ABSTRACT
Pastoral agriculture is a major source for nitrate () contamination in surface and ground waters and for the greenhouse gas
emissions in New Zealand. Advances have been made in recent years in understanding the role of different ammonia oxidisers, including ammonia oxidising bacteria (AOB) and ammonia oxidising archaea (AOA) in nitrification, and in developing nitrification inhibitor (NI) mitigation technologies. Results showed that, in the N-rich soil environment under the animal urine patches in grazed grassland, AOB are the dominant microbes responsible for ammonia oxidation whereas AOA play a less important role. A number of laboratory and field studies have demonstrated conclusively that treating grazed pasture soils with a nitrification inhibitor (NI), such as dicyandiamide (DCD), which inhibits the growth and activity of AOB, is an effective means of reducing
leaching and
emissions.

Professor Hong Di.
Introduction
Nitrate () is a major water contaminant and
leaching from livestock production systems is a worldwide environmental problem. The livestock sector is responsible for 18% of global greenhouse gas emissions (CO2 equivalent) and accounts for 65% of the anthropogenic nitrous oxide (N2O) emissions (IPCC Citation2007). Nitrous oxide is a potent greenhouse gas with a long-term global warming potential 265–298 times that of carbon dioxide (IPCC Citation2007; Myhre et al. Citation2013). In New Zealand, pastoral agriculture is the dominant land use, where animals graze outdoor pastures throughout the year. In such grazed grassland systems, the dominant source for both
leaching and N2O emissions is animal excreta, particularly urine, deposited by the animal during grazing. As animals graze the pastures, between 70%–90% of the nitrogen (N) ingested is returned to pasture and about 80% of the N is in the urine (Haynes & Williams Citation1993; Jarvis et al. Citation1995; Di & Cameron Citation2002a; Di et al. Citation2002; Cameron et al. Citation2013; Selbie et al. Citation2014a, Citation2014b). For example, the N loading rate under a dairy cattle urine patch can be as high as 700–1000 kg N ha−1. This high rate of N deposited in a single urination is well in excess of that which can be utilised by plants. The surplus N is prone to leaching after it is converted to
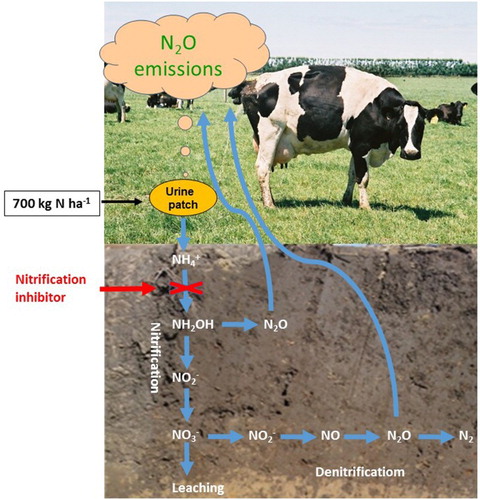
by nitrification (e.g. Scholefield et al. Citation1993; Ledgard et al. Citation1999; Silva et al. Citation1999; Di & Cameron Citation2000, Citation2002b), or lost as NO, N2O or N2 gases, from nitrification or denitrification (Allen et al. Citation1996; Di & Cameron Citation2003, Citation2006; Luo et al. Citation2007) ().
Figure 1. Simplified diagram showing animal urine-N as the main source for N2O emissions and leaching losses in a grazed grassland, and the point of intervention by a nitrification inhibitor (from Di & Cameron Citation2016).
Most of the N in the animal urine is urea which, when deposited in the soil, is quickly hydrolised to produce ammonium ():
(1) Most of the ammonium (
) is adsorbed onto the negatively charged soil cation exchange surfaces. Therefore,
leaching is usually negligible in most soils with a good cation exchange capacity (CEC).
However, in the soil, is rapidly oxidised, first to hydroxylamine, then to NO2− and then to
by the process known as nitrification:
(2)
(3)
(4)
Nitrate is an anion and is not retained by the negatively charged soil particles. Therefore, when drainage occurs it is leached out of the soil into groundwater or surface waters, contributing to water contamination.
Nitrous oxide is also produced from nitrification as a byproduct (Wrage et al. Citation2001). In addition to leaching, the produced from nitrification is subject to denitrification, where
is reduced step-wise to
, NO, N2O and N2, with N2O as an intermediate product:
(5)
If a nitrification inhibitor (NI) is applied to the soil to inhibit ammonia oxidation, the first step of the nitrification process, then this can lead to lower concentrations of and lower N2O emissions from both nitrification and denitrification ().
Significant advances have been made in recent years in understanding the role of different ammonia oxidisers, including ammonia oxidising bacteria (AOB) and ammonia oxidising archaea (AOA), and in developing mitigating technologies based on the use of NIs. This article provides a summary review of progress in these areas, with an emphasis on grazed grassland in New Zealand.
Ammonia oxidisers
The first and rate-limiting step of the nitrification process is the oxidation of NH3 to NH2OH (). It is performed by the key ammonia monooxygenase (AMO) enzyme, which is encoded by the subunits of amo genes. For over a century it was thought that this process was mostly performed by the chemolithoautotrophic AOB (Purkhold et al. Citation2000; Kowalchuk & Stephen Citation2001; Prosser & Nicol Citation2008). However, the recent discovery of the amoA gene in archaea populations has significantly revised our understanding (Venter et al. Citation2004; Francis et al. Citation2005; Könneke et al. Citation2005; Wuchter et al. Citation2006; Prosser & Nicol Citation2008). AOA populations were found to be more abundant than AOB in a range of soils, suggesting a potentially greater role for AOA than AOB in ammonia oxidation (Leininger et al. Citation2006; He et al. Citation2007; Chen et al. Citation2008; Prosser & Nicol Citation2008).
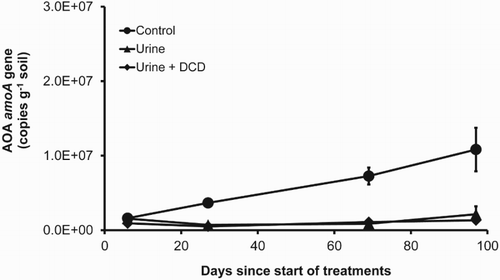
Recent advances in ammonia oxidiser research, using molecular biology techniques to target the functional amoA gene, indicate that the population abundance and activity of AOB and AOA in ammonia oxidation vary depending on soil and environmental conditions. In a study of intensively grazed dairy pasture soils in New Zealand, Di et al. (Citation2009a) showed that it was AOB, which grew in response to the application of dairy cattle urine (applied at 1000 kg N ha−1), that provided (). The AOB population abundance and activity increased significantly following the animal urine application and were significantly inhibited by the application of a NI, dicyandiamide (DCD). In contrast, neither the AOA population abundance nor activity increased following the application of the animal urine (Di et al. Citation2009a). The nitrification rate was shown to be significantly related to the AOB amoA gene copy numbers but not to that of AOA. In a separate study, Di et al. (Citation2010a) showed that the AOA communities in fact grew in the control treatment (no N applied) rather than in the urine treatment (). The AOA abundance was inhibited by the high dose of urine in some of the treatments. Other studies have validated these results (e.g. Offre et al. Citation2009; Shen et al. Citation2011; Dai et al. Citation2013; Robinson et al. Citation2014a, Citation2014b; Hill et al. Citation2015). These results demonstrate that in the high-N soil environment, such as that under the animal urine patch in intensively grazed grassland, it is AOB, particularly Nitrosospira, that are predominantly performing the NH3 oxidation. AOA may play a more important role in NH3 oxidation under low fertility or oligotrophic environments.
Figure 2. AOB amoA gene abundance as affected by the application of dairy cattle urine (1000 kg N ha−1) and the nitrification inhibitor DCD in a dairy pasture soil (from Di et al. Citation2009a).
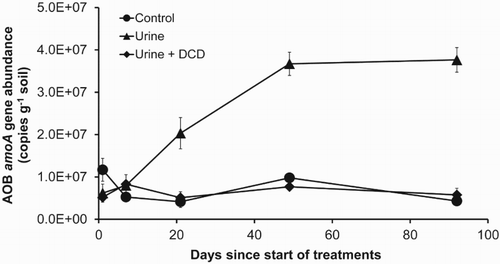
Figure 3. AOA amoA gene abundance as affected by the application of animal urine (1000 kg N ha−1) and the nitrification inhibitor DCD (Di et al. Citation2010b).
Soil pH may also affect the growth and activity of AOB and AOA in the soil (Nicol et al. Citation2008). Under strongly acidic conditions, if AOA growth is not inhibited by high N concentrations, then AOA may become important in NH3 oxidation (Nicol et al. Citation2008; Gubry-Rangin et al. Citation2010; He et al. Citation2012; Shen et al. Citation2012; Zhang et al. Citation2012; Hu et al. Citation2014; Robinson et al. Citation2014a).
Soil moisture content can also affect AOB and AOA growth. When the soil moisture was at 60% field capacity, both AOB and AOA growth was restricted (Di et al. Citation2014). AOB growth was significantly stimulated by the application of animal urine when the soil moisture content was close to, or above, field capacity (Di et al. Citation2014). However, AOA only grew in the control (no N applied) and was inhibited by the application of animal urine-N. Therefore, in the wet and heavily trampled winter grazing conditions where there is substantial input of animal urine, it is also AOB that are dominant.
Our understanding of the microbial ecology of AOB and AOA in different soil ecosystems is still limited and further research is needed to improve our knowledge. This knowledge is important for developing management tools, such as NI technologies, for sustainable N management.
Nitrification inhibitors
Although a number of compounds have been shown to have NI properties, only a few have been commercialised, including dicyandiamide (DCD), 3,4-dimethylpyrazole phosphate (DMPP) and nitrapyrin.
DCD inhibits the first-step of the nitrification process, the oxidation of ammonia. In soil studies, it has been shown that an application rate of 8–10 mg DCD kg−1 soil was effective in inhibiting the growth and activity of Nitrosospira (Di & Cameron Citation2004a; Di et al. Citation2009a, Citation2010a). DCD is bacteriostatic and thus does not kill the bacteria. It temporarily inhibits the growth and activity of ammonia oxidisers. DCD is completely degraded in the soil, first to urea and then to CO2 and NH3, leaving no other long-term residues (Amberger Citation1989). Most importantly, DCD is a very safe compound, with an oral LD50 being greater than 30,000 mg kg−1 body weight of female rats (more than 10 times that of table salt at 3000 mg kg−1 body weight), and no adverse reproductive/developmental effects, genotoxicity and carcinogenicity have been reported (OECD Citation2003). Therefore, the OECD (Citation2003, p. 4) concluded that ‘this chemical is currently of low priority for further work because of its low hazard potential’. Because of the widespread use of DCD in plastics that could be in contact with food, the European Commission has established a tolerable daily intake (TDI) for DCD at 1 mg DCD kg−1 body weight as a food standard guideline (European Commission Citation1995).
The inhibition action of DCD is also shown to be specific to ammonia oxidisers, and its use in the soil does not significantly affect the soil microbial biomass (Di & Cameron Citation2004a), methanotrophs (Di et al. Citation2011) or other agrobacteria (O’Callaghan et al. Citation2010), or other important enzymatic activities that are important for N cycling (Guo et al. Citation2013).
DMPP is a relatively new NI and has been through extensive toxicology and ecotoxicology tests (Zerulla et al. Citation2001). Its oral lethal dose (LD50) in rats ranges from 300 to 2000 mg kg−1 body weight. An application rate of 0.5–1.5 kg ha−1 is sufficient to provide effective inhibition. It has been tested for use in fertiliser formulations or mixing with slurries to mitigate NO3− leaching and N2O emissions (e.g. Barth et al. Citation2001; Pasda et al. Citation2001; Weiske et al. Citation2001; Hatch et al. Citation2005). Its performance in grazed grassland to treat urine patches in a liquid form was found to be comparable with DCD (Di & Cameron Citation2011, Citation2012).
Efficacy of nitrification inhibitors to reduce nitrate leaching
Di & Cameron (Citation2002c) showed that treating soil with DCD can reduce NO3− leaching by an average of 59%. A treatment technology was developed in New Zealand where DCD was applied in a solution form to cover the entire soil surface of a grazed grassland field so that as much of the AOB in the surface soil was treated and inhibited. The DCD was sprayed on to pastures shortly after grazing, before the urine-N was nitrified.
In many parts of New Zealand, leaching mainly occurs over the late autumn, winter and early spring period (e.g. from April to September). A study showed that during winter, when temperatures are low, the DCD effect lasted about 3 months (Di & Cameron Citation2004a). Therefore two applications of DCD, once in the autumn and once in the spring were sufficient to significantly inhibit AOB and decrease
leaching losses (Di & Cameron Citation2004b; Di et al. Citation2010b; Podolyan et al. Citation2014).
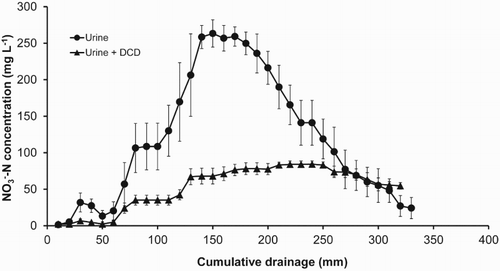
A series of studies were conducted to determine the optimum rate of DCD that was both effective and cost-effective (Di & Cameron Citation2002c, Citation2004a, Citation2004b, Citation2005; Di et al. Citation2009b). These studies showed that DCD applied at 10 kg DCD ha−1 was effective to give a significant reduction in NO3− leaching when two DCD applications were made between autumn and spring (). Molecular microbiology studies have shown that the 10 kg DCD ha−1 application was equivalent to the ED50 where it gave about 50% reduction in AOB growth and in nitrification rate (Guo et al. Citation2014).
Figure 4. Example breakthrough curve showing the effect of DCD applied at 10 kg DCD ha−1 on concentration from animal urine applied at 1000 kg N ha−1 (from Di et al. Citation2009b).
In order to reduce the volume of liquid needed, a fine particle spray formulation was developed where DCD was partly dissolved and partly in the form of fine particles (< 100 µn). This fine particle suspension spray was shown to be highly effective in reducing leaching losses (Di & Cameron Citation2004b, Citation2005).
The NI technology was not only applicable to grazed dairy pastures where the urine-N loading rate was high (about 700–1000 kg N ha−1), but also to beef cattle and sheep grazing systems where the urine-N loading rates were lower, around 500–700 and 300 kg N ha−1, respectively (Di & Cameron Citation2007; Moir et al. Citation2010).
The technology has subsequently been evaluated for its potential to mitigate leaching in a range of soil and environmental conditions in New Zealand and other countries (Di & Cameron Citation2016). The lysimeter studies which targeted the animal urine patch showed that DCD reduced
leaching by an average of about 50% (± 3 SEM). The suction cup and drainage plot studies provided an integrated measurement of the effect of DCD on urine and non-urine patches and showed a
reduction of about 30% (± 6 SEM) (Di & Cameron Citation2016).
Efficacy of nitrification inhibitors to reduce nitrous oxide emissions
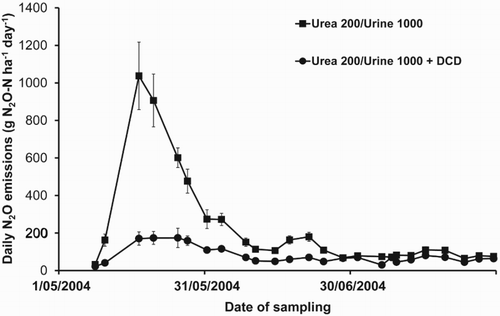
The treatment of grazed pasture soil with DCD has an additional benefit of reducing direct N2O emissions from urine patches (Di & Cameron Citation2002c, Citation2003, Citation2006) (). DCD decreased N2O emissions from both the nitrification and denitrification processes (Di & Cameron Citation2008), and the reductions in N2O emissions were largely the result of the inhibition of AOB by DCD in the high-N status urine patch soils (Di et al. Citation2010b). There was a positive relationship between N2O emissions and AOB amoA gene abundance, but not with that of the AOA amoA gene abundance (Di et al. Citation2010b). The efficacy of the DCD NI technology in reducing N2O emissions in grazed pastures has been assessed extensively under a range of soil and environmental conditions (Di & Cameron Citation2002c, Citation2003, Citation2006, Citation2008; Di et al. Citation2007, Citation2010b; Hoogendoorn et al. Citation2008; Ledgard et al. Citation2008, Citation2014; Smith et al. Citation2008a; Singh et al. Citation2009; Qiu et al. Citation2010; Zaman et al. Citation2010; de Klein et al. Citation2011, Citation2014; Ball et al. Citation2012; Dai et al. Citation2013; Cameron et al. Citation2014; Kim et al. Citation2014; Hu et al. Citation2015). These trials showed that DCD decreased N2O emissions from animal urine-N by an average of 57% (± 2 SEM) (Di & Cameron Citation2016). Based on these results, the NI technology was successfully incorporated into New Zealand’s greenhouse gas emissions inventory (Clough et al. Citation2007).
Figure 5. Daily N2O emissions from a Lismore soil from dairy cattle urine with and without DCD (10 kg DCD ha−1) showing the effect of DCD on N2O emissions (after Di & Cameron Citation2006).
The NI technology has also been shown in Canterbury to be effective in winter forage crop grazing systems where the soils are usually wet and heavily trampled by the grazing animal (Ball et al. Citation2012; Di et al. Citation2014). DCD applied nearly 3 weeks after winter grazing was still effective in reducing N2O emissions (Di et al. Citation2007).
The DCD effect did not diminish after repeated use, as DCD was equally effective in reducing N2O emissions after 4–5 years of consecutive use (de Klein et al. Citation2011).
Effect of nitrification inhibitor on pasture production
The treatment of grazed pasture soil with a nitrification inhibitor such as DCD can also increase N use efficiency and pasture production (e.g. Di & Cameron Citation2002c, Citation2004b, Citation2005, Citation2007; Moir et al. Citation2007, Citation2010; Carey et al. Citation2012). Di & Cameron (Citation2007) reported an average increase in N use efficiency of 32% and an average herbage dry matter yield increase of 25% when DCD was applied to urine patches. Moir et al. (Citation2007) reported a 21% increase in pasture dry matter following DCD application to an intensively grazed dairy pasture soil. Similarly, Carey et al. (Citation2012) reported a 19% increase in dry matter yield from 132 split paddock trials on dairy farms across New Zealand.
However, reported pasture responses to DCD application were more variable than the reported effects on leaching and N2O emissions (e.g. Monaghan et al. Citation2009; O’Connor et al. Citation2012; Cameron et al. Citation2014; de Klein et al. Citation2014; Kim et al. Citation2014; Ledgard et al. Citation2014). This is not surprising in view of the fact that pasture growth can be affected by many factors, including the supply of N and other nutrients, soil moisture status, temperature and other soil physical conditions. In addition, the random and uneven distribution of animal urine patches also complicates the pasture measurements (Cameron et al. Citation2014).
Other benefits of nitrification inhibitor use
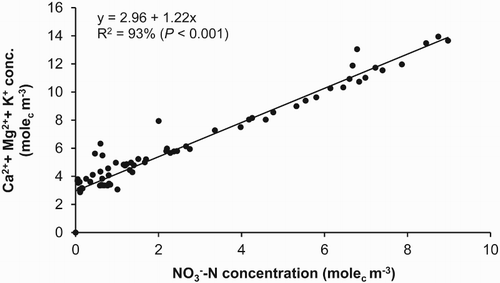
The reduction in leaching as a result of NI application to grazed pastures has also been shown to lead to lower leaching losses of counter cations (Di & Cameron Citation2004b, Citation2004c, Citation2005). As shown in , the Ca2+, Mg2+ and K+ leaching losses were positively correlated to
concentration. Due to decreased
leaching by the use of DCD, Ca2+, Mg2+ and K+ losses were correspondingly decreased by 50%, 52% and 65%, respectively (Di & Cameron Citation2004c). The reduction in leaching of these valuable macronutrients would reduce the need for application of these cation fertilisers where DCD is applied.
Figure 6. Relationship between the concentrations of and cations (Ca2+, Mg2+ and K+) in the leachate from the lysimeters (from Di & Cameron Citation2005).
The NI technology can reduce the risk of animal poisoning by high concentrations in pasture. Nitrate concentrations in pasture in the range of 1500–2000 mg
dry matter are considered risky for the grazing animal (Burgemeister Citation2003). The
concentration can be particularly high in the urine patch areas of pastures due to the high N-loading rate. The application of DCD can decrease
concentrations in pasture to below 800 mg
dry matter (safe for animals) (Moir et al. Citation2012). Therefore, DCD can increase pasture yield and improve pasture quality by decreasing
concentration in the pasture, without compromising the other pasture-quality indicators (Moir et al. Citation2007).
Factors that affect efficacy of nitrification inhibitors
Nitrification inhibitor properties
In addition to DCD, DMPP has also been evaluated for its efficacy. Di & Cameron (Citation2011) found that DCD and DMPP, when applied at the same rate of 10 kg ha−1, both in liquid formulations, were equally effective in inhibiting the growth of AOB. The cost of DMPP was significantly higher than that of DCD, so lower rates of DMPP were also assessed. It was shown that DMPP applied at the rate of 1 kg ha−1 was as effective at reducing leaching as DCD applied at 10 kg ha−1 (Di & Cameron Citation2012). The 1 kg DMPP ha−1 rate is close to the recommended rate for fertiliser formulations (Zerulla et al. Citation2001).
Temperature
Nitrification inhibitors such as DCD and DMPP are biodegradable in the soil and their longevity (as indicated by half-lives) and efficacy are temperature dependent. At 8°C (simulating winter temperatures in many parts of New Zealand) the half-life of DCD was about 110 days (Di & Cameron Citation2004a). However, when the temperature was at 20°C (simulating summer soil temperatures in New Zealand) the half-life was decreased to about 20 days. Gillingham et al. (Citation2012) summarised results from a series of field trials across New Zealand and found that the residence time ranged from 42 to 84 days in the North Island (warmer) and 40 to 160 days in the South Island (cooler). Qiu et al. (Citation2010) reported that the amount of N2O emitted (12.7 kg N2O–N ha−1) during the winter season was 1.6 times that from the summer season (7.8 kg N2O–N ha−1) and DCD decreased the N2O emissions by 69% and 40% for the winter and summer season, respectively. These results demonstrated the higher N2O emissions potential during the wet and cold winter period compared with the warm and dry summer period, and the higher efficacy of DCD during the winter compared with summer.
Soil properties
The reduction of leaching by NIs relies on the soil exchange complex to retain
from the animal urine or N fertiliser. Therefore an adequate cation exchange capacity (CEC) of the soil is important. However, Cameron et al. (Citation2007) reported that the use of DCD to treat animal urine patches was able to reduce
leaching by 23%–32% even in a very free-draining pumice soil with low CEC. Soil organic matter becomes important in increasing the CEC and retaining NH4+ in course-textured soils.
Soil compaction due to animal trampling in grazed pastures did not significantly affect the efficacy of DCD in reducing N2O emissions (Ball et al. Citation2012) or leaching (Hill et al. Citation2015). DCD was also found to be highly effective in decreasing N2O emissions in extremely wet soils, such as at 130% field capacity (Di et al. Citation2014).
Rainfall
DCD is water soluble and therefore may move through the soil under very high rainfall conditions (Shepherd et al. Citation2012). However, a study by Di et al. (Citation2009b) found that DCD reduced NO3− leaching by 44%–71% under two contrasting rainfall conditions (1260 mm vs 2145 mm) and the efficacy of DCD was not significantly affected by the different rainfall inputs. The importance of rainfall in affecting the efficacy of DCD may also depend on the soil drainage status, and the timing of rainfall in relation to DCD application.
Timing of application
To maximise effectiveness, it was found that the NI was most effective if applied shortly after grazing (i.e. when the pastures are short and the urine patches are fresh; and thus before significant nitrification has taken place). It is important to apply the NI when the pasture is short so that the NI can reach the soil. If, for practical reasons, the NI needs to be applied to tall pasture, it is important to ensure that there is sufficient rain or irrigation water (about 10 mm) to wash the NI off the foliage into the soil.
Concluding remarks
Research has clearly shown that AOB and AOA prefer different soil and nitrogen environments to grow and function. In the high-N soil environment of grazed grassland, it is the AOB that are the dominant players in performing ammonia oxidation, whereas AOA may play a role in low-N or strongly acidic soils. Research spanning more than a decade has conclusively demonstrated that treating grazed pasture soils with a NI is an effective technology for NO3− leaching and N2O emissions in grazed pastures. The large body of scientific evidence from a number of laboratory and field studies have proven the consistency and reliability of the technology across a wide range of soil and environmental conditions. The NI technology is one of the few environmental technologies that also provide an economic benefit by increasing the N use efficiency and pasture dry matter yield. The most widely evaluated NI, DCD, is one of the safest compounds, and has been shown to pose little or no health risks to either grazing animals or humans (OECD Citation2003). However, DCD is currently not in commercial use in New Zealand, awaiting the establishment of a standard for food in the Codex Alimentarius by the UN Food and Agriculture Organization (FAO). In view of the huge environmental benefits of this technology, and the low toxicity of DCD, it would seem a logical step to list DCD in the Codex Alimentarius so that these benefits can be captured.
Future research should be directed to:
The search for new NIs so that a range of such identified compounds may be designed to suit specific soils, environments or production systems.
Identification of AMO structure to aid the search for new NIs.
Understanding the variations of pasture response to NI applications.
Synthesis of research data into process- or management-based models.
Acknowledgements
We would like to thank Dr Barbara Brown for technical support with this review.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Allen AG, Jarvis SC, Headon DM. 1996. Nitrous oxide emissions from soils due to inputs of nitrogen from excreta return by livestock on grazed grassland in the U.K. Soil Biology and Biochemistry. 28:597–607. doi: 10.1016/0038-0717(95)00186-7
- Amberger A. 1989. Research on dicyandiamide as a nitrification inhibitor and future outlook. Communications in Soil Science and Plant Analysis. 20:1933–1955. doi: 10.1080/00103628909368195
- Ball BC, Cameron KC, Di HJ, Moore S. 2012. Effects of trampling of a wet dairy pasture soil on soil porosity and on mitigation of nitrous oxide emissions by a nitrification inhibitor, dicyandiamide. Soil Use and Management. 28:194–201. doi: 10.1111/j.1475-2743.2012.00389.x
- Barth G, von Tucher S, Schmidhalter U. 2001. Influence of soil parameters on the effect of 3,4-dimethylpyrazole-phosphate as a nitrification inhibitor. Biology and Fertility of Soils. 34:98–102. doi: 10.1007/s003740100382
- Burgemeister M. 2003. Nitrate and nitrite—an update. Dairy cattle. Proceedings of the Australian and New Zealand Combined Dairy Veterinarians Conference, Taupo, New Zealand; p. 505–516.
- Cameron KC, Di HJ, Moir J. 2013. Nitrogen losses from the soil/plant system: a review. Annals of Applied Biology. 162:145–173. doi: 10.1111/aab.12014
- Cameron KC, Di HJ, Moir JL. 2014. Dicyandiamide (DCD) effect on nitrous oxide emissions, nitrate leaching and pasture yield in Canterbury, New Zealand. New Zealand Journal of Agricultural Research. 57:251–270. doi: 10.1080/00288233.2013.797914
- Cameron KC, Di HJ, Moir JL, Roberts AHC. 2007. Reducing nitrate leaching losses from a Taupo Pumice soil using a nitrification inhibitor eco-n. Proceedings of the New Zealand Grassland Association. 69:131–135.
- Carey PL, Jiang S, Roberts AHC. 2012. Pasture dry matter responses to the use of a nitrification inhibitor: a national series of New Zealand farm trials. New Zealand Journal of Agricultural Research. 55:63–72. doi: 10.1080/00288233.2011.644628
- Chen XP, Zhu YG, Xia Y, Shen JP, He JZ. 2008. Ammonia-oxidizing archaea: important players in paddy rhizosphere soil? Environmental Microbiology. 10:1978–1987. doi: 10.1111/j.1462-2920.2008.01613.x
- Clough TJ, Di HJ, Cameron KC, Sherlock RR, Metherell AK, Clarke H, Rys G. 2007. Accounting for the utilization of a N2O mitigation tool in the IPCC inventory methodology for agricultural soils. Nutrient Cycling in Agroecosystems. 78:1–14. doi: 10.1007/s10705-006-9069-z
- Dai Y, Di HJ, Cameron KC, He J-Z. 2013. Effects of nitrogen application rate and a nitrification inhibitor dicyandiamide on ammonia oxidizers and N2O emissions in a grazed pasture soil. Science of The Total Environment. 465:125–135. doi: 10.1016/j.scitotenv.2012.08.091
- de Klein CAM, Cameron KC, Di HJ, Rys G, Monaghan RM, Sherlock RR. 2011. Repeated annual use of the nitrification inhibitor dicyandiamide (DCD) does not alter its effectiveness in reducing N2O emissions from cow urine. Animal Feed Science and Technology. 166-167:480–491. doi: 10.1016/j.anifeedsci.2011.04.076
- de Klein CAM, Letica SA, Macfie PI. 2014. Evaluating the effects of dicyandiamide (DCD) on nitrogen cycling and dry matter production in a 3-year trial on a dairy pasture in South Otago, New Zealand. New Zealand Journal of Agricultural Research. 57:316–331. doi: 10.1080/00288233.2014.941508
- Di HJ, Cameron KC. 2000. Calculating nitrogen leaching losses and critical nitrogen application rates in dairy pasture systems using a semi-empirical model. New Zealand Journal of Agricultural Research. 43:139–147. doi: 10.1080/00288233.2000.9513415
- Di HJ, Cameron KC. 2002a. Nitrate leaching in temperate agroecosystems: sources, factors and mitigating strategies. Nutr Cyc Agroecosyst. 46:237–256. doi: 10.1023/A:1021471531188
- Di HJ, Cameron KC. 2002b. Nitrate leaching and pasture production from different nitrogen sources on a shallow stony soil under flood irrigated dairy pasture. Australian Journal of Soil Research. 40:317–334. doi: 10.1071/SR01015
- Di HJ, Cameron KC. 2002c. The use of a nitrification inhibitor, dicyandiamide (DCD), to decrease nitrate leaching and nitrous oxide emissions in a simulated grazed and irrigated grassland. Soil Use and Management. 18:395–403. doi: 10.1111/j.1475-2743.2002.tb00258.x
- Di HJ, Cameron KC. 2003. Mitigation of nitrous oxide emissions in spray-irrigated grazed grassland by treating the soil with dicyandiamide, a nitrification inhibitor. Soil Use and Management. 19:284–290. doi: 10.1079/SUM2003207
- Di HJ, Cameron KC. 2004a. Effects of temperature and application rate of a nitrification inhibitor, dicyandiamide (DCD) on nitrification rate and microbial biomass in a grazed pasture soil. Australian Journal of Soil Research. 42:927–932. doi: 10.1071/SR04050
- Di HJ, Cameron KC. 2004b. Treating grazed pasture soil with a nitrification inhibitor, eco-n™, to decrease nitrate leaching in a deep sandy soil under spray irrigation—a lysimeter study. New Zealand Journal of Agricultural Research. 47:351–361. doi: 10.1080/00288233.2004.9513604
- Di HJ, Cameron KC. 2004c. Effects of the nitrification inhibitor dicyandiamide on potassium, magnesium and calcium leaching in grazed grassland. Soil Use and Management. 20:2–7. doi: 10.1079/SUM2003205
- Di HJ, Cameron KC. 2005. Reducing environmental impacts of agriculture by using a fine particle suspension nitrification inhibitor to decrease nitrate leaching from grazed pastures. Agriculture, Ecosystems & Environment. 109:202–212. doi: 10.1016/j.agee.2005.03.006
- Di HJ, Cameron KC. 2006. Nitrous oxide emissions from two dairy pasture soils as affected by different rates of a fine particle suspension nitrification inhibitor, dicyandiamide. Biology and Fertility of Soils. 42:472–480. doi: 10.1007/s00374-005-0038-5
- Di HJ, Cameron KC. 2007. Nitrate leaching losses and pasture yields as affected by different rates of animal urine nitrogen returns and application of a nitrification inhibitor—a lysimeter study. Nutrient Cycling in Agroecosystems. 79:281–290. doi: 10.1007/s10705-007-9115-5
- Di HJ, Cameron KC. 2008. Sources of nitrous oxide from 15N-labelled animal urine and urea fertiliser with and without a nitrification inhibitor, dicyandiamide (DCD). Australian Journal of Soil Research. 46:76–82. doi: 10.1071/SR07093
- Di HJ, Cameron KC. 2011. Inhibition of ammonium oxidation by a liquid formulation of 3,4-dimethylpyrazole phosphate (DMPP) compared with a dicyandiamide (DCD) solution in six New Zealand grazed grassland soils. Journal of Soils and Sediments. 11:1032–1039. doi: 10.1007/s11368-011-0372-1
- Di HJ, Cameron KC. 2012. How does the application of different nitrification inhibitors affect nitrous oxide emissions and nitrate leaching from cow urine in grazed pastures? Soil Use and Management. 28:54–61. doi: 10.1111/j.1475-2743.2011.00373.x
- Di HJ, Cameron KC. 2016. Inhibition of nitrification to mitigate nitrate leaching and nitrous oxide emissions in grazed grassland: a review. Journal of Soils and Sediments. 16:1401–1420. doi:10.1007/s11368-016-1403-8.
- Di HJ, Cameron KC, Podolyan A, Robinson A. 2014. Effect of soil moisture status and a nitrification inhibitor, dicyandiamide, on ammonia oxidizer and denitrifier growth and nitrous oxide emissions in a grassland soil. Soil Biology and Biochemistry. 73:59–68. doi: 10.1016/j.soilbio.2014.02.011
- Di HJ, Cameron KC, Shen JP, He J-Z, Winefield CS. 2009b. A lysimeter study of nitrate leaching from grazed grassland as affected by a nitrification inhibitor, dicyandiamide, and relationships with ammonia oxidizing bacteria and archaea. Soil Use and Management. 25:454–461. doi: 10.1111/j.1475-2743.2009.00241.x
- Di HJ, Cameron KC, Shen JP, Winefield CS, O’Callaghan M, Bowatte S, He J-Z. 2009a. Nitrification driven by bacteria and not archaea in nitrogen-rich grassland soils. Nature Geoscience. 2:621–624. doi: 10.1038/ngeo613
- Di HJ, Cameron KC, Shen JP, Winefield CS, O’Callaghan M, Bowatte S, He J-Z. 2010a. Ammonia-oxidizing bacteria and archaea grow under contrasting soil nitrogen conditions. FEMS Microbiology Ecology. 72:386–394. doi: 10.1111/j.1574-6941.2010.00861.x
- Di HJ, Cameron KC, Shen JP, Winefield CS, O’Callaghan M, Bowatte S, He J-Z. 2011. Methanotroph abundance not affected by applications of animal urine and a nitrification inhibitor, dicyandiamide, in six grazed grassland soils. Journal of Soils and Sediments. 11:432–439. doi: 10.1007/s11368-010-0318-z
- Di HJ, Cameron KC, Sherlock RR. 2007. Comparison of the effectiveness of a nitrification inhibitor, dicyandiamide, in reducing nitrous oxide emissions in four different soils under different climatic and management conditions. Soil Use Manag. 23:1–9. doi: 10.1111/j.1475-2743.2006.00057.x
- Di HJ, Cameron KC, Sherlock RR, Shen JP, He J-Z, Winefield CS. 2010b. Nitrous oxide emissions from grazed grassland as affected by a nitrification inhibitor, dicyandiamide, and relationships with ammonia-oxidizing bacteria and archaea. Journal of Soils and Sediments. 10:943–954. doi: 10.1007/s11368-009-0174-x
- Di HJ, Cameron KC, Silva RG, Russell JM, Barnett JW. 2002. A lysimeter study of the fate of 15N-labelled nitrogen in cow urine with or without farm dairy effluent in a grazed dairy pasture soil under flood irrigation. New Zealand Journal of Agricultural Research. 45:235–244. doi: 10.1080/00288233.2002.9513514
- European Commission. 1995. Reports of the scientific committee for food, 33rd series. Brussels: European Commission. 86 p.
- Francis CA, Roberts KJ, Beman JM, Santoro AE, Oakley BB. 2005. Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proceedings of the National Academy of Sciences. 102:14683–14688. doi: 10.1073/pnas.0506625102
- Gillingham AG, Ledgard SF, Saggar S, Cameron KC, Di HJ, de Klein CAM, Aspin MD. 2012. Initial evaluation of the effects of dicyandiamide (DCD) on nitrous oxide emissions, nitrate leaching and dry matter production from dairy pastures in a range of locations within New Zealand. In: Currie LD, Christensen CL, editors. Advanced Nutrient Management: Gains from the Past-Goals for the Future. Occasional Report No. 25, FLRC, Massey University, Palmerston North, New Zealand; p. 16.
- Gubry-Rangin C, Nicol GW, Prosser JI. 2010. Archaea rather than bacteria control nitrification in two agricultural acidic soils. FEMS Microbiology Ecology. 74:566–574. doi: 10.1111/j.1574-6941.2010.00971.x
- Guo YJ, Di HJ, Cameron KC, Li B. 2014. Effect of application rate of a nitrification inhibitor, dicyandiamide (DCD), on nitrification rate, and ammonia-oxidizing bacteria and archaea growth in a grazed pasture soil: an incubation study. Journal of Soils and Sediments. 14:897–903. doi: 10.1007/s11368-013-0843-7
- Guo YJ, Di HJ, Cameron KC, Li B, Podolyan A, Moir JL, Monaghan RM, Smith LC, O’Callaghan M, Bowatte S, et al. 2013. Effect of 7-year application of a nitrification inhibitor, dicyandiamide (DCD), on soil microbial biomass, protease and deaminase activities, and the abundance of bacteria and archaea in pasture soils. Journal of Soils and Sediments. 13:753–759. doi: 10.1007/s11368-012-0646-2
- Hatch D, Trindal H, Cardenas L, Carneiro J, Hawkins J, Scholefield D, Chadwick D. 2005. Laboratory study of the effects of two nitrification inhibitors on greenhouse gas emissions from a slurry-treated arable soil: impact of diurnal temperature cycle. Biology and Fertility of Soils. 41:225–232. doi: 10.1007/s00374-005-0836-9
- Haynes RJ, Williams PH. 1993. Advances in Agronomy. Adv Agron. 49:119–199. doi: 10.1016/S0065-2113(08)60794-4
- He JZ, Hu HW, Zhang LM. 2012. Current insights into the autotrophic thaumarchaeal ammonia oxidation in acidic soils. Soil Biology and Biochemistry. 55:146–154. doi: 10.1016/j.soilbio.2012.06.006
- He JZ, Shen JP, Zhang LM, Zhu YG, Zheng YM, Xu MG, Di HJ. 2007. Quantitative analyses of the abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea of a Chinese upland red soil under long-term fertilization practices. Environmental Microbiology. 9:2364–2374. doi: 10.1111/j.1462-2920.2007.01358.x
- Hill AJ, Di HJ, Cameron KC, Pololyan A. 2015. The effect of animal trampling and DCD on ammonia oxidisers, nitrification, and nitrate leaching under simulated winter forage grazing conditions. Journal of Soils and Sediments. 15:972–981. doi: 10.1007/s11368-014-1001-6
- Hoogendoorn CJ, de Klein CAM, Rutherford AJ, Letica S, Devantier BP. 2008. The effect of increasing rates of nitrogen fertiliser and a nitrification inhibitor on nitrous oxide emissions from urine patches on sheep grazed hill country pasture. Australian Journal of Experimental Agriculture. 48:147–151. doi: 10.1071/EA07238
- Hu HW, Chen DL, He JZ. 2015. Microbial regulation of terrestrial nitrous oxide formation: understanding the biological pathways for prediction of emission rates. FEMS Microbiology Reviews. 39:729–49. doi: 10.1093/femsre/fuv021
- Hu HW, Xu ZH, He JZ. 2014. Ammonia-oxidizing archaea play a predominant role in acid soil nitrification. Advances in Agronomy. 125:261–302.
- IPCC. 2007. Climate change 2007: the physical science basis, contribution of working group I to the fourth assessment report of the Intergovernmental Panel on Climate Change: frequently asked questions. In: S Solomon, M Qin, Z Manning, M Chen, K Marquis, T Avery, M Tignor, H Miller, editor. Intergovernmental Panel on Climate Change (IPCC). Cambridge, UK: Cambridge University Press; p. 996.
- Jarvis SC, Scholefield D, Pain B. 1995. Nitrogen cycling in grazing systems. In: Bacon PE, editor. Nitrogen fertilization in the environment. New York: Marcel Dekker; p. 381–419.
- Kim D-G, Giltrap DL, Saggar S, Hanly JA. 2014. Field studies assessing the effect of dicyandiamide (DCD) on N transformations, pasture yields, N2O emissions and N-leaching in the Manawatu region. New Zealand Journal of Agricultural Research. 57:271–293. doi: 10.1080/00288233.2013.855244
- Könneke M, Bernhard AE, de la Torre JR, Walker CB, Waterbury JB, Stahl DA. 2005. Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature. 437:543–546. doi: 10.1038/nature03911
- Kowalchuk GA, Stephen JR. 2001. Ammonia-oxidizing bacteria: a model for molecular microbial ecology. Annual Review of Microbiology. 55:485–529. doi: 10.1146/annurev.micro.55.1.485
- Ledgard SF, Luo J, Sprosen MS, Wyatt JB, Balvert BF, Lindsey SB. 2014. Effects of the nitrification inhibitor dicyandiamide (DCD) on pasture production, nitrous oxide emissions and nitrate leaching in Waikato, New Zealand. New Zealand Journal of Agricultural Research. 57:294–315. doi: 10.1080/00288233.2014.928642
- Ledgard SF, Menneer JC, Dexter MM, Kear MJ, Lindsey S, Peters JS, Pacheco D. 2008. A novel concept to reduce nitrogen losses from grazed pastures by administering soil nitrogen process inhibitors to ruminant animals: a study with sheep. Agriculture, Ecosystems & Environment. 125:148–158. doi: 10.1016/j.agee.2007.12.006
- Ledgard SF, Penno JW, Sprosen MS. 1999. Nitrogen inputs and losses from clover/grass pastures grazed by dairy cows, as affected by nitrogen fertilizer application. The Journal of Agricultural Science. 132:215–225. doi: 10.1017/S002185969800625X
- Leininger S, Urich T, Schloter M, Schwark L, Qi J, Nicol GW, Prosser JI, Schuster SC, Schleper C. 2006. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature. 442:806–809. doi: 10.1038/nature04983
- Luo J, Ledgard SF, Lindsey SB. 2007. Nitrous oxide emissions from application of urea on New Zealand pasture. New Zealand Journal of Agricultural Research. 50:1–11. doi: 10.1080/00288230709510277
- Moir J, Malcolm B, Cameron KC, Di HJ. 2012. The effect of dicyandiamide on pasture nitrate concentration, yield and N offtake under high N loading in winter and spring. Grass and Forage Science. 67:391–402. doi: 10.1111/j.1365-2494.2012.00857.x
- Moir J, Wild MA, Cameron KC, Di HJ. 2010. The effect of DCD on nitrogen losses from sheep urine patches applied to lysimeters in autumn. Proceedings of the New Zealand Grassland Association. 72:197–202.
- Moir JL, Cameron KC, Di HJ. 2007. Effects of the nitrification inhibitor dicyandiamide on soil mineral N, pasture yield, nutrient uptake and pasture quality in a grazed pasture system. Soil Use and Management. 23:111–120. doi: 10.1111/j.1475-2743.2006.00078.x
- Monaghan RM, Smith LC, Ledgard SF. 2009. The effectiveness of a granular formulation of dicyandiamide (DCD) in limiting nitrate leaching from a grazed dairy pasture. New Zealand Journal of Agricultural Research. 52:145–159. doi: 10.1080/00288230909510499
- Myhre G, Shindell D, Bréon F-M, Collins W, Fuglestvedt J, Huang J, Koch D, Lamarque J-F, Lee D, Mendoza B, et al. 2013. Anthropogenic and natural radiative forcing. In: TF Stocker, D Qin, G-K Plattner, M Tignor, SK Allen, J Boschung, A Nauels, Y Xia, V Bex, PM Midgley, editor. Climate change 2013: the physical science basis. Contribution of working group I to the fifth assessment report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press; p. 659–740.
- Nicol GW, Leininger S, Schleper C, Prosser JI. 2008. The influence of soil pH on the diversity, abundance and transcriptional activity of ammonia oxidizing archaea and bacteria. Environmental Microbiology. 10:2966–2978. doi:10.1111/j.1462-2920.2008.01701.x.
- O’Callaghan M, Gerard EM, Carter PE, Lardner R. 2010. Effect of the nitrification inhibitor dicyandiamide (DCD) on microbial communities in a pasture soil amended with bovine urine. Soil Biology and Biochemistry. 42:1425–1436. doi: 10.1016/j.soilbio.2010.05.003
- O’Connor PJ, Hennessy D, Brophy C, O’Donovan M, Lynch MB. 2012. The effect of the nitrification inhibitor dicyandiamide (DCD) on herbage production when applied at different times and rates in the autumn and winter. Agriculture, Ecosystems & Environment. 152:79–89. doi: 10.1016/j.agee.2012.02.014
- OECD. 2003. Cyanoguanidine. SIDS initial assessment report. Arona, Italy: UNEP Publications. p. 75.
- Offre P, Prosser JI, Nicol GW. 2009. Growth of ammonia-oxidizing archaea in soil microcosms is inhibited by acetylene. FEMS Microbiology Ecology. 70:99–108. doi: 10.1111/j.1574-6941.2009.00725.x
- Pasda G, Hähndel R, Zerulla W. 2001. Effect of fertilizers with the new nitrification inhibitor DMPP (3,4-dimethylpyrazole phosphate) on yield and quality of agricultural and horticultural crops. Biology and Fertility of Soils. 34:85–97. doi: 10.1007/s003740100381
- Podolyan A, Di HJ, Cameron KC, Clough T, de Klein C, Saggar S. 2014. Ammonia oxidising populations and relationships with N2O emissions in three New Zealand soils. New Zealand Journal of Agricultural Research. 57:228–243. doi: 10.1080/00288233.2014.924969
- Prosser JI, Nicol GW. 2008. Relative contributions of archaea and bacteria to aerobic ammonia oxidation in the environment. Environmental Microbiology. 10:2931–2941. doi: 10.1111/j.1462-2920.2008.01775.x
- Purkhold U, Pommerening-Röser A, Juretschko S, Schmid MC, Koops HJ, Wagner M. 2000. Phylogeny of all recognized species of ammonia oxidizers based on comparative 16S rRNA and amoA sequence analysis: Implications for molecular diversity surveys. Applied and Environmental Microbiology. 66:5368–5382. doi: 10.1128/AEM.66.12.5368-5382.2000
- Qiu W, Di HJ, Cameron KC, Chengxiao H. 2010. Nitrous oxide emissions from animal urine as affected by season and a nitrification inhibitor dicyandiamide. Journal of Soils and Sediments. 10:1229–1235. doi: 10.1007/s11368-010-0242-2
- Robinson A, Di HJ, Cameron KC, Podolyan A. 2014b. Effect of soil aggregate size and DCD on N2O emissions and ammonia oxidiser abundance in a grazed pasture soil. Soil Use and Management. 30:231–240.
- Robinson A, Di HJ, Cameron KC, Podolyan A, He JZ. 2014a. The effect of soil pH and dicyandiamide (DCD) on N2O emissions and ammonia oxidiser abundance in a stimulated grazed pasture soil. Journal of Soils and Sediments. 14:1434–1444. doi:10.1007/s11368-014-0888-2.
- Scholefield D, Tyson KC, Garwood EA, Armstrong AC, Hawkins J, Stone AC. 1993. Nitrate leaching from grazed grassland lysimeters: effects of fertilizer input, field drainage, age of sward and patterns of weather. Journal of Soil Science. 44:601–613. doi: 10.1111/j.1365-2389.1993.tb02325.x
- Selbie DR, Buckthought LE, Shepherd MA. 2014a. The challenge of the urine patch for managing nitrogen in grazed pasture systems. Advances in Agronomy. 129:229–292.
- Selbie DR, Cameron KC, Di HJ, Moir JL, Lanigan GJ, Richards KG. 2014b. The effect of urinary nitrogen loading rate and a nitrification inhibitor on nitrous oxide emissions from a temperate grassland soil. The Journal of Agricultural Science. 152:159–171. doi: 10.1017/S0021859614000136
- Shen JP, Zhang LM, Di HJ, He J-Z. 2012. A review of ammonia-oxidizing bacteria and archaea in Chinese agricultural soils. Frontiers in Microbiology. 3:1–7. doi:0.3389/fmicb.2012.00296.
- Shen XY, Zhang LM, Shen JP, Li LH, Yuan CL, He JZ. 2011. Nitrogen loading levels affect abundance and composition of soil ammonia oxidizing prokaryotes in semiarid temperate grassland. Journal of Soils and Sediments. 11:1243–1252. doi: 10.1007/s11368-011-0375-y
- Shepherd M, Wyatt J, Welten B. 2012. Effect of soil type and rainfall on dicyandiamide concentrations in drainage from lysimeters. Soil Research. 50:67–75. doi: 10.1071/SR11330
- Silva RG, Cameron KC, Di HJ, Hendry T. 1999. A lysimeter study of the impact of cow urine, dairy shed euent, and nitrogen fertiliser on nitrate leaching. Australian Journal of Soil Research. 37:357–369. doi: 10.1071/S98010
- Singh J, Saggar S, Bolan NS. 2009. Influence of dicyandiamide on nitrogen transformation and losses in cow-urine-amended soil cores from grazed pasture. Animal Production Science. 49:253–261. doi: 10.1071/EA08200
- Smith LC, de Klein CAM, Catto WD. 2008a. Effect of dicyandiamide applied in a granular form on nitrous oxide emissions from a grazed dairy pasture in Southland, New Zealand. New Zealand Journal of Agricultural Research. 51:387–396. doi: 10.1080/00288230809510469
- Venter JC, Remington K, Heidelberg JF, Halpern AL, Rusch D. 2004. Environmental genome shotgun sequencing of the Sargasso Sea. Science. 304:66–74. doi: 10.1126/science.1093857
- Weiske A, Benckiser G, Herbert T, Ottow JCG. 2001. Influence of the nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) in comparison to dicyandiamide (DCD) on nitrous oxide emissions, carbon dioxide fluxes and methane oxidation during 3 years of repeated application in field experiments. Biology and Fertility of Soils. 34:109–117. doi: 10.1007/s003740100386
- Wrage N, Velthof GL, van Beusichem ML, Oenema O. 2001. Role of nitrifier denitrification in the production of nitrous oxide. Soil Biology and Biochemistry. 33:1723–1732. doi: 10.1016/S0038-0717(01)00096-7
- Wuchter C, Abbas B, Coolen MJL, Herfort L, van Bleijswijk J, Timmers P, Strous M, Teira E, Herndl GJ, Middleburge JJ, et al. 2006. Archaeal nitrification in the ocean. Proceedings of the National Academy of Sciences. 103:12317–12322. doi: 10.1073/pnas.0600756103
- Zaman M, Blennerhassett JD. 2010. Effects of the different rates of urease and nitrification inhibitors on gaseous emissions of ammonia and nitrous oxide, nitrate leaching and pasture production from urine patches in an intensive grazed pasture system. Agriculture, Ecosystems & Environment. 136:236–246. doi: 10.1016/j.agee.2009.07.010
- Zerulla W, Barth T, Dressel KE, von Locquenghien KH, Wissemeier AH. 2001. 3,4-Dimethylpyrazole phosphate (DMPP) - a new nitrification inhibitor for agriculture and horticulture. Biology and Fertility of Soils. 34:79–84. doi: 10.1007/s003740100380
- Zhang LM, Hu HW, Shen JP, He JZ. 2012. Ammonia-oxidizing archaea have more important role than ammonia-oxidizing bacteria in ammonia oxidation of strongly acidic soils. The ISME Journal. 6:1032–1045. doi: 10.1038/ismej.2011.168
