaller and proportionally more elongated. Although there is some damage to the ventromedial surface of the tuberculum brachiale, the ligamental scars do not appear to be adjacent (the ventromedial ligamental scar is not visible due to the damage), as the (undamaged) area separating the ventromedial and craniodorsal margins occupies most of the tuberculum brachiale. The larger, ventromedial scar would therefore appear to have been situated close to the tip of the tuberculum brachiale (M). As in chionoideans, the ridge separating the ligamental insertion area and the ventral surface beside the facies articularis humeralis is well developed.
As in all chionoideans, the processus acrocoracoideus is very prominent omally. The facies articularis clavicularis also lacks the dorsoventrally flat and medially protruding surface for the articulation of the raised facies articularis acrocoracoidea of the furcula, displaying a rounded surface instead (see description for N. sansomae). A recess below the facies articularis clavicularis is nearly absent.
Similar to species of Chionis, the ventral margin of the facies articularis humeralis projects markedly ventrolaterally, and displays sternally a pronounced angle (M). The impressio ligamenti acrocoracohumeralis is less elongated than in the Magellanic Plover and most other Charadrii, being almost as wide as it is long (O), similar to N. sansomae and sheathbills. The cotyla scapularis is proportionally not as large as that of sheathbills and N. sansomae, in which it is over half the length of the facies articularis humeralis (L). The area immediately medial to the omal end of the facies articularis clavicularis (K) is narrower in omal–sternal direction compared with N. sansomae and species of Chionis, in which the dorsal area between the facies articularis humeralis and the facies articularis clavicularis is broader and more robust (E, L). In this aspect, Chionoides australiensis is more similar to P. socialis.
The foramen nervi supracoracoidei is situated in the midline of the shaft in dorsal view rather than close to its medial margin (contrary to the condition in many charadriid plovers). It is only slightly more sternally situated than in species of Chionis and N. sansomae. The ventral opening of the foramen is situated on the shaft of the coracoid, at the base of the processus procoracoideus. At the sternal end, the projection on the margo medialis of the extremitas sternalis resembles that of species of Chionis, being more omally situated than in P. socialis. Both Chionoides australiensis and P. socialis display a tuberosity on the medial margin of the shaft, cranial to the projection on the margo medialis (K). The impressio m. sternocoracoidei is well marked but not as deep as that of most examined charadriid plovers. The angulus medialis and surrounding area are rather worn.
Discussion and conclusions
The postcranial elements here attributed to N. sansomae display a mosaic of features present in both sheathbills and the Magellanic Plover, and as such it is not possible, based on the available material, to confidently attribute this taxon to either lineage (but see below). Most elements, however, particularly the coracoid and the tarsometatarsus, are closer in morphology to sheathbills than to the Magellanic Plover. The chionoidean affinities of N. sansomae are supported, primarily, by the morphology of the coracoid. The coracoid displays a pronounced ventral deflection of the tuberculum brachiale (the medial projection of the acrocoracoid) that is characteristic of sheathbills and to a slightly lesser degree of the Magellanic Plover. The tarsometatarsus, although only tentatively referred to N. sansomae, agrees with that of sheathbills in the mediolaterally splayed trochleae metatarsorum, in the less conspicuous plantar retraction of trochlea metatarsi II compared with most other Charadrii, and in the presence of a well-marked fossa metatarsi I for the articulation of the hind toe. A hallux, which is either absent or reduced in most representatives of the Charadrii and is vestigial in some charadriid plovers and recurvirostrids, is present in chionoideans. The small size of N. sansomae agrees with that of many representatives of the Charadrii, such as charadriid plovers, the Egyptian Plover, and the Magellanic Plover, being considerably smaller than extant sheathbills ().
Chionoides australiensis is only the second shorebird to be described from the late Oligocene–early Miocene lake deposits of South Australia (see De Pietri et al. Citation2015). This rich, South Australian avifauna also includes palaeognaths (Boles Citation2001), a megapode (Boles & Ivison Citation1999), anseriforms (Worthy Citation2009; De Pietri et al. Citation2016b), flamingos and palaelodids (Miller Citation1963; Baird & Vickers-Rich Citation1998), and cormorants and other waterbirds (Miller Citation1966; Worthy Citation2011, Citation2012). As with N. sansomae, the chionoidean affinities of Chionoides australiensis are supported by the ventral deflection of the medial portion of the acrocoracoid (‘tuberculum brachiale’), among other features. The morphology of this coracoid, the only element described for this taxon, is very different from that of the only other charadriiform bird described from these deposits, the pedionomid Oligonomus milleri (see De Pietri et al. Citation2015).
Both Chionoides australiensis and N. sansomae extend the known distribution of chionoideans to continental Australia, and New Zealand, respectively. Extant sheathbills are only found on the Antarctic Peninsula and subantarctic islands of the Atlantic and Indian Oceans; C. albus migrates to coastal areas of southern South America during the non-breeding season (Burger Citation1996). Sheathbills are predator-scavengers and opportunistic feeders, and the smallest members of this guild in the subantarctic region (Burger Citation1996). The small, plover-like size of both Neilus sansomae and Chionoides australiensis, about half the size of sheathbills, suggests that these taxa did not occupy a similar ecological niche. The ecological preferences of N. sansomae and Chionoides australiensis are unknown, but their presence in inland lake deposits suggests that they foraged near lake shores, at least during the breeding season, as inferred from the presence of immature bones.
The fossil record of the clade that encompasses stone-curlews, sheathbills and the Magellanic Plover, which is the sister clade to all other Charadrii (e.g. Baker et al. Citation2007), is among the poorest within Charadriiformes, especially for the southern hemisphere. Before the records here described, the earliest, unambiguous, record of a member of this clade (Burhinidae + Chionoidea) was from the latest Oligocene (c. 23 Ma) of France (De Pietri & Scofield Citation2014). Genucrassum bransatensis was a small stone-curlew that was described based on few elements that already showed the highly derived and characteristic morphology known for burhinids. Other than G. bransatensis, the pre-Quaternary fossil record of burhinids comprises specimens from the early Miocene of North America (Bickart Citation1981) and undescribed remains from the Pliocene of South Africa (Manegold et al. Citation2013). The reported late Oligocene–early Miocene fossils from Australia attributed to a stone-curlew (Boles et al. Citation2013) have since been shown to belong to an early-diverging representative of the Anseriformes (swans, geese and allies; De Pietri et al. Citation2016b).
The late Oligocene and early Miocene fossils we here attribute to the Chionoidea indicate that the split between stone-curlews and the lineage including sheathbills and the Magellanic Plover must have taken place before the late Oligocene (26–24 Ma), which is further supported by the already derived, burhinid-like morphology of late Oligocene stone-curlews (De Pietri & Scofield Citation2014). Molecular divergence dates for this split have been estimated at 54 Ma (interval 66.6–41.5 Ma; Paton et al. Citation2003), but also at c. 80 Ma (Late Cretaceous; Baker et al. Citation2007, see however Mayr Citation2011). More recently, the divergence between burhinids and other Charadrii was estimated at c. 40 Ma (interval 55–20 Ma; Prum et al. Citation2015), and although chionoideans were not included in this study, it suggests that the burhinid-chionoidean split may be more recent than proposed in earlier molecular studies.
The split between the Magellanic Plover and species of Chionis appears to be among the most ‘recent’ family-level splits within Charadriiformes, and is one of the youngest genus-level splits within Charadrii (Paton et al. Citation2003; Baker et al. Citation2007), indicating that, despite their somewhat different behaviour, the genetic divergence between sheathbills and P. socialis is less than that found among members of the Charadriidae. The split was dated at c. 19 Ma (interval 28.5–11.7 Ma; Paton et al. Citation2003), but, as noted above, divergence dates within modern bird clades tend to be overall younger in recent studies (e.g. Prum et al. Citation2015). Combined evidence from molecular sequence data and the fossil record therefore indicate that N. sansomae and Chionoides australiensis are likely to be stem-group representatives of the Chionoidea. This is inferred from both the mosaic morphology of these taxa (i.e. they display a combination of features present either in sheathbills or the Magellanic Plover), and from the recent divergence of the crown-group, which, as suggested by molecular data, N. sansomae and Chionoides australiensis potentially pre-date. This emerging diversity in the fossil record of stem-group chionoideans is to be expected if the split between chionoideans and stone-curlews is at least 40 Ma, as then the chionoidean ‘ghost lineage’ would be as old.
The relatively recent adaptations of sheathbills to the Antarctic environment, therefore, may have been coupled to the rapid expansion of the Antarctic ice-sheet during the middle Miocene (Zachos et al. Citation2001; Shevenell et al. Citation2008; Knorr & Lohmann Citation2014). Stem-group chionoideans Chionoides australiensis and N. sansomae probably made use of similar habitats as the Magellanic Plover or most other members of the Charadrii. The Magellanic Plover is a coastal littoral zone feeder during the non-breeding season, feeding by lake shores during the breeding season. Similar seasonal, inland to coastal shoreline shifts are known for many charadriid plovers (Piersma Citation1996). Both Chionoides australiensis and N. sansomae were present in lacustrine or fluvio-lacustrine environments, but whether their inland presence was seasonal is still unknown. We note however that the feeding habits of the Magellanic Plover differ from those of all charadriid plovers; whereas charadriids feed by pecking at surface-dwelling prey (the Wrybill Anarhynchus frontalis is somewhat of an exception; see Pierce Citation1979; Piersma Citation1996), the Magellanic Plover uses its legs to dig in sandy substrates, making prey readily available (Jehl Citation1975; Piersma Citation1996). Although the morphology of the tarsometatarsus we attributed to N. sansomae differs from that of all examined charadriid plovers, the material is still too incomplete to know whether N. sansomae may have shown a feeding behaviour similar to that of the Magellanic Plover.
The causes for the disappearance of these chionid-like birds from the Australasian region are unknown, but are possibly related to climatic and environmental changes that took place after the early or middle Miocene (Byrne et al. Citation2011; Knorr & Lohmann Citation2014). In New Zealand, these changes contributed to the disappearance of many warm-adapted taxa, including crocodilians (Worthy et al. Citation2007; De Pietri et al. Citation2016a), whereas the increasing aridification of the Australian continent during this time (Martin Citation2006; Byrne et al. Citation2011; Cohen et al. Citation2011) supported a significant faunal turnover that made room for species that could better cope with the new environmental conditions (McGowran et al. Citation2000; Black et al. Citation2012; Schweizer et al. Citation2013). Mystacinid bats, also recovered from early Miocene St Bathans and late Oligocene Lake Palankarinna sediments among other Australian deposits, disappeared from Australia sometime after the middle Miocene (Hand et al. Citation2005) but today survive in wetter, cooler New Zealand as the only Australasian members of the Gondwanan bat superfamily Noctilionoidea (Teeling et al. Citation2005). Similar to sheathbills, the survival of mystacinid bats in restricted areas of the southern hemisphere may be linked to their opportunistic diet, which, despite their unique semi-terrestrial foraging behaviour (Hand et al. Citation2009, Citation2013), is among the most diverse for bats (Daniel Citation1976; Arkins et al. Citation1999; Lloyd Citation2001).
Australasia’s mystacinids are the sister group of the South American noctilionoids, a speciose and highly ecologically diverse radiation of one extinct and five extant Neotropical bat families (Simmons Citation2005; Czaplewski & Morgan Citation2012). According to molecular data, this divergence of Australasian and South American clades occurred 51–41 Ma (Miller-Butterworth et al. Citation2007). They therefore provide an interesting comparative framework for the distribution of chionoidean plovers in Australasia and, potentially, South America during the Palaeogene and early Neogene. Together with a better understanding of the evolutionary history of another Gondwanan shorebird clade, the Pedionomidae and Thinocoridae (see De Pietri et al. Citation2015; De Pietri et al. Citation2016a), the fossil record is providing increasing evidence for Australasian–South American faunal relationships to the exclusion of northern regions that date back to the Oligocene.
Acknowledgements
We thank AMNH staff, especially J Cracraft, P. Sweet and photographer M Shanley, for providing images and measurements of Pluvianellus socialis. We are grateful to Kyle Davis and the many volunteers who have repeatedly assisted us with fieldwork, and we are especially grateful to the landowners A and E Johnstone of Home Hills Station and J and T Enright, Dunstanburn Station, Southern Lakes Holdings Ltd, for access to the sites. We thank J Worthy for sorting the material. We thank G Mayr and one other anonymous reviewer for comments that improved the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCiD
Vanesa L De Pietri http://orcid.org/0000-0002-3786-9741
R Paul Scofield http://orcid.org/0000-0002-7510-6980
Trevor H Worthy http://orcid.org/0000-0001-7047-4680
Additional information
Funding
References
- Arkins AM, Winnington AP, Anderson S, Clout MN. 1999. Diet and nectarivorous foraging behaviour of the short-tailed bat (Mystacina tuberculata). J Zool. 247:183–187. doi: 10.1111/j.1469-7998.1999.tb00982.x
- Baird RF, Vickers-Rich P. 1998. Palaelodus (Aves: Palaelodidae) from the middle to late Cainozoic of Australia. Alcheringa Austral J Palaeontol. 22:135–151. doi: 10.1080/03115519808619196
- Baker AJ, Pereira S, Paton TA. 2007. Phylogenetic relationships and divergence times of Charadriiformes genera: multigene evidence for the Cretaceous origin of at least 14 clades of shorebirds. Biology Lett. 3:205–210. doi: 10.1098/rsbl.2006.0606
- Baumel JJ, Witmer L. 1993. Osteologia. In: Baumel JJ, Kings AS, Breazile JE, Evans HE, Vanden Berge JC, editors. Handbook of avian anatomy: nomina anatomica avium. Cambridge (MA): Nuttall Ornithological Club; p. 45–132.
- Bickart KJ. 1981. A new thick-knee, Burhinus, from the Miocene of Nebraska, with comments on the habitat requirements of the Burhinidae (Aves: Charadriiformes). J Vert Paleontol. 1:273–277. doi: 10.1080/02724634.1981.10011902
- Black KH, Archer M, Hand SJ, Godthelp H. 2012. The rise of Australian marsupials: a synopsis of biostratigraphic, phylogenetic, palaeoecologic and palaeobiogeographic understanding. In: Talent JA, editor. Earth and life. Dordrecht: Springer; p. 983–1078.
- Boles WE. 2001. A new emu (Dromaiinae) from the late Oligocene Etadunna Formation. Emu. 101:317–321. doi: 10.1071/MU00052
- Boles WE, Finch MA, Hofheins RH, Vickers-Rich P, Rich TH. 2013. A fossil stone-curlew (Aves: Burhinidae) from the Late Oligocene/Early Miocene of South Australia. In: Göhlich UB, Kroh A, editors. Paleornithological research. Proceedings volume of the 8th international meeting of the society of avian palaeontology and evolution; 2012 Jun 11–16; Vienna: Naturhistorisches Museum Wien; p. 43–61.
- Boles WE, Ivison TJ. 1999. A new genus of dwarf megapode (Galliformes: Megapodiidae) from the late Oligocene of central Australia. Smithson Contrib Paleobiol. 89:199–206.
- Burger AE. 1996. Family Chionidae. In: del Hoyo J, Elliott JA, Sargatal J, editors. Handbook of birds of the world, volume 3, Barcelona: Lynx Edicions; p. 546–555.
- Byrne M, Steane D, Joseph L, Yeates DK, Jordan GJ, Crayn D, Aplin K, Cantrill DJ, Cook LG, Crisp MD, et al. 2011. Decline of a biome: evolution, contraction, fragmentation, extinction and invasion of the Australian mesic zone biota. J Biogeogr. 38:1635–1656. doi: 10.1111/j.1365-2699.2011.02535.x
- Cassens I, Vicario S, Waddell VG, Balchowsky H, Van Belle D, Ding W, Fan C, Mohan RSL, Simões-Lopes PC, Bastida R, et al. 2000. Independent adaptation to riverine habitats allowed survival of ancient cetacean lineages. Proc Natl Acad Sci. 97:11343–11347. doi: 10.1073/pnas.97.21.11343
- Cohen TJ, Nanson GC, Jansen J, Jones BG, Jacobs Z, Treble P, Price DM, May J, Smith A, Ayliffe LK, Hellstrom JC. 2011. Continental aridification and the vanishing of Australia’s megalakes. Geology. 39:167–170. doi: 10.1130/G31518.1
- Czaplewski NJ, Morgan GS. 2012. New basal noctilionoid bats (Mammalia: Chiroptera) from the Oligocene of subtropical North America. In: Gunnell GF, Simmons NB, editors. Evolutionary history of bats. Boston: Cambridge University Press; p. 162–209.
- Daniel MJ. 1976. Feeding by the short-tailed bat Mystacina tuberculata on fruit and possibly nectar. New Zeal J Zool. 3:391–398. doi: 10.1080/03014223.1976.9517927
- De Pietri VL, Camens AB, Worthy TH. 2015. A Plains-wanderer (Pedionomidae) that did not wander plains: a new species from the Oligocene of South Australia. Ibis. 157:68–74. doi: 10.1111/ibi.12215
- De Pietri VL, Costeur L, Güntert M, Mayr G. 2011. A revision of the Lari (Aves, Charadriiformes) from the early Miocene of Saint-Gérand-le-Puy (Allier, France). J Vert Paleontol. 31:812–828. doi: 10.1080/02724634.2011.586663
- De Pietri VL, Güntert M, Mayr G. 2013. A Haematopus-like skull and other remains of Charadrii (Aves, Charadriiformes) from the early Miocene of Saint-Gérand-le-Puy (Allier, France). In: Göhlich UB, Kroh A, editors. Paleornithological research. Proceedings volume of the 8th international meeting of the society of avian palaeontology and evolution; 2012 Jun 11–16. Vienna: Naturhistorisches Museum Wien; p. 93–101.
- De Pietri VL, Mayr G. 2012. An assessment of the diversity of early Miocene Scolopaci (Aves, Charadriiformes) from Saint-Gérand-le-Puy (Allier, France). Palaeontology. 55:1177–1197. doi: 10.1111/j.1475-4983.2012.01182.x
- De Pietri VL, Scofield RP. 2014. The earliest European record of a Stone-curlew (Charadriiformes, Burhinidae) from the late Oligocene of France. J Ornithol. 155:421–426. doi: 10.1007/s10336-013-1022-8
- De Pietri VL, Scofield RP, Tennyson AJD, Hand SJ, Worthy TH. 2016a. Wading a lost southern connection: Miocene fossils from New Zealand reveal a new lineage of shorebirds (Charadriiformes) linking Gondwanan avifaunas. J Syst Palaeontol. 14:603–616. doi:10.1080/14772019.2015.1087064.
- De Pietri VL, Scofield RP, Zelenkov N, Boles WE, Worthy TH. 2016b. The unexpected survival of an ancient lineage of anseriform birds into the Neogene of Australia: the youngest record of Presbyornithidae. R Soc Open Sci. 3:150635. doi: 10.1098/rsos.150635
- del Hoyo J, Elliott A, Sargatal J, Christie DA, de Juana E, editors. 2016. Handbook of the birds of the world alive. Barcelona: Lynx Edicions; [cited 2016 March 22]. Available from: http://www.hbw.com
- Ericson PGP, Envall I, Irestedt M, Norman JA. 2003. Inter-familial relationships of the shorebirds (Aves: Charadriiformes) based on nuclear DNA sequence data. BMC Evol Biol. 3:1–14. doi: 10.1186/1471-2148-3-16
- Gray GR. 1849. The genera of birds comprising their generic characters, a notice of the habits of each genus, and an extensive list of species referred to their several genera. Volume 3. London: Longman, Brown, Green and Longmans.
- Graybeal A. 1998. Is it better to add taxa or characters to a difficult phylogenetic problem? Syst Biol. 47:9–17. doi: 10.1080/106351598260996
- Hand S, Archer M, Godthelp H. 2005. Australian Oligo-Miocene mystacinids (Microchiroptera): upper dentition, new taxa and divergence of New Zealand species. Geobios. 38:339–352. doi: 10.1016/j.geobios.2003.11.005
- Hand SJ, Weisbecker V, Beck RM, Archer M, Godthelp H, Tennyson AJ, Worthy TH. 2009. Bats that walk: a new evolutionary hypothesis for the terrestrial behaviour of New Zealand’s endemic mystacinids. BMC Evol Biol. 9:169. doi: 10.1186/1471-2148-9-169
- Hand SJ, Worthy TH, Archer M, Worthy JP, Tennyson AJD, Scofield RP. 2013. Miocene mystacinids (Chiroptera: Noctilionoidea) indicate a long history for endemic bats in New Zealand. J Vert Paleontol. 33:1442–1448. doi: 10.1080/02724634.2013.775950
- Hartlaub G. 1841. Novelle espèce de Bec-en-fourreau (Chionis). Revue Zool. 1841:5–6.
- Jablonski D. 2002. Survival without recovery after mass extinctions. Proc Natl Acad Sci. 99:8139–8144. doi: 10.1073/pnas.102163299
- Jehl JR. 1975. Pluvianellus socialis: Biology, ecology, and relationships of an enigmatic Patagonian shorebird. Trans San Diego Soc Nat Hist. 18:25–74.
- Knorr G, Lohmann G. 2014. Climate warming during Antarctic ice sheet expansion at the Middle Miocene transition. Nat Geosci. 7:376–381. doi: 10.1038/ngeo2119
- Lesson RP. 1828. Manuel d’ornithologie, ou description des genres et des principals espèces d’oiseaux. Volume 2. Paris: Roret.
- Lloyd BD. 2001. Advances in New Zealand mammalogy 1990–2000: short-tailed bats. J R Soc NZ. 31:59–81. doi: 10.1080/03014223.2001.9517639
- Manegold A, Louchart A, Carrier J, Elzanowski A. 2013. The early Pliocene avifauna of Langebaanweg (South Africa): a review and update. In: Göhlich UB, Kroh A, editors. Paleornithological research. Proceedings volume of the 8th international meeting of the society of avian palaeontology and evolution; 2012 Jun 11–16; Vienna. Vienna: Naturhistorisches Museum Wien; p. 135–152.
- Martin HA. 2006. Cenozoic climatic change and the development of the arid vegetation in Australia. J Arid Environ. 66:533–563. doi: 10.1016/j.jaridenv.2006.01.009
- Mayr G. 2000. Charadriiform birds from the early Oligocene of Céreste (France) and the middle Eocene of Messel (Hessen, Germany). Geobios. 33:625–636. doi: 10.1016/S0016-6995(00)80034-0
- Mayr G. 2009. Paleogene fossil birds. Heidelberg: Springer.
- Mayr G. 2011. The phylogeny of charadriiform birds (shorebirds and allies) – reassessing the conflict between morphology and molecules. Zool J Linn Soc. 161:916–934. doi: 10.1111/j.1096-3642.2010.00654.x
- Mayr G, Knopf CW. 2007. A stem lineage representative of buttonquails from the Lower Oligocene of Germany–fossil evidence for a charadriiform origin of the Turnicidae. Ibis. 149:774–782. doi: 10.1111/j.1474-919X.2007.00712.x
- McGowran B, Archer M, Bock P, Darragh TA, Godthelp H, Hageman S, Hand SJ, Hill R, Li Q, Maxwell PA, et al. 2000. Australasian palaeobiogeography: the Palaeogene and Neogene record. Mem Assoc Australas Palaeontol. 23:405–470.
- Miller AH. 1963. The fossil flamingos of Australia. Condor. 65:289–299. doi: 10.2307/1365355
- Miller AH. 1966. An evaluation of the fossil anhingas of Australia. Condor. 68:315–320. doi: 10.2307/1365447
- Miller-Butterworth CM, Murphy WJ, O’Brien SJ, Jacobs DS, Springer MS, Teeling EC. 2007. A family matter: conclusive resolution of the taxonomic position of the long-fingered bats, Miniopterus. Mol Biol Evol. 24:1553–1561. doi: 10.1093/molbev/msm076
- Olson SL. 1976. An erroneous fossil record of Chionis from Australia. Emu. 76:90. doi: 10.1071/MU9760090b
- Paton TA, Baker AJ. 2006. Sequences from 14 mitochondrial genes provide a well-supported phylogeny of the charadriiform birds congruent with the nuclear RAG-1 tree. Mol Phylogenet Evol. 39:657–667. doi: 10.1016/j.ympev.2006.01.011
- Paton TA, Baker AJ, Groth JG, Barrowclough GF. 2003. RAG-1 sequences resolve phylogenetic relationships within charadriiform birds. Mol Phylogenet Evol. 29:268–278. doi: 10.1016/S1055-7903(03)00098-8
- Pierce RJ. 1979. Foods and feeding of the wrybill (Anarhynchus frontalis) on its riverbed breeding grounds. Notornis. 26:1–21.
- Piersma T. 1996. Family Charadriidae. In: del Hoyo J, Elliott JA, Sargatal J, editors. Handbook of birds of the world, volume 3. Barcelona: Lynx Edicions; p. 384–442.
- Prum RO, Berv JS, Dornburg A, Field DJ, Townsend JP, Lemmon EM, Lemmon AR. 2015. A comprehensive phylogeny of birds (Aves) using targeted next-generation DNA sequencing. Nature. 526:569–573. doi: 10.1038/nature15697
- Rabosky DL, Lovette IJ. 2008. Explosive evolutionary radiations: decreasing speciation or increasing extinction through time? Evolution. 62:1866–1875. doi: 10.1111/j.1558-5646.2008.00409.x
- Roux T. 2002. Deux fossiles d'oiseaux de l'oligocène inférieur du Luberon. Courr Sci Parc Nat Reg Luberon. 6:38–57.
- Schwarzhans W, Scofield RP, Tennyson AJD, Worthy JP, Worthy TH. 2012. Fish remains, mostly otoliths, from the non-marine early Miocene of Otago, New Zealand. Acta Palaeontol Pol. 57:319–350. doi: 10.4202/app.2010.0127
- Schweizer M, Guentert M, Hertwig ST. 2013. Out of the Bassian province: historical biogeography of the Australasian platycercine parrots (Aves, Psittaciformes). Zool Scripta. 42:13–27. doi: 10.1111/j.1463-6409.2012.00561.x
- Shevenell AE, Kennett JP, Lea DW. 2008. Middle Miocene ice sheet dynamics, deep-sea temperatures, and carbon cycling: a southern ocean perspective. Geochem Geophys. 9:Q02006. doi:10.1029/2007GC001736
- Simmons NB. 2005. Order Chiroptera. In: Wilson DE, Reeder DM, editors. Mammal species of the world: a taxonomic and geographic reference. Washington D.C.: Smithsonian Institution Press; p. 312–529.
- Smith NA, Clarke JA. 2015. Systematics and evolution of the Pan-Alcidae (Aves, Charadriiformes). J Avian Biol. 46:125–140. doi: 10.1111/jav.00487
- Stejneger L. 1885. A natural history of birds. In: Kingsley JS, editor. The standard natural history, volume 4. Boston: SE Cassino and Company; p. 1–195.
- Strauch JG. 1978. The phylogeny of the Charadriiformes (Aves): a new estimate using the method of character compatibility analysis. Trans Zool Soc London. 34:263–345. doi: 10.1111/j.1096-3642.1978.tb00375.x
- Teeling EC, Springer MS, Madsen O, Bates P, O’Brien SJ, Murphy WJ. 2005. A molecular phylogeny for bats illuminates biogeography and the fossil record. Science. 307:580–584. doi: 10.1126/science.1105113
- Thomas GH, Wills MA, Székely T. 2004. A supertree approach to shorebird phylogeny. BMC Evol Biol. 4:28.
- van Tets GF, Smith MJ. 1974. Small fossil vertebrates from Victoria Cave, Naracoorte, South Australia. III. Birds (Aves). Trans R Soc S Aust. 98:225–227.
- Wagner PJ. 2000. The quality of the fossil record and the accuracy of phylogenetic inferences about sampling and diversity. System Biol. 49:65–86. doi: 10.1080/10635150050207393
- Wheeler WC. 1992. Extinction, sampling, and molecular phylogenetics. In: Novacek MJ, Wheeler QD, editors. Extinction and phylogeny. New York: Columbia University Press; p. 205–215.
- Wiens JJ. 2005. Can incomplete taxa rescue phylogenetic analyses from long-branch attraction? System Biol. 54:731–742. doi: 10.1080/10635150500234583
- Woodburne MO, MacFadden BJ, Case JA, Springer MS, Pledge NS, Power JD, Woodburne JM, Springer KB. 1994. Land mammal biostratigraphy and magnetostratigraphy of the Etadunna Formation (Late Oligocene) of South Australia. J Vert Paleontol. 13:483–515. doi: 10.1080/02724634.1994.10011527
- Worthy TH. 2009. Descriptions and phylogenetic relationships of two new genera and four new species of Oligo-Miocene waterfowl (Aves: Anatidae) from Australia. Zool J Linn Soc. 156:411–454. doi: 10.1111/j.1096-3642.2008.00483.x
- Worthy TH. 2011. Descriptions and phylogenetic relationships of a new genus and two new species of Oligo-Miocene cormorants (Aves: Phalacrocoracidae) from Australia. Zool J Linn Soc. 163:277–314. doi: 10.1111/j.1096-3642.2011.00693.x
- Worthy TH. 2012. A new species of Oligo-Miocene darter (Aves: Anhingidae) from Australia. Auk. 129:96–104. doi: 10.1525/auk.2012.11204
- Worthy TH, Tennyson AJD, Jones C, McNamara JA, Douglas BJ. 2007. Miocene waterfowl and other birds from Central Otago, New Zealand. J System Palaeontol. 5:1–39. doi: 10.1017/S1477201906001957
- Zachos J, Pagani M, Sloan L, Thomas E, Billups K. 2001. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science. 292:686–693. doi: 10.1126/science.1059412
