ABSTRACT
Probiotics for preventing illness or improving health have become increasingly popular with consumers, researchers, and producers, both dairy companies and alternative medicine providers. They are widely available in foods such as yoghurt and as freeze dried capsules. They are used both for their general health effects on the gut including reducing travellers and antibiotic related diarrhoea. Their most important and best researched use is in the treatment of necrotising enterocolitis in premature babies where they reduce incidence, severity and mortality. The ultimate ‘probiotic’ mixture might be healthy human faeces which have been used for transplantation in patients with antibiotic resistant Clostridioides difficile infections with some success. Studies of allergy prevention using probiotics have shown mixed results. In New Zealand however, a specific strain of Lactobacillus rhamnosus (HN001) given from birth for two years has been shown to reduce the prevalence of allergic disease in infants throughout childhood. Further studies have shown a reduction in gestational diabetes amongst mothers who consume the probiotic during pregnancy. An important feature of the clinical use of probiotics is that different strains within the same species may have very different effects.
Introduction
Some 2,500 papers on a variety of probiotic topics are currently published annually. The purpose of this article is not to provide a detailed or extensive review of probiotics (there have been at least 3000 in the last five years), but rather to:
Outline a brief history of their use in human health
Explore some of the more controversial strands of the evidence supporting the clinical effectiveness, of probiotics in the prevention or treatment of two human gut diseases, necrotising enterocolitis and Clostridiodes difficile infection.
Outline the New Zealand experience with two probiotics in the prevention of allergic disease and gestational diabetes, Definitions
Probiotics are predominantly (but not exclusively) gram-positive bacterial microorganisms from the genus Lactobacillus or Bifidobacterium and many of their species occur naturally in mammalian milk and as commensals in body microbiomes, but predominantly in the gut. They are facultative anaerobes and use lactose or milk oligosaccharides as their major substrates, producing lactic acid, which tends to inhibit the growth of other bacteria. Many species of these two genera are considered as probiotics, with some 200 species of Lactobacillus (Salvetti et al. Citation2018) and more than 420 species of Bifidobacterium (Odamaki et al. Citation2018) described and sequenced, though more are being identified each year. Within each species there are a large number of stable genetic variants or strains with specific probiotic effects sometimes limited to specific strains within a species.
It is worth considering yoghurt at this point given that it was the interest in yoghurt that spawned interest in probiotics. Commercial yoghurt is made from milk fermentation by only two species of bacteria, Lactobacillus delbrueckii subspecies bulgaricus (the organism originally described by Grigorov (see below) and popularised by Mechnikov) and Streptococcus thermophilus, these two organisms being capable of producing the appropriate firm consistency and the required acidity for both preservation and gel formation (Panagiotis and Constatnina Citation2014). It is largely the partially denatured milk proteins (denatured by both heating during preparation and by acidification from the lactic acid fermentation) that gives yoghurt its gel structure. Other organisms deemed probiotics are often added to yoghurt following the fermentation but play no part in the actual yoghurt fermentation itself (Panagiotis and Constatnina Citation2014). Many other fermented milk products are used in various communities such as Kefir and Filmjolk and there are many fermented foods such as sauerkraut, miso and fermented fish. Some of these would be considered as foods with probiotic properties for which some health benefit might be obtained.
Brief history
Modern interest in probiotics is often traced to Ilya Mechnikov, working at the Pasteur Institute. Latterly, Mechnikov developed a theory that aging was associated with ‘toxic’ bacteria in the human gut and could be ameliorated by the regular ingestion of lactic acid bacteria. The theory arose from his observation of many centenarians in mountain villages in Eastern Europe whose diet relied heavily on milk products fermented with a specific microbe, Lactobacillus bulgaricus, (yoghurt) identified in 1905 (Grigoroff Citation1905) by the Bulgarian microbiologist Stamen Grigorov, and to which Mechnikov attributed their longevity (Mechnikov Citation1907). Fermented milk though has a much older and important history that dates to the dawn of dairying in agriculture and has been recently investigated with modern chemical analytics. Milk use in human nutrition has been dated to at least 7000 BC in south east Europe (Evershed et al. Citation2008) and preserved milk products to ∼5000 BC (Salque et al. Citation2013). Milk from domestic animals would have been an important year round food source that did not require the animals to be slaughtered. Milk as a food presented two major problems. Firstly it could not be stored for any length of time and secondly many individuals could not metabolise milk lactose as the lactase enzyme mechanism was switched off after weaning in the majority of individuals. It has been suggested that individuals who were able to metabolise lactose into adulthood experienced a significant survival advantage and hence a selective pressure for their survival over those who were lactose intolerant (Simoons Citation1981). However, archaeological evidence suggests that milk was being processed into cheese as early as 5000 BC (Evershed et al. Citation2008). These fermented product would be suitable for long term storage and could be consumed by lactose intolerant individuals, as most of the lactose is metabolised during the fermentation. Much of the evidence for this comes from the analysis of residues found on perforated pottery shards that were deliberately used as sieves in milk processing. Fatty acid residues on the shards that have been found in Europe, the near East and North Africa, with carbon signatures commonly found in various animal milks (Salque et al. Citation2013). Thus the consumption of fermented milk product has a long human history at least amongst some communities.
Probiotics and human health
Firstly, probiotics have almost exclusively been used in the clinical setting for maintenance of ‘good’ health or in prevention of specific conditions rather than for the treatment of established disease. Secondly, and not surprisingly, the major uses and hence studies, have been in the area of preventing or modifying gastrointestinal disorders. More recently studies have explored probiotic effects on immunological conditions, particularly allergic disease, and metabolic disorders such as obesity and type 2 diabetes. The International Scientific Association for Probiotics and Prebiotics in their 2014 consensus statement summarised the scope and many of the clinical uses of probiotics according to specificity and frequency of probiotic effects (Hill et al. Citation2014) ().
Figure 1. A hierarchy of probiotic effects. (from Hill et al. Citation2014 with permission).
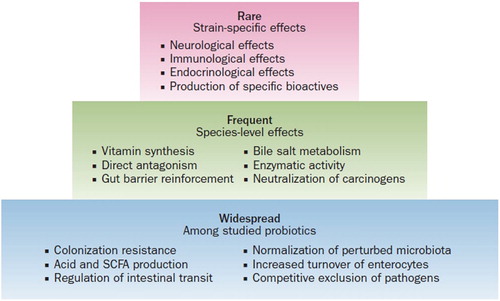
This summary of effects which essentially comes down to those associated with altering the gut bacterial milieu, described above as ‘Widespread’, those associated with specific host metabolic benefits described as ‘Frequent’ and those that appear strain specific and are associated with some special metabolic function, ‘Rare’. Thus the widespread bacterial effects apply to many species and benefits are likely to be greater with cocktails of organisms, (see Necrotising Enterocolitis (NEC) below). Whereas effects on the immune system, gut brain interactions and endocrine effects appear very strain specific and suggest some specific metabolic pathway might be involved in exerting the clinical effect. Having said that the mechanisms by which probiotics exert health effects are poorly understood.
Probiotics are marketed as health foods usually in the form of capsule supplements or as additions to formula milks or most commonly in the form of yoghurt or probiotic drinks. They are marketed directly to consumers and are generally available as over the counter products. They are not subject to the same stringent production requirements or quality assurance as pharmaceuticals (van den Berg et al. Citation2013) and in most jurisdictions do not carry any claims for therapeutic efficacy.
Gastrointestinal disorders
One of the most common reasons that consumers use probiotics is to maintain and improve their gut health. This may be because of non-specific symptoms such as Irritable Bowel Syndrome, for antibiotic related diarrhoea, traveller’s diarrhoea or non-specific infective episodes of diarrhoea, or simply taken in the belief that probiotics will maintain a healthy gut microbiota, though as yet it is unknown exactly what a healthy microbiota should look like, although diversity, richness and microbial resilience are thought to be important features for health (Tuddenham and Sears Citation2015). Ritchie and Romanuk, undertook a meta-analysis of eight gastrointestinal conditions (Pouchitis, Infectious diarrhoea, Irritable Bowel Syndrome, Helicobacter pylori, Clostridiodes difficile Disease, Antibiotic Associated Diarrhoea, Traveller’s Diarrhoea, and Necrotizing Enterocolitis (NEC)) involving 11 probiotic species in 74 studies of more than 10,000 subjects (Ritchie and Romanuk Citation2012). They found a significant effect across all eight conditions, pooled RR 0.59 (CI 0.51–0.65). They also observed a positive effect of the length of probiotic treatment. Interestingly when looking separately at individual conditions they found that Traveller’s Diarrhoea and NEC did not show a significant effect of probiotics.
Necrotizing enterocolitis
Necrotizing enterocolitis (NEC), a condition mainly seen in preterm infants, whereby part of the immature infant gut suffers serious mucosal damage, often leading to perforation and sometimes septicaemia has been the most controversial probiotic sensitive disorder over the last decade and highlights many of the problems associated with the uptake of probiotics in the clinical setting. NEC in very preterm infants (<32 weeks gestation) occurs in 5%–10% of cases, is inversely related to prematurity, and is associated with a ∼20%–30% all-cause mortality. It occurs some days after birth and is thought in part to be associated with inappropriate gut colonisation suggesting a condition entirely appropriate for probiotic intervention (Neu and Pammi Citation2018). Over the last decade there have been many clinical trials of probiotics in NEC, observational studies and experimental animal studies and to date 25 systematic reviews and meta-analyses involving ∼12,000 infants. Pooling data from all of these reviews suggests that the development of NEC is reduced by about 50%, and all-cause mortality by at least 20% with the provision of probiotics(Athalye-Jape and Patole Citation2019). The observational studies show similar protective effects as do the experimental animal intervention studies. Yet probiotics have not been regularly taken up in the management of premature babies. For example, in 2014 a national UK survey of neonatal units showed that only 12% of units were using probiotics to prevent NEC, a repeat survey in 2018 revealed that only 17% were using them despite the evidence of benefit from systematic reviews and meta analyses (Duffield and Clarke Citation2019). Part of the UK reluctance to use probiotics stemmed from a well conducted, large (1315 infants) completely null UK study (the PiPS study) published in 2016 which showed no effect on sepsis or mortality, contrary to the prevailing Randomised Controlled Trials (RCT) evidence (Costeloe et al. Citation2016). In correspondence following publication of the trial a number of issues common to probiotic studies were raised, the most important of which was that the species and strain are critically important, an issue that is important for all studies and complicates all of the meta analytic reviews of different species and strains. A single strain Bifidobacterium breve BBG-01, was used in the PiPS study which simply may not be effective whereas many of the RCT’s had used multiple species. Part of the problem was that many studies have used probiotics that are not manufactured or quality assured to a standard that would allow regulatory approval for a study, at least in the UK. Other possible reasons for the negative results included cross contamination with the probiotic such that it was recovered in almost half of the placebo infants though almost no other studies had even looked at this. Lastly, the study despite being the largest RCT to date was probably underpowered (Christopher et al. Citation2016). Interestingly, a secondary analysis of the data with adjustment comparing infants that were colonised versus those who weren’t did show benefit for all three outcomes, NEC, sepsis and mortality but was not significant. Similarly, a large observational cohort study in the US failed to show a protective effect of Lactobacillus rhamnosus GG in NEC (Kane et al. Citation2018). In contrast studies using combinations of probiotics such as Lactobacillus acidophilus and Bifidobacterium infantis marketed as InfloranTM show strong protective effects (Underwood Citation2019).
So, for the single most important probiotic sensitive disease most would consider that the jury is now in (some authors have been very emphatic that they should be used routinely (Athalye-Jape and Patole Citation2019)) but the verdict is not unanimous and translation far from comprehensive. The use of probiotics is supported by the most recent Cochrane review (AlFaleh and Anabrees Citation2014) and in Germany (and New Zealand) for example most premature infants now receive a probiotic cocktail (Härtel et al. Citation2014).
The problems with the use of probiotics in NEC and many other disorders include which species and within species, which strains, how much in terms of cfu’s, and assurances regarding the quality of the probiotic powders. While all of the RCT’s so far reported including the negative PiPS study have not shown any adverse effects at least one fatal fungal contamination of a probiotic supplement has been reported (Vallabhaneni et al. Citation2015) and a reported case of septicaemia with a treatment probiotic Bifidobacterium longum, strain infantis (Jenke et al. Citation2012). The increased exclusive use of human milk in this setting has also had a strong positive effect on reducing NEC and on survival (Talavera et al. Citation2016).
Clostridiodes difficile infection
Less controversial than NEC in terms of outcomes, but technically more challenging and currently the subject of much research interest, is faecal microbiota transplantation (FMT). Such ‘probiotic’ treatment might be considered the ultimate in such therapy (Mackowiak Citation2013). FMT has a long history first described in China in the fourth century. Bedouin were known to consume camel dung for dysenteric illnesses. During World War 2 in Africa, German soldiers were successfully treated for dysentery with Bacillus subtilis isolated from camel faeces following the observation that local Bedouin gained considerable benefit from consuming their own camel’s dung (de Groot et al. Citation2017). The first reported modern usage was in 1958 when it was used to successfully treat C. difficile infection (Eiseman et al. Citation1958). Infection with C. difficile which may become chronic and highly resistant to antibiotics and gives rise to considerable symptomatology often arises from the disturbed microbiota induced by previous broad spectrum antibiotic therapy. FMT has proved spectacularly successful in treating chronic C. difficile infections, especially when repeated FMT’s are administered. A recent systematic review of five RCT’s (284 patients) using fresh faecal transplantation showed a significant benefit of FMT in eradicating C. difficile, RR 0.41 (95% CI, 0.22–0.74) compared to placebo or vancomycin. The best route of administration, oral via nasogastric tube or as freeze dried capsulated faecal material, or colonic, is yet to be firmly established. Studies of FMT in Inflammatory Bowel Disease are ongoing with some preliminary evidence that they may be helpful in promoting remission.
Immunological effect – allergic disease
The first studies of probiotics and their effects on immunity suggested that they could enhance immunoglobulin production in germ free animals with differential effects of live and heat killed organisms (Bloksma et al. Citation1979) and that yoghurt cultures without additional probiotics in vitro could potentiate the production of the Th1 cytokine Interferon-γ (IFN- γ) (Desimone et al. Citation1986).
One of the first clinical studies saw healthy young and older adults randomised to three groups, live yoghurt (base culture only), pasteurised yoghurt and no yoghurt, consumed for one year. The study showed no effects on IgE or IFN- γ but in both age groups a significant effect of live yoghurt on self-reported symptoms of allergic rhinitis (Trapp et al. Citation1993). A later study amongst 15 adults with moderately severe asthma showed that one month of yoghurt plus L. acidophilus led to a borderline increase in IFN- γ from stimulated lymphocytes and a non-significant decrease in blood eosinophils but no change in peak flow, spirometry or quality of life measures (Wheeler et al. Citation1997).
Probiotics for eczema prevention
In 2001 the first small RCT of probiotics in the prevention of eczema amongst newborns was published (Kalliomaki et al. Citation2001). The study randomised 159 infants with a family history of allergic disease to placebo or 1 × 1010 L. rhamnosus GG (LGG) daily. Mothers took the capsules daily for 4 weeks before delivery and while they breast fed their infants. If the infants were not breast fed then they received the probiotic daily in a water slurry for 6 months. By 2 years there was a 50% reduction in eczema prevalence in the probiotic group with no difference in total IgE, or positive skin prick tests for atopy. The authors suggested that the probiotic might help reverse gut permeability, thereby reducing allergen exposure, and alter gut microflora in a way that might reduce Th2 mediated immunity. Since then there have been at least four further RCT’s of this specific strain of L. rhamnosus GG exploring effects on eczema, asthma/wheeze and allergic rhinitis analysed last year in a meta-analysis (Szajewska and Horvath Citation2018). Overall the study found no reduction in eczema with LGG supplementation and no reduction in asthma/wheeze or allergic rhinitis.
There have now been at least four systematic reviews and meta-analyses of probiotics in the prevention of infant eczema. All four (each containing many of the same studies) have shown a significant protective effect of probiotic supplementation for the development of eczema (Mansfield et al. Citation2014; Cuello-Garcia et al. Citation2015; Zuccotti et al. Citation2015; Li et al. Citation2018). The most recent meta-analysis and systematic review, by Lin et al published in 2018, examined 28 studies of probiotics given to mothers, infants or both, to prevent eczema using a variety of probiotics including combinations. They found an overall protective effect using a random effects model, OR 0.68, (95%CI 0.58–0.82, p < 0.0001). They noted significant heterogeneity in the studies and that studies that had given the probiotics to both mother and infant were protective. They also observed the protective effects in high risk and normal risk infants. In the reviews that also explored asthma/wheeze and allergic rhinitis no significant protective effects were observed for these outcomes. Despite this evidence, clinicians and various Societies and Academies have been reluctant to recommend their use to help prevent eczema. Only the World Allergy Organisation supports the use of probiotics for infants at high risk of developing eczema (Fiocchi et al. Citation2015). The problem (as discussed above) is predominantly the uncertainty regarding which species and within a species, which strain or strains are effective. There is uncertainty around dose in terms of numbers of live organisms and duration and target (mother and/or child) of probiotic administration. These uncertainties have added to the heterogeneity of studies and reduced support from mainstream clinical groups for the use of probiotics.
Probiotics for prevention of food allergy
This aspect of probiotic use has not been explored mainly because proving food allergy requires a blind food challenge which is difficult to undertake and requires hospital attendance. A number of studies have looked at the effects of probiotics on food sensitisation which is a pre-requisite for IgE mediated food allergy and these have recently been reviewed (Zhang et al. Citation2016). A review of 17 trials with ∼3000 infants showed a protective effect of probiotics against food sensitisation when given to both mother and infant RR 0.77 (95% CI 0.61–0.98). One small study (31 participants) of oral peanut desensitisation combined with a probiotic supplement showed that 80% obtained a sustained unresponsiveness to peanut allergen (Tang et al. Citation2015). However there was no relevant control group with which to compare the effects of the additional probiotic. In a small study of infants with cow’s milk allergy treated with extensively hydrolysed casein formula the addition of Lactobacillus GG to the formula significantly increased the rate of tolerance to cow’s milk protein at 6 and 12 months of treatment (Canani et al. Citation2012).
New Zealand experience with Lactobacillus rhamnosus HN001 and Bifidobacterium lactis HN019 for the prevention of allergic disease
In the early 2000s a series of murine studies showed that two lactic acid producing probiotics L. rhamnosus HN001 and B. lactis HN019 (organisms genotyped by Fonterra Ltd) had effects on natural and acquired immunity in animal models. Both strains were capable of enhancing phagocytic activity and increasing IFN- γ from stimulated spleen cells and both enhanced serum antibody responses to both oral and systemically administered antigens (Gill et al. Citation2000). In a further murine study Cross et al showed that HN001 increased lymphocyte IFN- γ and Th2 cytokines (IL-12 and IL-18) in antigen sensitised BALB/c mice (Cross et al. Citation2002).
During 2004–5 we recruited 494 infants (at high risk of developing allergic disease as a result of a positive family history) in Wellington and Auckland and randomised them to three groups, to receive either L rhamnosus HN001, Bifidobacterium animalis subsp lactis HN019 (109 cfu’s) or placebo. Treatments were given daily to mothers for the last few weeks of pregnancy and until 6 months postpartum if breast feeding and daily to infants soon after birth, for two years. We have followed the children at 2, 4, 6, and 11 years with the main outcomes, eczema, wheezing and allergic rhinitis (Wickens et al. Citation2008; Wickens et al. Citation2012; Wickens et al. Citation2013; Wickens, Barthow, Mitchell, Kang, et al. Citation2018). We found a 40%–50% reduction in eczema which has persisted to age 11 in those taking HN001 with some evidence that this group have also experienced a reduction in wheezing, atopic sensitisation and allergic rhinitis,that has become more evident with age. shows hazard ratios for eczema, atopy and wheeze at the various study time points from 2 to 11 years, while summarises the cumulative prevalence over the whole 11 years. This study was unique amongst clinical trials of probiotics in two ways. Firstly, we gave the treatments to the infants for two years, all other studies have given them for 6 months or less. The rationale being that the peak incidence of eczema in infants is at 1 year and if the effect was transitory we wanted to cover the most vulnerable period. Secondly, we compared two different species of probiotic with placebo in the one study and showed very clearly that the Lactobacillus had a significant effect and the Bifidobacterium almost no effect.
Figure 2. Hazard Ratios and 95% CI, HN001 vs Placebo for eczema, atopy and wheeze to 2,4,6 and 11 years.
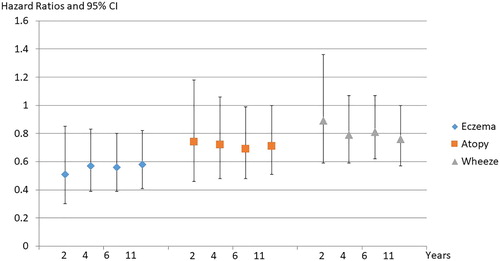
Table 1. Hazard ratios showing the associations between the probiotics HN001 and HN019 on the cumulative prevalence to 11 years of atopic sensitisation, eczema, wheeze and rhinitis.
In 2013–2014 we undertook a second RCT of L rhamnosus HN001, this time giving the probiotic (or placebo) to mothers only and much earlier in pregnancy (14–16 weeks) and while they were breast feeding for 6 months post-partum. Our interest was both maternal health (gestational diabetes, (GDM), vaginal infections and post-partum depression and anxiety) and infant eczema and allergy. HN001 given to the mother only had no effect on infant eczema or allergy at one year (Wickens, Barthow, Mitchell, Stanley, et al. Citation2018). HN001 reduced GDM (Wickens et al. Citation2017) and reduced post-partum depression and anxiety (Slykerman et al. Citation2017). GDM was non significantly reduced by 40% using the International definition of GDM (International Association of Diabetes and Pregnancy Study Groups Consensus Panel Citation2010) (13·8%–8·2%) and by 68% using the NZ definition (Ministry of Health Citation2014) (6·5%–2·1%), the latter effect reaching significance. The protective effect was greater amongst older women and those with a previous history of GDM and appeared to be reduced amongst women who had used antibiotics during pregnancy. We are currently looking at the possibilities of HN001 reducing the progression of pre-diabetes to Type 2 diabetes amongst adults with pre-diabetes. Interestingly, while HN001 had no effect on infant eczema when given to the mother only, daily consumption of yoghurt between 6 and 12 months of age by the infant was associated with significantly reduced eczema OR 0.22 (95% CI 0.11–0.47) and atopic sensitisation OR 0.27 (95% CI 0.11–0.66) at 12 months (Crane et al. Citation2018). Both probiotics are widely available commercially.
Safety of probiotics
In general, probiotics are considered safe and adverse effects have almost always been encountered by consumers who have some form of immune suppression or some structural vascular problem such as damaged heart valves. Cases of bacteraemia and endocarditis have been occasionally reported for probiotic bacteria. A recent systematic review of probiotic use in patients with cancer (25 studies with 2242 participants) showed 5 case reports with probiotic related bacteraemia or fungaemia and two deaths in the probiotic treated group though these deaths were unrelated to the probiotic (Hassan et al. Citation2018). In our own studies of HN001 and HN019 we have not found any evidence of adverse effects on growth, wheezing, or antibiotic treatment over the two years of the study (Dekker et al. Citation2009). It is perhaps a testament to probiotic safety that the many studies of cocktails of organisms given to premature infants who already have severe gastrointestinal and immunological problems show significantly improved survival. There are however two caveats. The first, from a randomised controlled trial of a cocktail of probiotics in patients with severe acute pancreatitis. Mortality was significantly higher (2.5 fold) in the treated group as was bowel ischaemia (Besselink et al. Citation2008). The second, is the ability of some probiotics to transfer antibiotic resistance genes to gut pathogens suggesting that potential probiotics should be screened for antibiotic resistance genes.
Conclusion
Fermented foods and their associated probiotics have been used by human populations for thousands of years, more recently in the form of freeze dried capsuled products. There is a large literature of variable quality that supports the use of a variety of probiotics for the treatment and prevention of a number of conditions. Arguably, the prevention of NEC by probiotics is the most important condition for which probiotics have been used, though their use remains somewhat controversial. Faecal transplants similarly have provided a unique and effective treatment modality for antibiotic resistant Clostridial infections.
The prevention of eczema and allergic diseases with probiotics has proved less clear cut though for eczema the evidence from meta analyses supports an effect. Species and strain within species appear to be particularly important. One such effective strain, Lactobacillus rhamnosus (HN001) has shown a 50% reduction in eczema and significant reductions in other allergic diseases through early childhood.
Financial support
The New Zealand probiotic RCTs discussed in this manuscript were funded primarily by the Health Research Council of New Zealand with supplementary grants from Fonterra Cooperative Ltd, who also supplied the two probiotics used in the New Zealand studies.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- AlFaleh K, Anabrees J. 2014. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev (4):CD005496.
- Athalye-Jape G, Patole S. 2019. Probiotics for preterm infants – time to end all controversies. Microbial Biotechnology. 12(2):249–253.
- Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HM, Nieuwenhuijs VB, Bollen TL, van Ramshorst B, Witteman BJ, et al. 2008. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 371(9613):651–659.
- Bloksma N, de Heer E, van Dijk H, Willers JM. 1979. Adjuvanticity of lactobacilli. I. Differential effects of viable and killed bacteria. Clin Exp Immunol. 37(2):367–375.
- Canani RB, Nocerino R, Terrin G, Coruzzo A, Cosenza L, Leone L, Troncone R. 2012. Effect of Lactobacillus GG on tolerance acquisition in infants with cow's milk allergy: A randomized trial. Journal of Allergy and Clinical Immunology. 129(2):580–U482. English.
- Christopher JDM, Rebello C, Tarnow-Mordi W. 2016. Probiotics in very preterm infants: the PiPS trial. Letter. 388(10045):655. DOI: 10.1016/S0140-6736(1016)31270-31273.
- Costeloe K, Hardy P, Juszczak E, Wilks M, Millar MR, Probiotics in Preterm Infants Study Collaborative G. 2016. Bifidobacterium breve BBG-001 in very preterm infants: a randomised controlled phase 3 trial. Lancet. 387(10019):649–660.
- Crane J, Barthow C, Mitchell EA, Stanley TV, Purdie G, Rowden J, Kang J, Hood F, Barnes P, Fitzharris P, et al. 2018. Is yoghurt an acceptable alternative to raw milk for reducing eczema and allergy in infancy? Clin Exp Allergy. 48(5):604–606.
- Cross ML, Mortensen RR, Kudsk J, Gill HS. 2002. Dietary intake of Lactobacillus rhamnosus HNOO1 enhances production of both Th1 and Th2 cytokines in antigen-primed mice. Med Microbiol Immunol. 191(1):49–53.
- Cuello-Garcia CA, Brozek JL, Fiocchi A, Pawankar R, Yepes-Nunez JJ, Terracciano L, Gandhi S, Agarwal A, Zhang Y, Schunemann HJ. 2015. Probiotics for the prevention of allergy: A systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. 136(4):952–961.
- de Groot PF, Frissen MN, de Clercq NC, Nieuwdorp M. 2017. Fecal microbiota transplantation in metabolic syndrome: history, present and future. Gut Microbes. 8(3):253–267.
- Dekker J, Wickens K, Black P, Stanley T, Mitchell E, Fitzharris P, Tannock G, Purdie G, Crane J. 2009. Safety aspects of probiotic bacterial strains Lactobacillus rhamnosus HN001 and Bifidobacterium animalis subsp. lactis HN019 in human infants aged 0–2 years. International Dairy Journal. 19(3):149–154.
- Desimone C, Salvadori BB, Negri R, Ferrazzi M, Baldinelli L, Vesely R. 1986. The Adjuvant effect of Yogurt on production of Gamma-interferon by Con-a-stimulated human peripheral-blood lymphocytes. Nutr Rep Int. 33(3):419–433. English.
- Duffield S, Clarke P. 2019. Current use of probiotics to prevent necrotising enterocolitis (Letter). Arch Dis Child Fetal Neonatal Ed. 104:F228.
- Eiseman B, Silen W, Bascom GS, Kauvar AJ. 1958. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. 44(5):854–859.
- Evershed RP, Payne S, Sherratt AG, Copley MS, Coolidge J, Urem-Kotsu D, Kotsakis K, Özdoğan M, Özdoğan AE, Nieuwenhuyse O, et al. 2008. Earliest date for milk use in the near east and southeastern Europe linked to cattle herding. Nature. 455:528.
- Fiocchi A, Pawankar R, Cuello-Garcia C, Ahn K, Al-Hammadi S, Agarwal A, Beyer K, Burks W, Canonica GW, Ebisawa M, et al. 2015. World allergy organization-McMaster University guidelines for allergic disease prevention (GLAD-P): probiotics. World Allergy Organ J. 8(1):4.
- Gill HS, Rutherfurd KJ, Prasad J, Gopal PK. 2000. Enhancement of natural and acquired immunity by Lactobacillus rhamnosus (HN001), Lactobacillus acidophilus (HN017) and Bifidobacterium lactis (HN019). Br J Nutr. 83(2):167–176.
- Grigoroff S. 1905. Étude sur une lait fermentée comestible. Le ‘Kissélo mléko’ de Bulgarie. Revue Médicale de la Suisse Romande.
- Härtel C, Pagel J, Rupp J, Bendiks M, Guthmann F, Rieger-Fackeldey E, Heckmann M, Franz A, Schiffmann J-H, Zimmermann B, et al. 2014. Prophylactic use of lactobacillus acidophilus/bifidobacterium infantis probiotics and outcome in very low birth Weight infants. The Journal of Pediatrics. 165(2):285–289.e281.
- Hassan H, Rompola M, Glaser AW, Kinsey SE, Phillips RS. 2018. Systematic review and meta-analysis investigating the efficacy and safety of probiotics in people with cancer. Support Care Cancer. 26(8):2503–2509.
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, et al. 2014. Expert consensus document. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 11(8):506–514.
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel. 2010. Recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 33:676–682.
- Jenke A, Ruf EM, Hoppe T, Heldmann M, Wirth S. 2012. Bifidobacterium septicaemia in an extremely low-birthweight infant under probiotic therapy. Arch Dis Child Fetal Neonatal Ed. 97(3):F217–F218.
- Kalliomaki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. 2001. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 357(9262):1076–1079.
- Kane AF, Bhatia AD, Denning PW, Shane AL, Patel RM. 2018. Routine supplementation of lactobacillus rhamnosus GG and risk of necrotizing enterocolitis in very Low birth weight infants. The Journal of Pediatrics. 195:73–79.e72.
- Li L, Han Z, Niu X, Zhang G, Jia Y, Zhang S, He C. 2018. Probiotic supplementation for prevention of atopic dermatitis in infants and children: a systematic review and meta-analysis. American Journal of Clinical Dermatology. 20(3):367–377.
- Mackowiak P. 2013. Recycling metchnikoff: probiotics, the intestinal Microbiome and the quest for long life. Frontiers in Public Health. 1:52. DOI:10.3389/fpubh.2013.00052.
- Mansfield JA, Bergin SW, Cooper JR, Olsen CH. 2014. Comparative probiotic strain efficacy in the prevention of eczema in infants and children: a systematic review and meta-analysis. Mil Med. 179(6):580–592.
- Mechnikov I. 1907. The prolongation of life. London: Heinemann.
- Ministry of Health. 2014. Screening, diagnosis and management of gestational diabetes in New Zealand: a clinical practice guideline.
- Neu J, Pammi M. 2018. Necrotizing enterocolitis: the intestinal microbiome, metabolome and inflammatory mediators. Semin Fetal Neonatal Med. 23(6):400–405.
- Odamaki T, Bottacini F, Kato K, Mitsuyama E, Yoshida K, Horigome A, Xiao J-z, van Sinderen D. 2018. Genomic diversity and distribution of Bifidobacterium longum subsp. longum across the human lifespan. Scientific Reports. 8(1):85.
- Panagiotis S, Constatnina T. 2014. Conventional and innovative processing of milk for Yogurt manufacture; development of Texture and flavor: a review. Foods. 3:176–193.
- Ritchie ML, Romanuk TN. 2012. A meta-analysis of probiotic efficacy for gastrointestinal diseases. PLoS One. 7(4):e34938.
- Salque M, Bogucki PI, Pyzel J, Sobkowiak-Tabaka I, Grygiel R, Szmyt M, Evershed RP. 2013. Earliest evidence for cheese making in the sixth millennium BC in northern Europe. Nature. 493(7433):522–525.
- Salvetti E, Harris HMB, Felis GE, O'Toole PW. 2018. Comparative genomics of the genus lactobacillus reveals Robust phylogroups that provide the basis for reclassification. Applied and Environmental Microbiology. 84. DOI:10.1128/AEM.00993-18.
- Schlundt J. 2001. Joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including Powder milk with live lactic acid bacteria. WHO. http://www.who.int/foodsafety/publications/fs_management/en/probiotics.pdf.
- Simoons FJ. 1981. Geographic patterns of primary adult lactose malabsorption. In: Paige DM, Bayless TM, editor. Lactose digestion: clinical and nutritional implications. Baltimore: Johns Hopkins University Press; p. 23–48.
- Slykerman RF, Hood F, Wickens K, Thompson JMD, Barthow C, Murphy R, Kang J, Rowden J, Stone P, Crane J, et al. 2017. Effect of Lactobacillus rhamnosus HN001 in pregnancy on postpartum symptoms of depression and anxiety: a randomised double-blind placebo-controlled trial. EBioMedicine. 24:159–165.
- Szajewska H, Horvath A. 2018. Lactobacillus rhamnosus GG in the primary prevention of eczema in children: a systematic review and meta-analysis. Nutrients. 10(9):1319.
- Talavera MM, Bixler G, Cozzi C, Dail J, Miller RR, McClead R Jr, Reber K. 2016. Quality improvement initiative to reduce the necrotizing enterocolitis rate in premature infants. Pediatrics. 137(5):e20151119.
- Tang ML, Ponsonby AL, Orsini F, Tey D, Robinson M, Su EL, Licciardi P, Burks W, Donath S. 2015. Administration of a probiotic with peanut oral immunotherapy: a randomized trial. J Allergy Clin Immunol. 135(3):737–744 e738.
- Trapp CL, Chang CC, Halpern GM, Keen CL, Gershwin ME. 1993. The influence of chronic Yogurt consumption on populations of young and elderly adults. Int J Immunother. 9(1):53–64. English.
- Tuddenham S, Sears CL. 2015. The intestinal microbiome and health. Curr Opin Infect Dis. 28(5):464–470. eng.
- Underwood MA. 2019. Probiotics and the prevention of necrotizing enterocolitis. J Pediatr Surg. 54(3):405–412.
- Vallabhaneni S, Walker TA, Lockhart SR, Ng D, Chiller T, Melchreit R, Brandt ME, Smith RM, Centers for Disease C, Prevention. 2015. Notes from the field: fatal gastrointestinal mucormycosis in a premature infant associated with a contaminated dietary supplement–connecticut, 2014. MMWR Morbidity and Mortality Weekly Report. 64(6):155–156.
- van den Berg F, Lyndgaard CB, Sørensen KM, Engelsen SB. 2013. Process analytical technology in the food industry. Trends in Food Science & Technology. 31(1):27–35.
- Vergin F. 1954. Antibiotics and probiotics. Hippokrates. 25(4):116–119.
- Waksman SA. 1941. Antagonistic relations of microorganisms. Bacteriol Rev. 5(3):231–291.
- Waksman SA, Woodruff HB. 1940. The soil as a source of microorganisms antagonistic to disease-producing bacteria. J Bacteriol. 40(4):581–600.
- Wheeler JG, Shema SJ, Bogle ML, Shirrell MA, Burks AW, Pittler A, Helm RM. 1997. Immune and clinical impact of Lactobacillus acidophilus on asthma. Ann Allergy Asthma Immunol. 79(3):229–233.
- Wickens K, Barthow C, Mitchell EA, Kang J, van Zyl N, Purdie G, Stanley T, Fitzharris P, Murphy R, Crane J. 2018. Effects of Lactobacillus rhamnosus HN001 in early life on the cumulative prevalence of allergic disease to 11 years. Pediatr Allergy Immunol. 29(8):808–814.
- Wickens K, Barthow C, Mitchell EA, Stanley TV, Purdie G, Rowden J, Kang J, Hood F, van den Elsen L, Forbes-Blom E, et al. 2018. Maternal supplementation alone with Lactobacillus rhamnosus HN001 during pregnancy and breastfeeding does not reduce infant eczema. Pediatr Allergy Immunol. 29(3):296–302.
- Wickens KL, Barthow CA, Murphy R, Abels PR, Maude RM, Stone PR, Mitchell EA, Stanley TV, Purdie GL, Kang JM, et al. 2017. Early pregnancy probiotic supplementation with Lactobacillus rhamnosus HN001 may reduce the prevalence of gestational diabetes mellitus: a randomised controlled trial. Br J Nutr. 117(6):804–813.
- Wickens K, Black P, Stanley T, Mitchell E, Barthow C, Fitzharris P, Purdie G, Crane J, the Probiotic Study Group. 2012. A protective effect of Lactobacillus rhamnosus HN001 against eczema in the first two years of life persists to age 4 years. Clin Exp Allergy. 42(7):1071–1079.
- Wickens K, Black P, Stanley T, Mitchell E, Fitzharris P, Tannock G, Purdie G, Crane J, the Probiotic Study Group. 2008. A differential effect of two probiotics in the prevention of eczema and atopy: a double-blind randomized placebo-controlled trial. J Allergy CIin Immunol. 122:788–794.
- Wickens K, Stanley T, Mitchell E, Barthow C, Fitzharris P, Purdie G, Siebers R, Black P, Crane J. 2013. Early supplementation with Lactobacillus rhamnosus HN001 reduces eczema prevalence to 6 years: does it also reduce atopic sensitization? Clin Exp Allergy. 43(9):1048–1057. DOI:1010.1111/cea.12154.
- Zhang G-Q, Hu H-J, Liu C-Y, Zhang Q, Shakya S, Li Z-Y. 2016. Probiotics for prevention of atopy and food Hypersensitivity in early childhood: a PRISMA-compliant systematic review and meta-analysis of randomized controlled trials. Medicine. 95(8):e2562–e2562. eng.
- Zuccotti G, Meneghin F, Aceti A, Barone G, Callegari ML, Di Mauro A, Fantini MP, Gori D, Indrio F, Maggio L, et al. 2015. Probiotics for prevention of atopic diseases in infants: systematic review and meta-analysis. Allergy. 70(11):1356–1371.
