ABSTRACT
The goal to make New Zealand predator-free by 2050 has drawn strong praise and criticism, but these critiques have focused largely on economic or technological feasibility of long-term large-scale eradication. We suggest that achieving this goal is not a simple ‘scaling-up’ of current eradication efforts, but requires enduring co-ordination and integration of research, management and societal elements if a predator-free goal is to become a reality. Here we ask what are the key impediments to eradicating invasive species on a national scale? We highlight four interlinked issues that must be addressed to accomplish a predator-free New Zealand: (1) improved ecological understanding of interactive effects; (2) refinement and development of operational methods; (3) overcoming social and bioethical challenges; and (4) improving governance and partnerships with Māori. Understanding the linkages among these issues can also provide new insights into the biology and ecology of invasions, development of improved eradication methods, and social support or involvement in large-scale conservation management. Addressing these challenges will ultimately improve policy and management of biological invaders and set new international precedents.
Introduction
Biological invasions by non-native species are a major driver of global change, causing major shifts in both community structure and ecosystem processes (Atkinson and Cameron Citation1993; Vitousek et al. Citation1997; King Citation2005; Tylianakis et al. Citation2008; Vilà and Hulme Citation2017); however, few places are as profoundly invaded as New Zealand (NZ). For example, about half of the flora is composed of naturalised non-native plant species, and numerous other species of non-native animals including mammals, birds and invertebrates established in the wild (King Citation2005; Allen and Lee Citation2006). Overall, the proportion of naturalised non-native species in New Zealand exceeds nearly all other countries or regions except Hawai’i (see Table 1 in Vitousek et al. Citation1996). In addition, because non-native species in NZ often appear to be functionally distinct from the endemic flora and fauna, their effects likely interact with other drivers of global change such as deforestation driven by historical fires (Kelly and Sullivan Citation2010; Perry et al. Citation2014b). Several approaches to manage this tide of invaders have been vigorously pursued including border risk assessment systems to prevent new invasions, and national co-ordination of landscape-level management of diseases (e.g. Bovine tuberculosis) and mammals (Allen and Lee Citation2006; Hutchings et al. Citation2013; King Citation2017; Edney-Browne et al. Citation2018). Management of invasive non-native species has provided new and often generalisable insights into the biology and ecology of invasions, as well as the ability of different communities to mobilise in response to invasions to contain or eradicate unwanted species (Simberloff et al. Citation2013).
Invasive non-native mammalian predators are widespread in NZ, and have well-documented impacts on native species (e.g. Jones et al. Citation2016; Bombaci et al. Citation2018). As a consequence, there have been major conservation efforts to reduce or eradicate these predators from islands, fenced areas or sanctuaries, and in open systems such as mainland islands (Parkes and Murphy Citation2003; Eason et al. Citation2011; Goldson et al. Citation2015; Russell and Broome Citation2016; Parkes et al. Citation2017a). Status quo management of invasive predators relies on a combination of methods including aerial application of toxins, trapping and predator exclusion fences; but there is general agreement that greater co-ordination and scaling up of these efforts is needed to secure the biodiversity benefits of these management activities (Russell et al. Citation2015). Accelerating the widespread eradication of invasive non-native mammalian predators thus underpins the ambitious goal to make NZ predator free by 2050 (NZ Government Citation2016; Owens Citation2017), and this has drawn national and international praise and criticism regarding the economic or technological feasibility of large-scale eradication (e.g. Glen et al. Citation2013; Holmes et al. Citation2015; Russell et al. Citation2015; Owens Citation2017; Linklater and Steer Citation2018). The key goal of Predator-Free New Zealand 2050 (hereafter ‘PFNZ 2050’) is to protect and restore NZ’s native biological diversity, but also the relationship and values that Māori and non-Māori have with that flora and fauna.
If PFNZ 2050 becomes a reality, the technological and social advances required will have major implications for improving large-scale conservation and pest management worldwide and over the long-term (Kopf et al. Citation2017). However, the size of the challenge should not be underestimated. The spatial scale of eradication being attempted is about two orders of magnitude larger than any current success, and this will involve new challenges for predator eradication such as a greater range of refugia for predators, and immigration or reinvasion from multiple sources (Doherty and Ritchie Citation2017). PFNZ 2050 will not be achieved by a simple ‘scaling-up’ of current eradication efforts and application of new technologies, but will require enduring integration of research, management and societal elements to succeed. The focus of PFNZ 2050 on a subset of predators is premised on their documented effects as a major driver of extinctions or declines in abundance of indigenous species (Towns et al. Citation2006; Bellingham et al. Citation2010; Smith et al. Citation2010; Wood et al. Citation2017), and assumes that removing this driver will result in long-lasting conservation benefits (e.g. for diverse invertebrate communities Barker Citation2016). This approach is supported by recent reviews of predator eradications on islands that demonstrate, with few exceptions, widespread benefits of native species to predator removal (e.g. Jones et al. Citation2016; Bombaci et al. Citation2018).
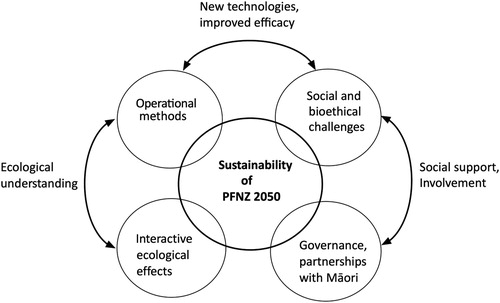
Here we ask what are the key impediments to eradicating invasive predator species at a national scale? Although there is ongoing debate about whether the aspirational goals of PFNZ 2050 are realistic (e.g. Linklater and Steer Citation2018), our aim is to highlight four interlinked issues that must be addressed for PFNZ 2050 to achieve its goal: (1) improved ecological understanding of interactive effects; (2) refinement and development of operational methods; (3) overcoming social and bioethical challenges; and (4) improving governance and partnerships with Māori (). We also illustrate linkages among these issues that can provide new insights into ecological understanding, development of improved eradication methods, and social support or involvement in large-scale conservation management that, in combination, are needed for achieving the goal of making NZ predator-free by 2050. Addressing these challenges is a widespread and urgent issue given the need to apply ecological understanding to develop improved policy and management of biological invaders and the species or ecosystems affected by invasive species and their management.
Figure 1. Predator free New Zealand 2050 (PFNZ 2050) has the ambitious goal of eradicating multiple non-native mammal predators at a national scale. In this paper we outline four interlinked issues (in external circles) that underpin the long-term success or sustainability of PFNZ 2050. Interactions between these issues are also illustrated in the text, shown here as bidirectional arrows. For example, the successful adoption of new eradication technologies depends on whether these fill a gap or improve on current operational methods that are also socially and ethically acceptable (see text for details).
Ecological understanding
New Zealand’s unique fauna at risk
The unique and unusual characteristics of NZ’s native fauna have long been recognised, and have arisen as the product of a long period of physical isolation from other landmasses, major marine inundation, significant climate fluctuations and evolution within a geologically dynamic landscape (Fleming Citation1979; Gibbs Citation2006, Citation2010). These characteristics include high rates of endemism, which for terrestrial arthropods and dicotyledons is ca. 90%, and for amphibians, reptiles and bats is 100%; gigantism, such as in moa (Dinorthiformes), land snails, earthworms; and flightlessness, as in kākāpō (Strigops habroptilus) and kiwi (Apteryx spp.; McGlone et al. Citation2001; Duncan and Blackburn Citation2004; Wallis and Trewick Citation2009). Moreover, the NZ fauna included a large number of relictual or phylogenetically-basal taxa such as tuatara (Sphenodon punctatus), acathasittid wrens, leiopelmid frogs, and many species exhibiting adaptations characteristic of mammals elsewhere (e.g. kiwi). At the time of initial human settlement by Māori (NZ’s Indigenous peoples) in the 13th Century AD (Wilmshurst et al. Citation2008) the NZ fauna had a near-absence of terrestrial mammals (except for three species of bat). Birds dominated the vertebrate fauna, which also included tuatara, >100 species of skinks and geckos, and six species of frog (Worthy and Holdaway Citation2002; Wood Citation2013). With one of the world’s most comprehensive late Quaternary fossil records, the prehistoric fauna of NZ has been relatively well-characterised, yet the use of DNA analyses to study these specimens continues to uncover previously unknown taxa, such as the Waitaha penguin (Megadyptes waitaha), as reviewed by Cole and Wood (Citation2018). Such detailed records of past biota can serve as important baselines for restoration and help inform conservation management (Willis and Birks Citation2006; Froyd and Willis Citation2008; Drake and Hunt Citation2009). For example, in NZ these historic insights are used to better understand the natural states of transformed native plant communities (Wilmshurst et al. Citation2014), guide restoration goals (Lyver et al. Citation2015), and identify lost ecological interactions that may have resulted in the decline of rare species (Wood et al. Citation2012).
The arrival of human settlers initiated a period of sustained erosion of NZ’s native biota and ecosystems through hunting (Perry et al. Citation2014a), fires (McWethy et al. Citation2014) and land drainage (Robertson et al. Citation2019). However, the introduction of the Pacific rat (kiore, Rattus exulans) and Polynesian dog (kurī, Canis lupus familiaris) by Māori were followed by rapid extinctions of many small, ground-nesting bird species, frogs and insects (Worthy and Holdaway Citation2002; Gibbs Citation2010; Wood Citation2013). Later introductions of a wide range of predatory mammals following European contact and settlement further impacted remaining native species (Wood Citation2013). Introductions of predatory mammals and their devastating effects on native species are not unique to NZ but have occurred across other island systems (Wood et al. Citation2017). New Zealand has, however, led other island nations in developing novel approaches to conserving remaining biodiversity and halting species declines (e.g. Russell et al. Citation2015; Norton et al. Citation2016). These approaches have included direct intervention with declining species such as translocations to establish new populations of declining bird species (Merton Citation1992; Elliott et al. Citation2001), control of predatory invasive mammals such as stoats (Mustela ermina) and weasels (M. nivalis) that have no analogues in the native fauna (Parkes and Murphy Citation2003; Goldson et al. Citation2015), and the eradication or removal of all invasive mammals from 143 islands (Parkes et al. Citation2017b; ).
Figure 2. Eradication of invasive non-native mammal predators have had growing success on ever-larger islands such Te Hauturu-o-Toi/Little Barrier Island (top left, 3083 ha) on which rats and cats were eradicated over a decade ago spurring on eradication efforts on larger areas such as Auckland island/Motu Maha (right panel, 46 000 ha) requiring by some estimates $40–50M NZD of management over at least a decade. Beyond island eradications, management of multiple predator species nationally will increasingly require management in ever closer proximity to people, necessitating additional consideration of social support and public engagement (bottom left). Photo credits. Copyright Rob Suisted / Nature’s Pic (Hauturu island, top left); NASA -http://eol.jsc.nasa.gov/scripts/sseop/photo.pl?mission=STS089&roll=743&frame=5, Public Domain, https://commons.wikimedia.org/w/index.php?curid=41494 (Motu Maha island); Nicola Toki, New Zealand Department of Conservation (pest control, bottom left).

Interactive ecological effects
What is eradication meant to accomplish beyond removal of undesirable species per se? Invasive non-native predator species (hereafter, ‘predators’) can have direct negative impacts on their prey, but can also exert important indirect effects such as vectoring diseases or disrupting mutualisms. For example, the Australian brushtail possum (Trichosurus vulpecula) is thought to increase tree mortality and drive population-level dieback of palatable native species in some areas through consumption of canopy foliage (Nugent et al. Citation2001; Holland et al. Citation2013), can act as a vector for bovine tuberculosis (TB) transmission (Nugent Citation2011), and is a predator of native birds (Innes et al. Citation2010). Eradication is viewed as a long-term solution to mitigate against these negative impacts, but these goals are not always articulated explicitly as the long-term desired benefits or positive outcomes of eradication efforts. In other words, the proximate goal of management to remove predators is clear, but the ultimate outcome and motivation of management is to restore biodiversity, reduce risks of disease, or renew ecological or ecosystem processes, which are less well articulated outcomes (Russell et al. Citation2015; Innes et al. Citation2019). Recent initiatives in conservation management, such as sanctuaries, have excluded pests including predators from discrete or bounded areas, and thus provide model systems for understanding the outcomes of predator removal, and in many cases, translocations, for native species recovery (Innes et al. Citation2010, Citation2019; Tanentzap and Lloyd Citation2017). Current eradication success from islands is to be scaled up (Russell et al. Citation2015; Pech and Maitland Citation2016), first by removing a subset of predators from a set of relatively large islands by 2025, and then by eradicating rats, mustelids and possums nationally by 2050 (see Supplemental Figure S1). The explicit goal of PFNZ 2050 is to eradicate mustelids, possums and rats (ship rats, Rattus rattus, and Norway rats, Rattus norvegicus), which are present on >94% of NZ’s land area. Some other major predators such as feral cats (Felis catus) and pigs (Sus scrofa) are not targeted nationally, in part because of the potential social and political conflicts involved with managing feral and pet cats in a country with relatively high rates of cat ownership (van Heezik et al. Citation2010; Kikillus et al. Citation2017), or pigs which are both a predator and food resource for rural New Zealanders.
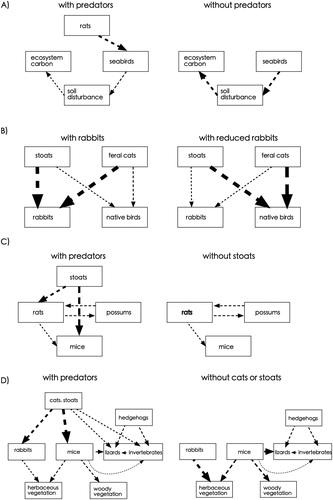
The removal of mammalian predators can have complex effects in ecosystems (e.g. O’Dowd et al. Citation2003; Wardle et al. Citation2007; Ruscoe et al. Citation2011; Russell Citation2011) because of their influence on trophic interactions and the ecosystem processes driven by such interactions (Mulder et al. Citation2009; Polis and Holt Citation1992; see ). PFNZ 2050 selectively removes predators such as mustelids, possums and rats across different systems, but their removal may have contrasting outcomes for other species including non-native mammals, native birds or other taxa (Towns et al. Citation2006; Norbury et al. Citation2015; Thoresen et al. Citation2017; Wardle and Peltzer Citation2017; ). For example, ‘mesopredator release’ (i.e. increases in the abundance of smaller predators or omnivores following predator control sensu lato) can negatively affect prey species, although the strength and generality of this effect is uncertain; this mechanism has been shown in models and for native predators on continents, but more empirical data on islands is needed (Courchamp et al. Citation1999; Rayner et al. Citation2007; Ritchie and Johnson Citation2009; Ruscoe et al. Citation2011). Regardless of whether mesopredator release per se follows eradication, the removal of selected mammalian predators highlights that the responses of other species such as mice (Mus musculus), European hedgehogs (Erinaceus europaeus), and feral cats, need to be considered in the context of predator eradication (Angel et al. Citation2009). Removal of predators can also result in releases of herbivores, which in turn can alter plant community structure and composition (e.g. Mason et al. Citation2010; Gormley et al. Citation2012; Ripple and Beschta Citation2012; Wardle and Peltzer Citation2017). For example, reducing the abundance of mustelids and cats in a dry shrubland ecosystem (Alexandra, NZ) drove increases in non-native herbivores (European rabbits Oryctolagus cuniculus, and hares Lepus europaeus) that in turn reduced grass productivity and shrub establishment (Norbury et al. Citation2013). An unanticipated consequence of this herbivore-induced vegetation change was to create habitat less suitable for mice, thus releasing populations of native lizards and invertebrates from predation by mice (Norbury et al. Citation2013). On the other hand, rabbits are generally considered to be more strongly limited by disease or primary production than by predation, but sufficient data to demonstrate which driver(s) work at the population-level are uncommon (Norbury and Jones Citation2015). Such indirect effects of predator removal on trophic interactions are broadly predictable, and can be used to reduce uncertainty and unanticipated responses of species or ecological processes to eradication (David et al. Citation2017). Whether indirect effects of predator management are of sufficient magnitude to undermine the potential conservation gains of eradication are unlikely but poorly understood; based on current knowledge and studies mentioned above we predict that the outcomes will be context-dependent and vary among taxa. More generally, the deliberate eradication of some species coupled with restoration of other species provides new opportunities for revealing the outcomes of both species gains and losses in ecosystems at unprecedented scales (Wardle et al. Citation2011; see also Wallach et al. Citation2015).
Figure 3. Summary of energy (biological carbon, C) flows though different ecosystem trophic levels. Both panels depict the predicted major pathways of influence of invasive predator species (A) or removing predators (B) on ecosystem C changes. Width of arrow depicts the relative importance of a pathway with respect to C flow. Most effects of predators on NEP (net ecosystem production) are mediated indirectly through herbivores, which in turn can regulate primary producers (net primary productivity, NPP). External gains and losses of C represent subsidies or losses to an ecosystem of C via non-biological activities (e.g. fire). (Modified from Peltzer et al. Citation2010).
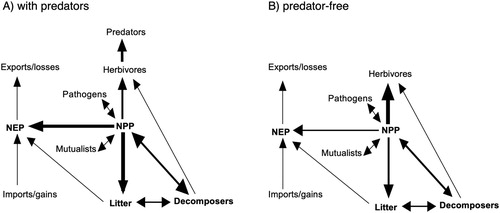
Figure 4. Illustrative examples of a systems approach to understanding the trophic interactions or ecosystem consequence of mammal eradication (redrawn and modified from original sources). In all cases dashed lines show negative effects; line thickness represents the relative strength of effects; boxes represent species or ecosystem properties. (A) Rat eradication from islands increases the number of burrowing seabirds, and this soil disturbance ultimately lowers ecosystem carbon (Wardle et al. Citation2007). (B) Reductions in important prey species such as rabbits (themselves a major invasive species) can cause prey switching to native bird species (Norbury Citation2001; Doherty et al. Citation2015). Rabbits are often resource or disease-limited rather than regulated by predators, in which case the arrows (causal pathways) can be in the opposite direction (Norbury and Jones Citation2015). (C) The sequential removal or one or more top predators can have contrasting effects on remaining invasive omnivores (Tompkins and Veltman Citation2006; Ruscoe et al. Citation2011). (D) predator removal can have complex knock-on effects in dryland ecosystems, mediated both through herbivore or mesopredator release (Norbury et al. Citation2009, Citation2013). Although mesopredator release is often assumed it is rarely documented (Ringler et al. Citation2015; Norbury et al. Citation2013); nevertheless the above illustrates only one of many potential indirect biological effects of predator removal. Negative interactions shown as causal pathways or arrows above do not necessarily imply population regulation (sufficient data to demonstrate population regulation is uncommon).
New approaches and methods for eradication
Operational methods
Pest or predator eradication has rapidly developed over the past two decades through refined management of environmental toxins or biocides, better spatial deployment or targeting of control efforts, and improved methods for detecting target populations and the effectiveness of eradication (Courchamp et al. Citation2003; Russell et al. Citation2015; Shepherd et al. Citation2018). Rapid progress in predator control has also been made through innovation of novel traps (e.g. automated resetting traps that can kill multiple individuals), improvements in surveillance and detection methods to understand when and where to deploy management, and more network-enabled systems to generate data (e.g. networked sensors, web-enabled tunnel traps; see also https://predatorfreenz.org/category/research/techniques-technologies/). The range of operational tools used to remove or eradicate predators is well-documented and not reviewed further here (e.g. Parkes and Murphy Citation2003; Brown et al. Citation2015; Goldson et al. Citation2015; Russell et al. Citation2015; Parkes et al. Citation2017a; see also https://www.doc.govt.nz/nature/pests-and-threats/predator-free-2050/toolkit-predator-free-2050/trapping-and-poisoning/choose-traps-and-toxins/). Much of this progress is based on predator removal from islands, and eradication has proceeded on ever-larger areas in these systems (Bellingham et al. Citation2010). However, increasing the area over which eradication can succeed will be a non-linear effort (i.e. involving thresholds of management effort), not just because of biological processes, but also driven by societal support for these activities (Towns et al. Citation2012; Simberloff et al. Citation2013; Niemiec et al. Citation2016; Yletyinen et al. Citation2019). As a consequence, eradication at larger spatial scales cannot be achieved solely through an expansion of current management approaches, but also relies in part on development or deployment of socially acceptable new methods or techniques, which itself is dynamic and interacts in complex ways across communities (discussed further in sections below).
Some potential future tools for eradicating mammalian predators such as novel gene technologies, viral biological control and new toxins, will challenge public perception of what is acceptable. For example, despite decades of research, use and refinement of using the toxin sodium monoflouracetate (i.e. 1080; Eason et al. Citation2002, Citation2011), there is ongoing debate, distrust and disagreement about the landscape-scale use of this poison despite its well-documented short-term effectiveness (e.g. PCE Citation2011; Green and Rohan Citation2012; Van Vianen et al. Citation2018). Deliberate introduction of additional species or diseases as potential biocontrol agents of predators is also scrutinised by government and the public alike (Wilkinson and Fitzgerald Citation1997; Suckling et al. Citation2014; Goldson et al. Citation2015), which seems somewhat ironic given that some of the predators (mustelids) now being controlled were themselves originally introduced as biocontrol agents (King Citation2017). Similarly, emerging genetic technologies that could be considered as potential tools for predator control have attracted early debate about their potential as a sustained alternative to the repeated and large-scale use of biocides, but also scrutiny of both the feasibility, safety, and effectiveness of such approaches. For example, gene-editing using CRISPR (an enzyme-based DNA editing tool) has been promoted as a targeted method for inducing heritable male sterility (Esvelt and Gemmell Citation2017). There are, however, significant risks of gene drive technologies leading to possible uncontrolled effects, including species extinctions (Noble et al. Citation2017, Citation2018). Emerging genetic methods depend on overcoming a series of non-trivial technical barriers including maintaining genetic changes in declining populations; targeting of methods across different mammal species and a marsupial (possums); and successfully operationalising these methods under field conditions (Esvelt and Gemmell Citation2017; Moro et al. Citation2018). More localised approaches to population control using gene-editing such as daisy-drives (Noble et al. Citation2018) are possible. The latter is based on the theory CRISPR editing can be modulated by a cascade of genetic elements, where the primary trigger is not operating under a drive, and therefore lost from the population within a few generations, breaking the chain of drive elements. While this method would have the advantage of having a limited lifespan in the environment, the technical barriers to this approach are likely considerably higher than standard gene-drive systems (Noble et al. Citation2018). More generally, the transfer of any of these technologies from in silico studies or laboratory cultures to the field is not a trivial undertaking. While there is evidence of success for ‘inundation’ methods of genetic control of populations, such as via the Sterile Insect Technique (Alphey et al. Citation2010), the logistics of implementing even gene-drive systems with mammalian pests would be more challenging (Moro et al. Citation2018). Furthermore, there may be danger that gene drive technologies could be developed or deployed by non-governmental agents, but would be difficult to regulate or police. If a major shift in public attitudes toward the use of such technologies, and concerns raised in domestic and international regulatory environments are achieved, this could result in much wider application of novel technologies to support pest management in agriculture and human health (Wilson et al. Citation2018; https://www.technologyreview.com/s/612415/united-nations-considers-a-test-ban-on-evolution-warping-gene-drives/), but will this come on stream in time for 2050?
Given the scale, complexity and multiple species involved with PFNZ 2050, a combination of approaches is likely to be pursued including toxins, advances in detection and trapping or control technologies, or new genetic or biological tools. Furthermore, these methods are likely to be deployed in combination and in different ways to the status quo in order to achieve eradication at ever-larger spatial scales. In all cases, the translation from a new potentially effective tool to social acceptance of this combination of approaches goes far beyond simple ethics into stronger and sustained engagement within a social-ecological system (Kendal and Ford Citation2018; see section on ‘societal considerations’ below and report of the BioHeritage Challenge Bioethics Panel Citation2019).
What are the broad outcomes of eradication efforts?
Although the explicit goal of PFNZ 2050 is national eradication of multiple predator species, there are implied benefits of achieving this goal that are less well articulated. One unequivocal benefit of predator eradication is for improving biodiversity, such as increasing the abundance of indigenous bird species. However, demonstrating benefits of predator eradication requires including response variables that capture how biodiversity or ecosystem functions change as a consequence of predator removal; in other words, management effectiveness of eradication. If, for example, increases in abundance of native bird species populations (or benefits for our diverse snail communities; e.g. Barker Citation2016) are a broad benefit resulting from PFNZ 2050, then their responses should be measured and reported as part of this programme or through closely-aligned efforts such as systematic monitoring (O'Donnell and Hoare Citation2012; PCE Citation2017). Data or evidence are needed in different systems to overcome ‘ecological surprises’ (Doak et al. Citation2008) and to better understand the complexity of indirect or interactive effects of predator invasion and management (e.g. Towns Citation2009). For example, recent reviews demonstrate that even the most intensive management of predators in fenced sanctuaries (Byrom et al. Citation2016; Bombaci et al. Citation2018; Miskelly Citation2018; Innes et al. Citation2019) have context-dependent benefits that vary among taxa. Precedents of linking large-scale management and responses are in place (e.g. ‘battle for our birds’, Elliott and Kemp Citation2016), but would require additional development for multiple species management and responses under PFNZ 2050. More generally, beyond quantifying catch effort and predator numbers (i.e. direct costs of eradication management), a long-standing issue is what additional information and effort is needed to demonstrate management effectiveness and benefits (Rands et al. Citation2010; Redpath et al. Citation2013)? We anticipate that widening the measures of success would also capture unanticipated benefits or surprises of large-scale management efforts (see also Doherty et al. Citation2015, Citation2018).
Will PFNZ 2050 be a net benefit for native biodiversity? Undoubtedly yes, if perverse effects are avoided and management efforts are not diverted away from other drivers of decline. Ensuring management efforts are not diverted away from other activities such as habitat restoration relies on clear governance structures and co-ordination at the meso- and national scales; for example, by linking PFNZ 2050 with the ‘billion trees’ programme, which seeks to increase forest cover throughout NZ and will thus have consequences for habitat availability and quality. On the other hand, predator removal alone many not result in the expected benefits if it undermines other efforts or other major drivers of change are not also considered; Hare et al. (Citation2019; their Table 2) highlight that across a range of NZ taxa, there are multiple stressors or threats other than predators including ecosystem changes, pollution, climate change, habitat fragmentation or exploitation. For example, Ruffell and Didham (Citation2017) demonstrate for northern NZ that only a few bird species responded positively to intense predator control, and that this response was conditional on having sufficient forest cover (ca. 10%), suggesting that restoration of woody plant cover is needed for the benefits of predator control to be realised (see also Norton et al. Citation2016). Moreover, Towns (Citation2009) suggested that rodent eradication from islands is not simply the inverse of invasion, but may require additional efforts to restore species that have been extirpated or are unable to recolonise (see also Jones Citation2010). Treating predator removal as the single or main driver of changes in diversity may be treating the symptoms rather than the causes of change in complex social-ecological systems (SES). Because social and ecological systems are inextricably linked, understanding the dynamics of SES provides insights into system-level changes such as resilience or environmental tipping points (Berkes et al. Citation2008; Lyver et al. Citation2019b). A simple example would be that removing predation on bird populations might favour non-native species over indigenous bird species through several ecological mechanisms (i.e. habitat modification favouring non-native species, Atkinson and Campbell Citation1966; Didham et al. Citation2007), and an expansion of bird populations could include both positive and negative consequences (e.g. greater rates of pollination or seed dispersal, but greater damage to crops). Rat removal can increase the abundance of some native bird species (e.g. kākā, Nestor meridionalis, and mohua, Mohoua ochrocephala; O'Donnell and Hoare Citation2012; Bombaci et al. Citation2018), but whether native species will benefit more than non-native species from predator eradication outside of forest vegetation generally is unknown. Possum removal will help to eradicate bovine TB, may increase the abundance of some bird species, and should have spillover benefits into primary production sectors (e.g. pasture and orchard production; Byrom et al. Citation2016). Understanding the ultimate costs and benefits of PFNZ 2050 thus reinforces the need to take a systems-level approach to biodiversity and biosecurity.
Societal considerations of eradication efforts
Beyond understanding the biological system implications of large-scale predator management, complex social issues will also need to be addressed (Glen et al. Citation2013, Citation2017; Estévez et al. Citation2015). For example, one potential conflict involves non-native species that are simultaneously valued and despised by different communities (Dickie et al. Citation2014; Schlaepfer Citation2018); in NZ, non-native species can underpin primary production, many economic activities, and recreational values. On the other hand, there are numerous pests, weeds and diseases that are viewed as overwhelmingly negative. Similarly, there are also global debates over the value of non-native species that result in conflicts over their use and management (Pejchar and Mooney Citation2009; Pauchard et al. Citation2018). Non-native predator species in NZ span the range from universally unwanted pests (e.g. rats, mustelids, possums, wasps (Vespula spp.)) though to valued resources (cats, dogs, pigs) and even to legally protected (e.g. salmonid fish) (Rouco et al. Citation2017). Furthermore, non-native species such as pigs and deer (Cervus elaphus) have become culturally significant to Māori as a source of food. Some non-native species such as salmonid fish are not necessarily widely invasive, but are managed as a resource because of their value to people.
What is remarkable about non-native species is the sustained long-term efforts that communities have taken to either remove species or manage them as a resource, sometimes simultaneously (e.g. pigs). Eradication requires long-term control and investment by diverse communities and this necessitates resolving potential conflicts among groups, and maintaining interest and investment despite declining returns from long-term control (i.e. the long-tail of eradication whereby management effort is relative greater as population density declines). There are excellent examples of aggregating community efforts to achieve greater benefits of activities at the landscape scale for native bird restoration (e.g. Innes et al. Citation2012, Citation2015; see also Niemiec et al. Citation2016). However, will PFNZ 2050 also require sustained behavioural changes to maintain management over a multi-decadal time scale? Some specific changes required to achieve PFNZ 2050 could have wider benefits including better mechanisms for conflict resolution or co-ordination among communities, and internal biosecurity or quarantine to prevent reinvasions or to contain predators (King et al. Citation2011; Omondiagbe et al. Citation2017; Parkes et al. Citation2017a).
Finally, urban areas are particularly complex social-ecological systems that will require renewed effort to understand ecological dynamics within (Pickett et al. Citation2001; Swan et al. Citation2011; Charles and Linklater Citation2015; Gaertner et al. Citation2017). Nowhere in the world have rats been eradicated from an urban area and if NZ can achieve this it will have major benefits for urban health worldwide (Wilson et al. Citation2018). Eradication of even feral cats is particularly challenging in urban areas due to social considerations, as well as the risk of increased mouse populations (van Heezik et al. Citation2010; Kikillus et al. Citation2017; see also Russell and Stanley Citation2018), but like removing rats, could have health benefits such as reduced transmission of toxoplasmosis.
The success of large-scale management for conservation depends on sustained community support including of urban areas. For example, a major rat eradication effort on Lord Howe island originally planned for in 2015 was deferred because of public resistance to the widespread use of rodenticides despite >15 years of research and planning that demonstrated the technical feasibility of this operation (http://lhirodenteradicationproject.org/; The Guardian Citation2016). Additional engagement with the community continued about the values and potential risks of this operation resulting in a narrow majority of the islanders (52%) voting in its favour. Rodent eradication on the island finally began in May 2019; this will be the largest undertaken on a permanently inhabited island (permanent population of 380; Pickrell Citation2019). This example highlights that engagement and negotiation with communities are essential for large-scale eradications to succeed. There are several positive examples of good social and community engagement that have supported scaling-up of management and conservation efforts such as the Cape to city landscape-scale predator control, Greater Wellington community groups co-ordinating bird count and predator removal efforts, and Predator-free Dunedin. For example, landscape-scale predator management in the Cape to City programme has included social science analyses to better understand landowners’ values and motivations, and this reveals that a series of more proximate management goals are needed to sustain landowners participation in, and value of, predator management (e.g. Neimiec et al. Citation2017; Glen et al. Citation2019). When considering the eradication of multiple species using several methods, and at a national scale for PFNZ 2050, potential communities of support comprise a diverse set of audiences. Social acceptability of eradication has been a relatively minor issue for many, but not all (e.g. Hauturu, ), offshore island eradications to date, but will become increasingly important as eradication efforts move from more outlying, to rural, and finally urban environments.
An unresolved issue is what level of community acceptance is needed to allow effective eradication? Even if a small minority of the population object, what then is the likelihood of eradication success? Social engagement or considerations of impacts are non-trivial given that NZ is highly urbanised (i.e. in 2014, 72% of the population live in centres with >30,000 people, Statistics NZ Citation2018; see also Fitzgerald et al. Citation2005). Accomplishing the PFNZ 2050 goal thus requires widespread engagement, and appropriate methods to consider social impacts of management activities (Russell et al. Citation2015, Citation2018; Glen et al. Citation2017), particularly in urban areas where alternative approaches to aerial poison drops will need to be developed. Furthermore, there is also a widespread move towards greater support for, and reliance on, community partnerships to accomplish conservation goals that rely on voluntary public interest in pest control, management or restoration activities (e.g. Curtis et al. Citation2014; Peters et al. Citation2015). For example, the recovery plan for an endemic flightless bird, the rail takahē (Porphyrio hochstetteri), has involved long-term captive breeding programme, management of deer (Cervus elaphus) as a putative competitor with takahē, and co-ordinating management efforts amongst conservation groups, hunters and volunteers for > 60 years (Lee and Jamieson Citation2001). Similarly, there are sustained efforts by many groups that manage fenced sanctuaries, who have co-ordinated efforts to build predator-proof fences, often reintroduce bird species, and have ongoing educational, monitoring and predator management efforts over decades (Innes et al. Citation2019).
Aside from working across the rural-urban continuum with communities that will have potentially different values or perceptions of predator removal, social impacts and values are dynamic and are likely to shift through multiple mechanisms. For example, there is growing corporate or international ownership of NZ’s rural lands with attendant declines in small farms and resident land-owners (Johnsen Citation2004; Nuthall and Old Citation2017), with the result that decisions about land management are increasingly remote from site; whether this results in different decisions from resident land owners is not known. In addition, there have also been major shifts in land use during the last decade (e.g. from pine plantations and sheep farming to intensive dairy farming; Burton and Peoples Citation2014; Sparling et al. Citation2014), and these changes will likely continue in the coming decades (e.g. through the Billion Trees Initiative, MPI Citation2018; see also MacLeod and Moller Citation2006).
External communities also shape acceptable management practices. NZ hosts a rapidly growing number of international visitors through tourism (e.g. 3.79 million international arrivals in 2018, an 53% increase since 2008; tourism contributes ca. 5.6% of NZ’s GDP), and the ongoing success of tourism, in part, relies on environmental credentials and meeting visitors’ expectations. Thus, international perceptions and acceptance of our management is essential for sustaining the tourism industry. Similarly, international opinion, values, and agreements also need to be taken into consideration to avoid perverse outcomes. For example, internationally, there is a growing intolerance of widespread use of toxins, driven by high-profile human health related cases, but likely to catalyse a growing concern and scrutiny of large-scale use of biocides sensu lato (e.g. Richmond Citation2018). Given the scaling-up of eradication efforts, there will inevitably be increasing confrontation of tourism with eradication activities starting from the point of arrival. Overall, we can expect that the social costs and benefits of achieving PFNZ 2050 will be different across communities, requiring a pluralistic approach to be used for engagement and support of any large-scale management (i.e. different approaches are needed among communities). Quite separately from social engagement (Kendal and Ford Citation2018), if this initiative is to succeed, it will be essential to incorporate the perspectives and values of Māori, who are partners with the Crown in the Treaty of Waitangi (NZ’s constitutional framework that guides the relationship between Māori and the government; Orange Citation2011; Jones Citation2016), major land owners, and kaitiaki (environmental guardians) for NZ’s biodiversity (Roberts et al. Citation1995).
Māori, PFNZ 2050 and governance
Māori acutely recognise the role that non-native mammalian predators play in the biological and cultural diversity crises confronting their communities (Lyver et al. Citation2008). Declines in iconic birds, such as kererū (Hemiphaga novaeseelandiae), kākā kōkō (tūī, Prosthemadera novaeseelandiae) and tītī (Puffinus griseus), and many other terrestrial, marine and freshwater native species threaten highly valued customary food sources, cultural heritage practices and expressions, and ultimately tribal identity (Lyver et al. Citation2017). Predators have significantly impacted the key cultural construct of whakapapa (sequential system that portrays the genealogical connections between the natural world, including humans, and cosmological domain), which has degraded the mauri (condition and representativeness of connections within whakapapa) and mana (authority and prestige drawn from the condition and representativeness of those connections) of NZ’s environment and people (Timoti et al. Citation2017). The eradication of predators from the landscape, therefore, represents significant gains and opportunities for Māori. For Māori, however, the detrimental effect of predators on biodiversity is entangled with the ongoing impact of the European-dominated political process on their relationship with the environment (Ellison Citation2001; Ruru Citation2004; Solomon Citation2014; see also Mawyer and Jacka Citation2018 for additional international examples).
Unrealised rights to, and ownership of, decision-making over the environment guaranteed within the Treaty of Waitangi still constrains the relationship Māori have with their environment (Ruru et al. Citation2017), and hence Māori do not enter the PFNZ 2050 initiative as equal partners with the Crown. Aspirational goals and outcomes significant for Māori that could potentially emerge from a PFNZ 2050 initiative are not necessarily the same as the Crown’s, nor are they currently recognised by the Crown. For example, customary rights to protect, restore and use native biodiversity remain poorly instituted within current policy and legislative mechanisms (Lyver et al. Citation2019a). Reformed laws that empower tangata whenua (people of the land) and enable Māori worldview representations, values, knowledge and solutions for managing the environment are needed alongside national initiatives like PFNZ 2050. For example, re-classification of Te Urewera National Park to a place with its own legal entity and accorded the legal rights of a person (NZ Government Citation2014) has made possible a pluralistic place-based governance framework for implementing biocultural approaches to managing the environment. However, the PFNZ 2050 initiative is being developed and applied over a fragile social fabric still mired in historical cross-cultural political turmoil, which weakens its capacity for success.
Achieving the PFNZ 2050 vision is heavily reliant on the government’s appeal to NZ society, including Māori, to act in a coordinated fashion nationally to eradicate predators. However, little consideration has been given to how the PFNZ 2050 initiative impacts Māori, nor what ramifications its implementation might have for tribal groups. For example, future PFNZ 2050-related policy may place the responsibility and cost of predator eradication directly on land owners. Māori are the owners of large tracts of remote marginal lands in NZ that typically would be the most difficult and expensive from which to eradicate predators. Responsibility for having to eradicate predators from those lands would represent a significant ecological debt passed from government to Māori. If this was the case, legislated obligations for Māori to pay for eradication on their lands may lead to future treaty grievances and treaty claims. Extensive discussions with Māori leaders and communities throughout the country are therefore needed well ahead of enacting PFNZ 2050 policy or allocating funds.
Because the government in partnership with Māori aims to make decisions about predator eradication, PFNZ 2050 also needs to be responsive to Māori ecological, economic, and cultural values and aspirations for the environment (Ruru et al. Citation2017; Lyver et al. Citation2019a). For example, plucked possum fur that is woven with merino wool to produce high quality yarn (e.g. Merino mink™, Perino™) can be valuable additions individuals’ and families incomes in small, predominantly rural Māori communities with high unemployment high (ca. 15%) and low annual incomes (Jones et al. Citation2012). Eradicating possums would remove this income source, so alternative local industry opportunities that might arise from a PFNZ 2050 need to be considered in consultation with those who may be affected. Similarly, kiore introduced to NZ by Māori are considered a culturally-significant species and valued source of meat and pelts for korowai (traditional cloaks) by individuals within some tribes (e.g. Ngātiwai; Department of Conservation and Ngātiwai Trust Board Citation2010). Although kiore may not be directly targeted by PFNZ 2050, this species will be susceptible to many control methods, especially if gene-based manipulation methods specific to rodents are deployed. Methods and processes to eradicate predators would also need to recognise a Māori worldview (Timoti et al. Citation2017). Negative attitudes towards the aerial application of toxins and genetic engineering techniques within some tribal groups emerge from interpretations of a cultural worldview (Ogilvie et al. Citation2007; see also Lambert et al. Citation2018 for parallel issues around emerging forest pathogens). Investment into consultation processes with Māori ahead of potential method development would engage Māori communities and also help alleviate potential concerns over eradication techniques in the future. To support and enable these engagement and leadership processes, and to realise outcomes PFNZ 2050, goals should be aligned to legislated reforms in conservation law that empower Māori in their role and obligations as kaitiaki of the environment (Ruru et al. Citation2017).
Discussion
The expanding scale and multiple management approaches being implemented by PFNZ 2050 mean that diverse communities and agencies need to co-ordinate their actions if they are to overcome impediments to large-scale eradication or conservation efforts. National predator eradication requires a new funding or resource allocation model that brings government, philanthropists, industry, Māori and the general public together to support management that must endure over several decades (Goldson et al. Citation2015; see also Epanchin-Niell Citation2017.). More generally, co-ordinating efforts and resources among the different groups contributing to PFNZ 2050 will require using explicit approaches to prioritise and allocate resources amongst different activities to minimise conflicts (e.g. Estévez et al. Citation2015). Sustaining eradication efforts, or most larger-scale conservation efforts, also necessitates better understanding of how complex social-ecological systems operate, and can be used to maintain support and engagement across communities (Liu et al. Citation2012; Lyver et al. Citation2016, Citation2019a; Yletyinen et al. Citation2019). What PFNZ 2050 provides is a tangible long-term goal and aspiration that could stimulate a collective approach to management action (e.g. Adger Citation2003; Steffen et al. Citation2011; Graham et al. Citation2019).
Current large-scale management provides insights into the magnitude of effort required for eradication, and what is required to resolve issues of scaling-up these efforts. For example, discrete spatial eradication efforts have been achieved using fenced sanctuaries or focal management areas, and these can provide ‘spillover’ benefits for biodiversity by providing core populations (e.g. see Innes et al. Citation2012 and earlier rejoinder by Scofield et al. Citation2011; Byrom et al. Citation2016; Tanentzap and Lloyd Citation2017; review of Hayward and Kerley Citation2009). Because eradication management efforts and effectiveness are likely to be spatially heterogeneous, how can management be deployed to minimise real or perceived risks driven by this spatial variability (Mahoney et al. Citation2018)? One approach is to have sufficient monitoring in place across sites to demonstrate management progress by first initially detecting residual target predators or changes in populations directly following control treatments, and then subsequent reinvasion at the decadal scale (e.g. Tompkins et al. Citation2013; Holland et al. Citation2015; Holden et al. Citation2016; see also Russell et al. Citation2015). Landscape-scale approaches to conservation also require greater acceptance of risk and uncertainty in management practice or outcomes at local or regional scales, despite these efforts being designed to propagate up to achieving a large-scale goal. For example, visualisation approaches such as graphic maps of changes in population abundance have been used to forecast the likely outcomes of management, can incorporate estimates of risk or uncertainty, and be an effective way to communicate across communities (Clark et al. Citation2001; Luo et al. Citation2011; Dietze et al. Citation2018). However, whether greater awareness or engagement across communities can be designed to stimulate collective action and ultimately progress towards a shared goal is an emerging field (Bodin Citation2017; Graham et al. Citation2019). PFNZ 2050 could be thought of as implicitly designing a shift in social-ecological systems towards a country without (a subset) of non-native predators through unprecedented scaling up of technical advances integrated with social dynamics. Regardless of the ultimate outcome of efforts towards PFNZ 2050, the attempts and progress towards such an ambitious goal should shed new light on how collective action can be sustained to achieve long-term environmental goals more generally (Norton et al. Citation2016; Graham et al. Citation2019).
A related but unresolved issue involves the relative magnitude of different drivers influencing the feasibility of eradication efforts, and the consequence of broad scale species removals for other taxa or ecosystem processes. Historical forest losses and long-term declines in the distribution and abundance of many native species have fundamentally changed NZ ecosystems. Concurrently, increases in multiple non-native taxa are thought to have sometimes profound, but poorly understood, additional or synergistic effects (Allen and Lee Citation2006). There is a growing awareness of interactions between different drivers of global change including biological invasions, land use, eutrophication, altered disturbance regimes and climate (Tylianakis et al. Citation2008; Côté et al. Citation2016). Thus, a shift in emphasis in NZ from focussing on the importance of habitat availability for sustaining species populations, toward predators or biological invasions as the primary drivers of change may be overly simplistic. Put another way, assigning cause and effect to individual species does not capture the multiple or interacting drivers of changes in species populations, community structure and ecosystems (Brook et al. Citation2008; Peltzer et al. Citation2014). Thus, achieving the predator-free goal across the diverse range native ecosystems in NZ will require greatly improved ecological understanding and ability to restore ecosystems following eradication (Norton et al. Citation2016; Clement and Standish Citation2018). Application of new conceptual approaches such as network theory can be used to better predict the direct and indirect effects of multiple drivers in systems, but these methods rely on sufficient knowledge and data to make informed predictions and to aid in management decision making (Tylianakis et al. Citation2008; Yletyinen et al. Citation2019). The clear history of introduction, relatively good understanding of distribution and abundance of both native and non-native taxa, and active management provide opportunities for disentangling the role of species in ecosystems.
Finally, the long-term success of PFNZ 2050 or any large-scale conservation issue, largely depends on support for, and participation by, civil society. Management of invasive species can galvanise but simultaneously produce conflicts among communities (Dickie et al. Citation2014; Crowley et al. Citation2017). For PFNZ 2050, Linklater and Steer (Citation2018) have suggested that imposing a policy of predator eradication could escalate conflicts over pest control operations such as the contested use of biocides. Moreover, predators are not always the primary driver of declines in biodiversity; thus if predator eradication displaces other activities such as habitat protection or restoration, then this could inadvertently cause overall declines in biodiversity. Several approaches could be taken to resolve potential conflicts and to scale-up conservation efforts. First, co-ordination among different landowners and managers is essential for landscape-level control of biological invaders (e.g. Epanchin-Niell et al. Citation2010; Cape to City example above). This approach includes both top-down and meso-level (e.g. regional rather than only local or national) education, regulation, incentives and clear communication amongst stakeholders. Second, widespread social support or acceptance of eradication goes far beyond the concept of ‘social licence to operate’ (Garnett et al. Citation2018; Kendal and Ford Citation2018), but rather requires understanding broader community relationships or complex governance arrangements that typify large-scale conservation efforts. For PFNZ 2050, we suggest that a greater awareness of people’s motivation and skills for making decisions should be guided by institutions and behaviourally-informed policy at the regional or national scale (Madrian Citation2014; Hertwig and Grüne-Yanoff Citation2017) rather than relying on changes in individual behaviours to resolve the collective problem of national-scale pest eradication. Third, several techniques have emerged that seek to shift individual choices or behaviour that can then propagate up to greater social welfare (Reijula et al. Citation2018), for example through ‘nudging’ (i.e. decisions are influenced by choice architecture) or ‘boosting’ (i.e. promote better capability for decision-making through education). Hertwig and Grüne-Yanoff (Citation2017) highlight that boosts ultimately increase people’s competencies to make positive behavioural choices, and should be more stable and longer-term than behavioural nudges (see also Mols et al. Citation2015). Finally, network governance can also be applied to conservation issues to better understand how behavioural changes can be designed or planned for (Hukkinen Citation2016). For example, network administrative organisation (Provan and Kenis Citation2007; Lubell et al. Citation2017) has been successfully used as a governance approach to eradicate the weed Spartina (several species) from San Francisco Bay; this approach requires clearly defining authority, expertise and resource across stakeholders to facilitate effective co-operative behaviour and co-ordinate management. Thus the management of biological invaders relies not only on resolving methods, biological and economic issues (e.g. as highlighted in Linklater and Steer (Citation2018) and elsewhere), but also governance arrangements that support co-operative behaviour across networks of communities.
We do not believe that a single approach can lead to sustained large-scale management of invasive species, but the approaches and examples provided throughout this paper illustrate how large-scale and long-term conservation efforts could be supported. An emerging principle is that multiple approaches are needed to generate and sustain the broad societal support needed to achieve a PFNZ 2050. For example, whether shifts in individual behaviours can be designed to propagate up to large-scale collective outcomes remains largely unknown, whereas large-scale co-ordination and clear lines of responsibility are recognised as necessary elements for promoting cooperative behaviour (e.g. Meinzen-Dick et al. Citation2004; Pollard et al. Citation2019). For PFNZ 2050, national and meso-scale governance are needed to co-ordinate activities and to stimulate a collective approach to national-scale predator eradication rather than relying on top-down policy. Collective action approaches to the management of invasive species are becoming more widespread internationally, but can be driven by communities, co-management or organisational coalitions (Graham et al. Citation2019). Encouragingly, there are examples of all of these approaches already in place as part of PFNZ 2050; now the challenge is in scaling these up in a way that is flexible enough to accommodate the complexity and uncertainties involved (see also Kappes et al. Citation2019; Russell and Taylor Citation2019). An underpinning principle to all of the above is that sustained engagement, collaborative consultation, or negotiation across civil society is an essential principle of conservation governance regimes (Berkes Citation2007) and will help to ensure the success of PFNZ 2050.
Conclusions
Large-scale eradication of multiple invasive species attempted by the PFNZ 2050 initiative deliberately manipulates the biota of an entire country, and in so doing provides unprecedented opportunities for understanding the biological and ecological processes of invasion, reinvasion, secondary invasions, and the responses of complex ecosystems. However, the long-term success and potential benefits implied by achieving PFNZ 2050 are driven largely by social and cultural processes. Empowering, mobilising and sustaining large-scale eradication efforts across diverse communities is non-trivial. For example, post-Treaty of Waitangi settlement governance requires public support and legal changes to facilitate their development, and implies longer-term societal norms must change because no one governance system will suffice at a national scale. Addressing these challenges is widespread and urgent given the need to apply ecological understanding to develop improved policy and management of biological invaders, the species or ecosystems affected by invasive species and their management. NZ is embarking on one of the largest social and environmental experiments ever envisaged and, if well designed, will deliver conservation insights of worldwide relevance.
Supplemental material
Download TIFF Image (8.4 MB)Acknowledgements
We thank Dave Towns for thoughtful comments and editorial inputs to this manuscript, and James Russell and an anonymous referee for helpful comments.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Duane A. Peltzer http://orcid.org/0000-0001-7724-3738
Peter J. Bellingham http://orcid.org/0000-0001-9646-4819
Ian A. Dickie http://orcid.org/0000-0002-2740-2128
Gary Houliston http://orcid.org/0000-0003-0553-993X
Philip E. Hulme http://orcid.org/0000-0001-5712-0474
Sarah J. Richardson http://orcid.org/0000-0002-4097-0381
Jamie Wood http://orcid.org/0000-0001-8008-6083
Additional information
Funding
References
- Adger WN. 2003. Social capital, collective action, and adaptation to climate change. Economic Geography. 79:387–404.
- Allen RB, Lee WG, editors. 2006. Biological invasions in New Zealand (Vol. 186). Berlin: Springer Science and Business Media.
- Alphey L, Benedict M, Bellini R, Clark GG, Dame DA, Service MW, Dobson SL. 2010. Sterile-insect methods for control of mosquito-borne diseases: an analysis. Vector Borne Zoonotic Dis. 10:295–311.
- Angel A, Wanless RM, Cooper J. 2009. Review of impacts of the introduced house mouse on islands in the Southern Ocean: are mice equivalent to rats? Biological Invasions. 11:1743–1754.
- Atkinson IAE, Cameron EK. 1993. Human influence on the terrestrial biota and biotic communities of New Zealand. Trends in Ecology and Evolution. 8:447–451.
- Atkinson IAE, Campbell DJ. 1966. Habitat factors affecting saddlebacks on Hen island. Proceedings of the New Zealand Ecological Society. 13:35–40.
- Barker GM. 2016. Land snail communities respond to control of invasive rats in New Zealand forests. New Zealand Journal of Ecology. 40:310–320.
- Bellingham PJ, Towns DR, Cameron EK, Davis JJ, Wardle DA. 2010. New Zealand island restoration: seabirds, predators, and the importance of history. New Zealand Journal of Ecology. 34:115–136.
- Berkes F. 2007. Community-based conservation in a globalized world. Proceedings of the National Academy of Sciences. 104:15188–15193.
- Berkes F, Colding J, Folke C. 2008. Navigating social-ecological systems: building resilience for complexity and change. Cambridge: Cambridge University Press.
- BioHeritage Challenge Bioethics Panel. 2019. Report on “Predator-free New Zealand: Social, Cultural, and Ethical Challenges ”.
- Bodin Ö. 2017. Collaborative environmental governance: achieving collective action in social-ecological systems. Science. 357:eaan1114.
- Bombaci S, Pejchar L, Innes J. 2018. Fenced sanctuaries deliver conservation benefits for most common and threatened native island birds in New Zealand. Ecosphere. 9:e02497.
- Brook BW, Sodhi NS, Bradshaw CJ. 2008. Synergies among extinction drivers under global change. Trends in Ecology and Evolution. 23:453–460.
- Brown K, Elliott G, Innes J, Kemp J. 2015. Ship rat, stoat and possum control on mainland New Zealand. An overview of techniques, successes and challenges. Wellington: Department of Conservation.
- Burton RJF, Peoples S. 2014. Market liberalisation and drought in New Zealand: A case of ‘double exposure’ for dryland sheep farmers? Journal of Rural Studies. 33:82–94.
- Byrom AE, Innes J, Binny RN. 2016. A review of biodiversity outcomes from possum-focused pest control in New Zealand. Wildlife Research. 43:228–253.
- Charles KE, Linklater WL. 2015. The role of environmental engagement in tolerating urban bird problems. Human Dimensions of Wildlife. 20:99–111.
- Clark JS, Carpenter SR, Barber M, Collins S, Dobson A, Foley JA, Lodge DM, Pascual M, Pielke R, Pizer W, et al. 2001. Ecological forecasts: an emerging imperative. Science. 293:657–660.
- Clement S, Standish RJ. 2018. Novel ecosystems: governance and conservation in the age of the Anthropocene. Journal of Environmental Management. 208:36–45.
- Cole TL, Wood JR. 2018. The ancient DNA revolution: the latest era in unearthing New Zealand’s faunal history. New Zealand Journal of Zoology. 45:91–120.
- Côté IM, Darling ES, Brown CJ. 2016. Interactions among ecosystem stressors and their importance in conservation. Proceedings of the Royal Society B: Biological Sciences. 283:20152592.
- Courchamp F, Chapuis JL, Pascal M. 2003. Mammal invaders on islands: impact, control and control impact. Biological Reviews. 78:347–383.
- Courchamp F, Langlais M, Sugihara G. 1999. Cats protecting birds: modelling the mesopredator release effect. Journal of Animal Ecology. 68:282–292.
- Crowley SL, Hinchliffe S, McDonald RA. 2017. Conflict in invasive species management. Frontiers in Ecology and the Environment. 15:133–141.
- Curtis A, Ross H, Marshall GR, Baldwin C, Cavaye J, Freeman C, Carr A, Syme GJ. 2014. The great experiment with devolved NRM governance: lessons from community engagement in Australia and New Zealand since the 1980s. Australasian Journal of Environmental Management. 21:175–199.
- David P, Thebault E, Anneville O, Duyck PF, Chapuis E, Loeuille N. 2017. Impacts of invasive species on food webs: a review of empirical data. Advances in Ecological Research. 56:1–60.
- Department of Conservation and Ngātiwai Trust Board. 2010. Memorandum of agreement for the control and management of an area of a Nature Reserve. Mauitaha and Araara Islands Operational Management Agreement. Department of Conservation, Wellington, New Zealand; 19 p.
- Dickie IA, Bennett BM, Burrows LE, Nuñez MA, Peltzer DA, Porté A, Richardson DM, Rejmánek M, Rundel PW, Van Wilgen BW. 2014. Conflicting values: ecosystem services and invasive tree management. Biological Invasions. 16:705–719.
- Didham RK, Tylianakis JM, Gemmell NJ, Rand TA, Ewers RM. 2007. Interactive effects of habitat modification and species invasion on native species decline. Trends in Ecology and Evolution. 22:489–496.
- Dietze MC, Fox A, Beck-Johnson LM, Betancourt JL, Hooten MB, Jarnevich CS, Keitt TH, Kenney MA, Laney CM, Larsen LG, et al. 2018. Iterative near-term ecological forecasting: needs, opportunities, and challenges. Proceedings of the National Academy of Sciences. 115:1424–1432.
- Doak DF, Estes JA, Halpern BS, Jacob U, Lindberg DR, Lovvorn J, Monson DH, Tinker MT, Williams TM, Wootton JT, et al. 2008. Understanding and predicting ecological dynamics: are major surprises inevitable? Ecology. 89:952–961.
- Doherty TS, Bland LM, Bryan BA, Neale T, Nicholson E, Ritchie EG, Driscoll DA. 2018. Expanding the role of targets in conservation policy. Trends in Ecology and Evolution. 33:809–812.
- Doherty TS, Dickman CR, Nimmo DG, Ritchie EG. 2015. Multiple threats, or multiplying the threats? Interactions between invasive predators and other ecological disturbances. Biological Conservation. 190:60–68.
- Doherty TS, Ritchie EG. 2017. Stop jumping the gun: a call for evidence-based invasive predator management. Conservation Letters. 10:15–22.
- Drake DR, Hunt TL. 2009. Invasive rodents on islands: integrating historical and contemporary ecology. Biological Invasions. 11:1483–1487.
- Duncan RP, Blackburn TM. 2004. Extinction and endemism in the New Zealand avifauna. Global Ecology and Biogeography. 13:509–517.
- Eason CT, Miller AM, Ogilvie SC, Fairweather A. 2011. An updated review of the toxicology and ecotoxicology of sodium fluoroacetate (1080) in relation to its use as a pest control tool in New Zealand. New Zealand Journal of Ecology. 35:1–20.
- Eason CT, Murphy EC, Wright GR, Spurr EB. 2002. Assessment of risks of brodifacoum to non-target birds and mammals in New Zealand. Ecotoxicology. 11:35–48.
- Edney-Browne E, Brockerhoff EG, Ward D. 2018. Establishment patterns of non-native insects in New Zealand. Biological Invasions. 20:1657–1669.
- Elliott G, Kemp J. 2016. Large-scale pest control in New Zealand beech forests. Ecological Management and Restoration. 17:200–209.
- Elliott GP, Merton DV, Jansen PW. 2001. Intensive management of a critically endangered species: the kākāpo. Biological Conservation. 99:121–133.
- Ellison E. 2001. Deficiencies in Conservation Subsidiary Legislation. Report to the New Zealand Conservation Authority, 25 July 2001. Unpublished.
- Epanchin-Niell RS. 2017. Economics of invasive species policy and management. Biological Invasions. 19:3333–3354.
- Epanchin-Niell RS, Hufford MB, Aslan CE, Sexton JP, Port JD, Waring TM. 2010. Controlling invasive species in complex social landscapes. Frontiers in Ecology and the Environment. 8:210–216.
- Estévez RA, Anderson CB, Pizarro JC, Burgman MA. 2015. Clarifying values, risk perceptions, and attitudes to resolve or avoid social conflicts in invasive species management. Conservation Biology. 29:19–30.
- Esvelt KM, Gemmell NJ. 2017. Conservation demands safe gene drive. PLoS Biology. 15:e2003850.
- Fitzgerald G, Fitzgerald N, Wilkinson R. 2005. Social acceptability of stoats and stoat control methods: A survey of the New Zealand public. Science for Conservation 253, 40 pgs. Wellington, New Zealand: Department of Conservation.
- Fleming CA. 1979. The geological history of New Zealand and its life. Auckland: Auckland University Press.
- Froyd CA, Willis KJ. 2008. Emerging issues in biodiversity & conservation management: the need for a palaeoecological perspective. Quaternary Science Reviews. 27:1723–1732.
- Gaertner M, Wilson JRU, Cadotte MW, MacIvor JS, Zenni RD, Richardson DM. 2017. Non-native species in urban environments: patterns, processes, impacts and challenges. Biological Invasions. 19:3461–3469.
- Garnett ST, Zander KK, Robinson CJ. 2018. Social license as an emergent property of political interactions: response to Kendal and Ford 2017. Conservation Biology. 32:734–736.
- Gibbs GW. 2006. Ghosts of Gondwana: the history of life in New Zealand. Nelson: Craig Potton Publishing.
- Gibbs GW. 2010. Do New Zealand invertebrates reflect the dominance of birds in their evolutionary history? New Zealand Journal of Ecology. 34:152–157.
- Glen AS, Atkinson R, Campbell KJ, Hagen E, Holmes ND, Keitt BS, Parkes JP, Saunders A, Sawyer J, Torres H. 2013. Eradicating multiple invasive species on inhabited islands: the next big step in island restoration? Biological Invasions. 15:2589–2603.
- Glen AS, Latham MC, Anderson D, Leckie C, Niemiec R, Pech RP, Byrom AE. 2017. Landholder participation in regional-scale control of invasive predators: an adaptable landscape model. Biological Invasions. 19:329–338.
- Glen AS, Perry M, Yockney I, Cave S, Gormley AM, Leckie C, Dickson R, Rakete-Stones W, Rakete-Stones P, Norbury GL, et al. 2019. Predator control on farmland for biodiversity conservation: a case study from Hawke's Bay, New Zealand. New Zealand Journal of Ecology. 43:1–7.
- Goldson SL, Bourdôt GW, Brockerhoff EG, Byrom AE, Clout MN, McGlone MS, Nelson WA, Popay AJ, Suckling DM, Templeton MD. 2015. New Zealand pest management: current and future challenges. Journal of the Royal Society of New Zealand. 45:31–58.
- Gormley AM, Pech RP, Holland EP, Thomson C, Reddiex B. 2012. Impacts of an invasive herbivore on indigenous forests. Journal of Applied Ecology. 49:1296–1305.
- Graham S, Metcalf AL, Gill N, Niemiec R, Moreno C, Bach T, Ikutegbe V, Hallstrom L, Ma Z, Lubeck A. 2019. Opportunities for better use of collective action theory in research and governance for invasive species management. Conservation Biology. 33:275–287.
- Green W, Rohan M. 2012. Opposition to aerial 1080 poisoning for control of invasive mammals in New Zealand: risk perceptions and agency responses. Journal of the Royal Society of New Zealand. 42:185–213.
- Hare KM, Borrelle SB, Buckley HL, Collier KJ, Constantine R, Perrott JK, Watts CH, Towns DR. 2019. Intractable: species in New Zealand that continue to decline despite conservation efforts. Journal of the Royal Society of New Zealand. 49(3):301–319. doi:10.1080/03036758.2019.1599967.
- Hayward MW, Kerley GIH. 2009. Fencing for conservation: restriction of evolutionary potential or a riposte to threatening processes? Biological Conservation. 142:1–13.
- Hertwig R, Grüne-Yanoff T. 2017. Nudging and boosting: Steering or empowering good decisions. Perspectives on Psychological Science. 12:973–986.
- Holden MH, Nyrop JP, Ellner SP. 2016. The economic benefit of time-varying surveillance effort for invasive species management. Journal of Applied Ecology. 53:712–721.
- Holland EP, James A, Ruscoe WA, Pech RP, Byrom AE. 2015. Climate-based models for pulsed resources improve predictability of consumer population dynamics: outbreaks of house mice in forest ecosystems. PLoS One. 10:e0119139.
- Holland EP, Pech RP, Ruscoe WA, Parkes JP, Nugent G, Duncan RP. 2013. Thresholds in plant–herbivore interactions: predicting plant mortality due to herbivore browse damage. Oecologia. 172:751–766.
- Holmes ND, Griffiths R, Pott M, Alifano A, Will D, Wegmann AS, Russell JC. 2015. Factors associated with rodent eradication failure. Biological Conservation. 185:8–16.
- Hukkinen JI. 2016. Addressing the practical and ethical issues of nudging in environmental policy. Environmental Values. 25:329–351.
- Hutchings SA, Hancox N, Livingstone PG. 2013. A strategic approach to eradication of bovine TB from wildlife in New Zealand. Transboundary and Emerging Diseases. 60:85–91.
- Innes J, Fitzgerald N, Binny R, Byrom A, Pech R, Watts C, Gillies C, Maitland M, Campbell-Hunt C, Burns B. 2019. New Zealand ecosanctuaries: types, attributes and outcomes. Journal of the Royal Society of New Zealand. 49(3):370–393. doi:10.1080/03036758.2019.1620297.
- Innes J, Kelly D, Overton JMC, Gillies C. 2010. Predation and other factors currently limiting New Zealand forest birds. New Zealand Journal of Ecology. 34:86–114.
- Innes J, King C, Bartlam S, Forrester G, Howitt R. 2015. Predator control improves nesting success in Waikato forest fragments. New Zealand Journal of Ecology. 39:245–253.
- Innes J, Lee WG, Burns B, Campbell-Hunt C, Watts C, Phipps H, Stephens T. 2012. Role of predator-proof fences in restoring New Zealand's biodiversity: a response to Scofield et al. (2011). New Zealand Journal of Ecology. 36:232–238.
- Johnsen S. 2004. The redefinition of family farming: agricultural restructuring and farm adjustment in Waihemo, New Zealand. Journal of Rural Studies. 20:419–432.
- Jones C. 2016. New treaty, new tradition: reconciling New Zealand and Māori law. Vancouver, Canada: University of British Columbia Press. 211 p.
- Jones HP. 2010. Prognosis for ecosystem recovery following rodent eradication and seabird restoration in an island archipelago. Ecological Applications. 20:1204–1216.
- Jones C, Barron M, Warburton B, Coleman M, Lyver POB, Nugent G. 2012. Serving two masters: reconciling economic and biodiversity outcomes of brushtail possum (Trichosurus vulpecula) fur harvest in an indigenous New Zealand forest. Biological Conservation. 153:143–152.
- Jones HP, Holmes ND, Butchart SH, Tershy BR, Kappes PJ, Corkery I, Aguirre-Muñoz A, Armstrong DP, Bonnaud E, Burbidge AA, et al. 2016. Invasive mammal eradication on islands results in substantial conservation gains. Proceedings of the National Academy of Sciences. 113:4033–4038.
- Kappes PJ, Bond AL, Russell JC, Wanless RM. 2019. Diagnosing and responding to causes of failure to eradicate invasive rodents. Biological Invasions. 21:2247–2254.
- Kelly D, Sullivan JJ. 2010. Life histories, dispersal, invasions, and global change: progress and prospects in New Zealand ecology, 1989–2029. New Zealand Journal of Ecology. 34:207–217.
- Kendal D, Ford RM. 2018. The role of social license in conservation. Conservation Biology. 32:493–495.
- Kikillus KH, Chambers GK, Farnworth MJ, Hare KM. 2017. Research challenges and conservation implications for urban cat management in New Zealand. Pacific Conservation Biology. 23:15–24.
- King CM. 2005. The Handbook of New Zealand mammals. Melbourne: Oxford University Press.
- King CM. 2017. The history of transportations of stoats (Mustela erminea) and weasels (M. nivalis) to New Zealand, 1883–1892. International Review of Environmental History. 3:51–87.
- King CM, Innes JG, Gleeson D, Fitzgerald N, Winstanley T, O’Brien B, Bridgman L, Cox N. 2011. Reinvasion by ship rats (Rattus rattus) of forest fragments after eradication. Biological Invasions. 13:2391–2408.
- Kopf RK, Nimmo DG, Humphries P, Baumgartner LJ, Bod M, Bond NR, Byrom AE, Cucherousset J, Keller RP, King A, et al. 2017. Confronting the risks of large-scale invasive species control. Nature Ecology and Evolution. 1:172–175.
- Lambert S, Waipara N, Black A, Mark-Shadbolt M, Wood W. 2018. Indigenous biosecurity: Māori responses to Kauri dieback and Myrtle Rust in Aotearoa New Zealand. In: The human Dimensions of forest and tree health. Cham: Palgrave Macmillan; p. 109–137.
- Lee WG, Jamieson IG. 2001. The takahē: fifty years of conservation management and research. Dunedin: Otago University Press.
- Linklater W, Steer J. 2018. Predator free 2050: A flawed conservation policy displaces higher priorities and better, evidence-based alternatives. Conservation Letters. 11:e12593.
- Liu S, Walshe T, Long G, Cook D. 2012. Evaluation of potential responses to invasive non-native species with structured decision making. Conservation Biology. 26:539–546.
- Lubell M, Jasny L, Hastings A. 2017. Network governance for invasive species management. Conservation Letters. 10:699–707.
- Luo Y, Ogle K, Tucker C, Fei S, Gao C, LaDeau S, Clark JS, Schimel DS. 2011. Ecological forecasting and data assimilation in a data-rich era. Ecological Applications. 21:1429–1442.
- Lyver POB, Akins A, Phipps H, Kahui V, Towns DR, Moller H. 2016. Key biocultural values to guide restoration action and planning in New Zealand. Restoration Ecology. 24:314–323.
- Lyver POB, Ruru J, Scott N, Tylianakis JM, Arnold J, Malinen SK, Bataille CY, Herse MT, Jones CJ, Gormley AM, et al. 2019a. Building biocultural approaches into Aotearoa – New Zealand’s conservation future. Journal of the Royal Society of New Zealand. 49(3):394–411. doi:10.1080/03036758.2018.1539405.
- Lyver POB, Taputu M, Kutia ST, Tahi B. 2008. Tūhoe Tuawhenua mātauranga of kererū (Hemiphaga novaseelandiae novaseelandiae) in Te Urewera. New Zealand Journal of Ecology. 32:7–17.
- Lyver POB, Timoti P, Davis T, Tylianakis JM. 2019b. Biocultural hysteresis inhibits adaptation to environmental change. Trends in Ecology and Evolution. (in press). https://doi.org/10.1016/j.tree.2019.04.002.
- Lyver POB, Timoti P, Gormley A, Jones CJ, Richardson SJ, Tahi BL, Greenhalgh S. 2017. Key Māori values strengthen the mapping of forest ecosystem services. Ecosystem Services. 27:92–102.
- Lyver PON, Wilmshurst JM, Wood JR, Jones CJ, Fromont M, Bellingham PJ, Stone C, Sheehan M, Moller H. 2015. Looking back for the future: local knowledge and palaeoecology inform bicultural restoration of coastal ecosystems in New Zealand. Human Ecology. 43:681–695.
- MacLeod CJ, Moller H. 2006. Intensification and diversification of New Zealand agriculture since 1960: An evaluation of current indicators of land use change. Agriculture, Ecosystems & Environment. 115:201–218.
- Madrian BC. 2014. Applying insights from behavioral economics to policy design. Annual Review of Economics. 6:663–688.
- Mahoney PJ, Young JK, Hersey KR, Larsen RT, McMillan BR, Stoner DC. 2018. Spatial processes decouple management from objectives in a heterogeneous landscape: predator control as a case study. Ecological Applications. 28:786–797.
- Mason NW, Peltzer DA, Richardson SJ, Bellingham PJ, Allen RB. 2010. Stand development moderates effects of ungulate exclusion on foliar traits in the forests of New Zealand. Journal of Ecology. 98:1422–1433.
- Mawyer A, Jacka JK. 2018. Sovereignty, conservation and island ecological futures. Environmental Conservation. 45:238–251.
- McGlone MS, Duncan RP, Heenan PB. 2001. Endemism, species selection and the origin and distribution of the vascular plant flora of New Zealand. Journal of Biogeography. 28:199–216.
- McWethy DB, Wilmshurst JM, Whitlock C, Wood JR, McGlone MS, Carcaillet C. 2014. A high-resolution chronology of rapid forest transitions following Polynesian arrival in New Zealand. PLoS One. 9:e111328.
- Meinzen-Dick R, DiGregorio M, McCarthy N. 2004. Methods for studying collective action in rural development. Agricultural Systems. 82:197–214.
- Merton D. 1992. The legacy of “Old Blue”. New Zealand Journal of Ecology. 16:65–68.
- Miskelly CM. 2018. Changes in the forest bird community of an urban sanctuary in response to pest mammal eradications and endemic bird reintroductions. Notornis. 65:132–151.
- Mols F, Haslam SA, Jetten J, Steffens NK. 2015. Why a nudge is not enough: A social identity critique of governance by stealth. European Journal of Political Research. 54:81–98.
- Moro D, Byrne M, Kennedy M, Campbell S, Tizard M. 2018. Identifying knowledge gaps for gene drive research to control invasive animal species: The next CRISPR step. Global Ecology and Conservation. 13:e00363.
- MPI. 2018. New Zealand Ministry for Primary Industry. https://www.teururakau.govt.nz/funding-and-programmes/forestry/planting-one-billion-trees/.
- Mulder CP, Grant-Hoffman MN, Towns DR, Bellingham PJ, Wardle DA, Durrett MS, Fukami T, Bonner KI. 2009. Direct and indirect effects of rats: does rat eradication restore ecosystem functioning of New Zealand seabird islands? Biological Invasions. 11:1671–1688.
- Neimiec RM, Pech RP, Norbury GL, Byrom AE. 2017. Landowners’ perspectives on coordinated, landscape-level invasive species control: the role of social and ecological context. Environmental Management. 59:477–489.
- New Zealand Government. 2014. Te Urewera Act 2014. public Act 2014 No 51. Wellington, New Zealand: New Zealand Government. 546 p.
- New Zealand government. 2016. https://www.beehive.govt.nz/release/new-zealand-be-predator-free-2050.
- Niemiec RM, Ardoin NM, Wharton CB, Asner GP. 2016. Motivating residents to combat invasive species on private lands: social norms and community reciprocity. Ecology and Society. 21:30–40.
- Noble C, Adlam B, Church GM, Esvelt KM, Nowak MA. 2018. Current CRISPR gene drive systems are likely to be highly invasive in wild populations. eLife. 7:e33423.
- Noble C, Olejarz J, Esvelt KM, Church GM, Nowak MA. 2017. Evolutionary dynamics of CRISPR gene drives. Science Advances. 3:e1601964.
- Norbury G. 2001. Conserving dryland lizards by reducing predator-mediated apparent competition and direct competition with introduced rabbits. Journal of Applied Ecology. 38:1350–1361.
- Norbury GL, Byrom AE, Pech RP, Smith J, Clarke D, Anderson DP, Forrester G. 2013. Invasive mammals and habitat modification interact to generate unforeseen outcomes for indigenous fauna. Ecological Applications. 23:1707–1721.
- Norbury G, Heyward R, Parkes J. 2009. Skink and invertebrate abundance in relation to vegetation, rabbits and predators in a New Zealand dryland ecosystem. New Zealand Journal of Ecology. 33:24–31.
- Norbury G, Jones C. 2015. Pests controlling pests: does predator control lead to greater European rabbit abundance in Australasia? Mammal Review. 45:79–87.
- Norbury GL, Pech RP, Byrom AE, Innes J. 2015. Density-impact functions for terrestrial vertebrate pests and indigenous biota: guidelines for conservation managers. Biological Conservation. 191:409–420.
- Norton DA, Young LM, Byrom AE, Clarkson BD, Lyver POB, McGlone MS, Waipara NW. 2016. How do we restore New Zealand's biological heritage by 2050? Ecological Management and Restoration. 17:170–179.
- Nugent G. 2011. Maintenance, spillover and spillback transmission of bovine tuberculosis in multi-host wildlife complexes: a New Zealand case study. Veterinary Microbiology. 151:34–42.
- Nugent G, Fraser W, Sweetapple P. 2001. Top down or bottom up? Comparing the impacts of introduced arboreal possums and ‘terrestrial’ruminants on native forests in New Zealand. Biological Conservation. 99:65–79.
- Nuthall PL, Old KM. 2017. Will future land based food and fibre production be in family or corporate hands? An analysis of farm land ownership and governance considering farmer characteristics as choice drivers. The New Zealand case. Land Use Policy. 63:98–110.
- O'Donnell CF, Hoare JM. 2012. Quantifying the benefits of long-term integrated pest control for forest bird populations in a New Zealand temperate rainforest. New Zealand Journal of Ecology. 36:131–140.
- O’Dowd DJ, Green PT, Lake PS. 2003. Invasional ‘meltdown’ on an oceanic island. Ecology Letters. 6:812–817.
- Ogilvie SC, Ataria JM, Waiwai J, Doherty J. 2007. Creating and publicizing a web-based database of 1080 and taonga species information. Lincoln University Wildlife Management Report No. 43, Lincoln University, Lincoln, New Zealand; 29 p.
- Omondiagbe HA, Towns DR, Wood JK, Bollard-Breen B. 2017. Stakeholders and social networks identify potential roles of communities in sustainable management of invasive species. Biological Invasions. 19:3037–3049.
- Orange C. 2011. The Treaty of Waitangi. Wellington, New Zealand: Bridget Williams Books. 356 p.
- Owens B. 2017. The big cull: Behind New Zealand’s wild plan to purge all pests. Nature. 541:148–150.
- Parkes JP, Byrom AE, Edge KA. 2017b. Eradicating mammals on New Zealand island reserves: what is left to do? New Zealand Journal of Ecology. 41:263–270.
- Parkes J, Murphy E. 2003. Management of introduced mammals in New Zealand. New Zealand Journal of Zoology. 30:335–359.
- Parkes JP, Nugent G, Forsyth DM, Byrom AE, Pech RP, Warburton B, Choquenot D. 2017a. Past, present and two potential futures for managing New Zealand’s mammalian pests. New Zealand Journal of Ecology. 41:151–161.
- Pauchard A, Meyerson LA, Bacher S, Blackburn TM, Brundu G, Cadotte MW, Courchamp F, Essl F, Genovesi P, Haider S, et al. 2018. Biodiversity assessments: Origin matters. PLoS Biology. 16:e2006686.
- PCE. 2011. Evaluating the use of 1080: predators, poisons and silent forests. Parliamentary Commissioner for the Environment report. New Zealand Government publication, Wellington, New Zealand; p. 1–85.
- PCE. 2017. Taonga of an island nation: Saving New Zealand's birds. Parliamentary Commissioner for the Environment report. New Zealand Government publication, Wellington, New Zealand; p. 1–139.
- Pech R, Maitland M. 2016. Conservation of native fauna in highly invaded systems: managing mammalian predators in New Zealand. Restoration Ecology. 24:816–820.
- Pejchar L, Mooney HA. 2009. Invasive species, ecosystem services and human well-being. Trends in Ecology and Evolution. 24:497–504.
- Peltzer DA, Allen RB, Bellingham PJ, Richardson SJ, Wright EF, Knightbridge PI, Mason NWH. 2014. Disentangling drivers of tree population size distributions. Forest Ecology and Management. 331:165–179.
- Peltzer DA, Allen RB, Lovett GM, Whitehead D, Wardle DA. 2010. Effects of biological invasions on forest carbon sequestration. Global Change Biology. 16:732–746.
- Perry GL, Wheeler A, Wood JR, Wilmshurst JM. 2014a. A high-precision chronology for the rapid extinction of New Zealand moa (Aves, Dinornithiformes). Quaternary Science Reviews. 105:126–135.
- Perry GL, Wilmshurst JM, McGlone MS. 2014b. Ecology and long-term history of fire in New Zealand. New Zealand Journal of Ecology. 38:157–176.
- Peters MA, Hamilton D, Eames C. 2015. Action on the ground: a review of community environmental groups’ restoration objectives, activities and partnerships in New Zealand. New Zealand Journal of Ecology. 39:179–189.
- Pickett ST, Cadenasso ML, Grove JM, Nilon CH, Pouyat RV, Zipperer WC, Costanza R. 2001. Urban ecological systems: linking terrestrial ecological, physical, and socioeconomic components of metropolitan areas. Annual Review of Ecology and Systematics. 32:127–157.
- Pickrell J. 2019. Rat eradication launched on populated island. Science. 364:915–916.
- Polis GA, Holt RD. 1992. Intraguild predation: The dynamics of complex trophic interactions. Trends in Ecology and Evolution. 7:151–154.
- Pollard CRJ, Redpath S, Bussière LF, Keane Aidan, Thompson DBA, Young JC, Bunnefeld N, Lee TM. 2019. The impact of uncertainty on cooperation intent in a conservation conflict. Journal of Applied Ecology. 56:1278–1288.
- Provan KG, Kenis P. 2007. Modes of network governance: structure, management, and effectiveness. Journal of Public Administration Research and Theory. 18:229–252.
- Rands MR, Adams WM, Bennun L, Butchart SH, Clements A, Coomes D, Entwistle A, Hodge I, Kapos V, Scharlemann JP, et al. 2010. Biodiversity conservation: challenges beyond 2010. Science. 329:1298–1303.
- Rayner MJ, Hauber ME, Imber MJ, Stamp RK, Clout MN. 2007. Spatial heterogeneity of mesopredator release within an oceanic island system. Proceedings of the National Academy of Sciences of the USA. 104:20862–20865.
- Redpath SM, Young J, Evely A, Adams WM, Sutherland WJ, Whitehouse A, Amar A, Lambert RA, Linnell JD, Watt A, et al. 2013. Understanding and managing conservation conflicts. Trends in Ecology and Evolution. 28:100–109.
- Reijula S, Kuorikoski J, Ehrig T, Katsikopoulos K, Sunder S. 2018. Nudge, Boost, or Design? Limitations of behaviorally informed policy under social interaction. Journal of Behavioral Economics for Policy. 2:99–105.
- Richmond ME. 2018. Glyphosate: A review of its global use, environmental impact, and potential health effects on humans and other species. Journal of Environmental Studies and Sciences. 8:416–434.
- Ringler D, Russell JC, Le Corre M. 2015. Trophic roles of black rats and seabird impacts on tropical islands: mesopredator release or hyperpredation? Biological Conservation. 185:75–84.
- Ripple WJ, Beschta RL. 2012. Trophic cascades in Yellowstone: The first 15 years after wolf reintroduction. Biological Conservation. 145:205–213.
- Ritchie EG, Johnson CN. 2009. Predator interactions, mesopredator release and biodiversity conservation. Ecology Letters. 12:982–998.
- Roberts M, Norman W, Minhinnick N, Wihongi D, Kirkwood C. 1995. Kaitiakitanga: Māori perspectives on conservation. Pacific Conservation Biology. 2:7–20.
- Robertson HA, Ausseil A, Range B, Betts H, Pomeroy E. 2019. Loss of wetlands since 1990 in Southland, New Zealand. New Zealand Journal of Ecology. 43. in press.
- Rouco C, de Torre-Ceijas R, Martín-Collado D, Byrom AE. 2017. New Zealand shouldn’t ignore feral cats. BioScience. 67:686.
- Ruffell J, Didham RK. 2017. Conserving biodiversity in New Zealand’s lowland landscapes: does forest cover or pest control have a greater effect on native birds? New Zealand Journal of Ecology. 41:23–33.
- Ruru J. 2004. Managing our treasured home: the conservation estate and the principles of the Treaty of Waitangi. New Zealand Journal of Environmental Law. 8:243–266.
- Ruru J, Lyver POB, Scott N, Edmunds D. 2017. Reversing the decline in New Zealand’s biodiversity: Empowering Māori within reformed conservation law. Policy Quarterly. 13:65–71.
- Ruscoe WA, Ramsey DSL, Pech RP, Sweetapple PJ, Yockney I, Barron MC, Perry M, Nugent G, Carran R, Warne R, et al. 2011. Unexpected consequences of control: competitive vs. predator release in a four-species assemblage of invasive mammals. Ecology Letters. 14:1035–1042.
- Russell JC. 2011. Indirect effects of introduced predators on seabird islands. In: Mulder CP, Anderson WB, Towns DR, Bellingham PJ, editors. Seabird islands: ecology, invasion, and restoration. Oxford: Oxford University Press; p. 261–279.
- Russell JC, Broome KG. 2016. Fifty years of rodent eradications in New Zealand: another decade of advances. New Zealand Journal of Ecology. 40:197–204.
- Russell JC, Innes JG, Brown PH, Byrom AE. 2015. Predator-Free New Zealand: conservation country. BioScience. 65:520–525.
- Russell JC, Stanley MC. 2018. An overview of introduced predator management in inhabited landscapes. Pacific Conservation Biology. 24:371–378.
- Russell JC, Taylor CN. 2019. Strategic environmental assessment for invasive species management on inhabited islands. Island invasives: scaling up to meet the challenge 62:692–697. In: Veitch R, Clout MN, Martin AR, Russell JC, West CJ, editor. Island invasives: scaling up to meet the challenge. Occasional Paper SSC no. 62. Gland, Switzerland: IUCN.
- Russell JC, Taylor CN, Aley JP. 2018. Social assessment of inhabited islands for wildlife management and eradication. Australasian Journal of Environmental Management. 25:24–42.
- Schlaepfer MA. 2018. Do non-native species contribute to biodiversity? PLOS Biology. 16:e2005568.
- Scofield RP, Cullen R, Wang M. 2011. Are predator-proof fences the answer to New Zealand's terrestrial faunal biodiversity crisis? New Zealand Journal of Ecology. 35:312–317.
- Shepherd JD, Gillingham S, Heuer T, Barron MC, Byrom AE, Pech RP. 2018. Multi-scale dynamic maps for the management of invading and established wildlife populations: brushtail possums in New Zealand. Wildlife Research. 45:336–343.
- Simberloff D, Martin JL, Genovesi P, Maris V, Wardle DA, Aronson J, Courchamp F, Galil B, García-Berthou E, Pascal M, et al. 2013. Impacts of biological invasions: what’s what and the way forward. Trends in Ecology and Evolution. 28:58–66.
- Smith RK, Pullin AS, Stewart GB, Sutherland WJ. 2010. Effectiveness of predator removal for enhancing bird populations. Conservation Biology. 24:820–829.
- Solomon M. 2014. Locked out of National Parks, A call to action from Kaiwhakahaere Ta Mark Solomon. Te Karaka (http://ngaitahu.iwi.nz/our_stories/locked-national-parks/).
- Sparling GP, Lewis R, Schipper LA, Mudge P, Balks M. 2014. Changes in soil total C and N contents at three chronosequences after conversion from plantation pine forest to dairy pasture on a New Zealand Pumice soil. Soil Research. 52:38–45.
- Statistics New Zealand. 2018. https://www.stats.govt.nz/news/migration-drives-local-population-growth.
- Steffen W, Persson Å, Deutsch L, Zalasiewicz J, Williams M, Richardson K, Crumley C, Crutzen P, Folke C, Gordon L, et al. 2011. The Anthropocene: from global change to planetary stewardship. Ambio. 40:739–745.
- Suckling DM, Sforza RFH, Marion-Poll F. 2014. What magnitude are observed non-target impacts from weed biocontrol? PLoS One. 9:e84847.
- Swan CM, Pickett ST, Szlavecz K, Warren P, Willey KT. 2011. Biodiversity and community composition in urban ecosystems: coupled human, spatial, and metacommunity processes. Handbook of urban ecology. New York: Oxford University Press. pp.179–186.
- Tanentzap AJ, Lloyd KM. 2017. Fencing in nature? predator exclusion restores habitat for native fauna and leads biodiversity to spill over into the wider landscape. Biological Conservation. 214:119–126.
- The Guardian. 2016. https://www.theguardian.com/environment/2016/feb/09/trouble-in-paradise-lord-howe-island-divided-over-plan-to-exterminate-rats
- Thoresen JJ, Towns D, Leuzinger S, Durrett M, Mulder CP, Wardle DA. 2017. Invasive rodents have multiple indirect effects on seabird island invertebrate food web structure. Ecological Applications. 27:1190–1198.
- Timoti P, Lyver POB, Matamua R, Jones CJ, Tahi BL. 2017. A representation of a Tuawhenua worldview guides environmental conservation. Ecology and Society. 22:20. [online] URL:https://www.ecologyandsociety.org/vol22/iss4/art20/.
- Tompkins DM, Byrom AE, Pech RP. 2013. Predicted responses of invasive mammal communities to climate-related changes in mast frequency in forest ecosystems. Ecological Applications. 23:1075–1085.
- Tompkins DM, Veltman CJ. 2006. Unexpected consequences of vertebrate pest control: predictions from a four-species community model. Ecological Applications. 16:1050–1061.
- Towns DR. 2009. Eradications as reverse invasions: lessons from Pacific rat (Rattus exulans) removals on New Zealand islands. Biological Invasions. 11:1719–1733.
- Towns DR, Atkinson IA, Daugherty CH. 2006. Have the harmful effects of introduced rats on islands been exaggerated? Biological Invasions. 8:863–891.
- Towns DR, Bellingham PJ, Mulder CP, Lyver POB. 2012. A research strategy for biodiversity conservation on New Zealand’s offshore islands. New Zealand Journal of Ecology. 36:1–20.
- Tylianakis JM, Didham RK, Bascompte J, Wardle DA. 2008. Global change and species interactions in terrestrial ecosystems. Ecology Letters. 11:1351–1363.
- van Heezik Y, Smyth A, Adams A, Gordon J. 2010. Do domestic cats impose an unsustainable harvest on urban bird populations? Biological Conservation. 143:121–130.
- Van Vianen J, Burge OR, MacFarlane AT, Kelly D. 2018. The effects of single aerial 1080 possum-control operations on common forest birds in the South Island, New Zealand. New Zealand Journal of Ecology. 42(2). in press.
- Vilà M, Hulme PE. 2017. Impact of biological invasions on ecosystem services. Cham, Switzerland: Springer.
- Vitousek PM, Dantonio CM, Loope LL, Westbrooks R. 1996. Biological invasions as global environmental change. American Scientist. 84:468–478.
- Vitousek PM, D’Antonio CM, Loope LL, Rejmanek M, Westbrooks R. 1997. Introduced species: a significant component of human-caused global change. New Zealand Journal of Ecology. 21:1–16.
- Wallach AD, Ripple WJ, Carroll SP. 2015. Novel trophic cascades: apex predators enable coexistence. Trends in Ecology and Evolution. 30:146–153.
- Wallis GP, Trewick SA. 2009. New Zealand phylogeography: evolution on a small continent. Molecular Ecology. 18:3548–3580.
- Wardle DA, Bardgett RD, Callaway RM, Van der Putten WH. 2011. Terrestrial ecosystem responses to species gains and losses. Science. 332:1273–1277.
- Wardle DA, Bellingham PJ, Fukami T, Mulder CPH. 2007. Promotion of ecosystem carbon sequestration by invasive predators. Biology Letters. 3:479–482.
- Wardle DA, Peltzer DA. 2017. Impacts of invasive biota in forest ecosystems in an aboveground–belowground context. Biological Invasions. 19:3301–3316.
- Wilkinson R, Fitzgerald G. 1997. Public perceptions of biological control of rabbits in New Zealand: some ethical and practical issues. Agriculture and Human Values. 14:273–282.
- Willis KJ, Birks HJB. 2006. What is natural? The need for a long-term perspective in biodiversity conservation. Science. 314:1261–1265.
- Wilmshurst JM, Anderson AJ, Higham TF, Worthy TH. 2008. Dating the late prehistoric dispersal of Polynesians to New Zealand using the commensal Pacific rat. Proceedings of the National Academy of Sciences. 105:7676–7680.
- Wilmshurst JM, Moar NT, Wood JR, Bellingham P, Findlater A, Robinson J, Stone C. 2014. Use of Pollen and Ancient DNA as conservation baselines for offshore islands in New Zealand. Conservation Biology. 28:202–212.
- Wilson N, McIntyre M, Blaschke P, Muellner P, Mansoor OD, Baker MG. 2018. Potential public health benefits from eradicating rats in New Zealand cities and a tentative research agenda. Journal of the Royal Society of New Zealand. 48:280–290.
- Wood JR. 2013. New Zealand, 500 years ago. In: MacLeod N, editor. Grzimek’s Animal Life Encyclopedia: Extinction. Farmington Hills, USA: Gale; p. 595–604.
- Wood JR, Alcover JA, Blackburn TM, Bover P, Duncan RP, Hume JP, Louys J, Meijer HJ, Rando JC, Wilmshurst JM. 2017. Island extinctions: processes, patterns, and potential for ecosystem restoration. Environmental Conservation. 44:348–358.
- Wood JR, Wilmshurst JM, Worthy TH, Holzapfel AS, Cooper A. 2012. A lost link between a flightless parrot and a parasitic plant and the potential role of coprolites in conservation paleobiology. Conservation Biology. 26:1091–1099.
- Worthy TH, Holdaway RN. 2002. Lost world of the moa. Christchurch New Zealand: Canterbury University Press.
- Yletyinen J, Brown P, Pech R, Byrom A, Gendig E, Hodges D, Hulme PE, Malcolm T, Maseyk F, Peltzer DA, et al. 2019. Understanding and managing social-ecological tipping points in primary industries. BioScience. 69:335–347.
