ABSTRACT
Pre-Miocene, stem odontocetes are known for their procumbent incisors and their function has been the subject of much speculation. Notable among these were Waipatia and several related taxa from New Zealand. Though some studies hypothesise the function of these teeth was for thrusting, the here described Aureia rerehua has unique teeth which might have formed a cage around small fish. These teeth, along with a weak vertex, flexible neck, and the smallest size among its relatives would make it a capable hunter in shallow waters. The addition of A. rerehua along with other taxa to phylogenetic analyses show three broad groups within taxa related to Waipatia and Otekaikea based on the divergence of the function of their teeth and the possible feeding strategies employed to catch prey.
Introduction
Early odontocetes were primarily raptorial feeders while most modern odontocetes are capable of suction feeding, minimally processing captured food (Werth Citation2000; Sinha et al. Citation2010; Schorr et al. Citation2014; Marx et al. Citation2016). Early odontocetes were heterodont with teeth possessing multiple denticles or shark-like dental crowns (Werth Citation2000; Marx et al. Citation2016; Fordyce Citation2018). Notable in early odontocetes were procumbent tusk-like incisors in many clades, including Squalodontidae, Platanistoidea, Kentriodontidae, and Waipatiidae (de Muizon Citation1987, Citation2009; Fordyce Citation1994, Citation2018; Bianucci et al. Citation2015; Tanaka and Fordyce Citation2015a, Citation2015b, Citation2014; Marx et al. Citation2016; Viglino et al. Citation2019; Boessenecker et al. Citation2020; Collareta et al. Citation2020; Coste et al. Citation2023a, Citation2023b).
New Zealand fossils have been instrumental in illuminating late Oligocene odontocetes, especially Waipatia maerewhenua. W. maerewhenua was in Platanistoidea, sensu de Muizon (Citation1987) and formed the base of the new family ‘Waipatiidae’ (Fordyce Citation1994). Specimens from Australia and Europe have been referred to as ‘waipatiid’ based on partial material (Bianucci et al. Citation2011; Fitzgerald Citation2016). Taxa with definitive relationships to W. maerewhenua have been described; Waipatia hectori, Otekaikea marplesi, Otekaikea huata, Awamokoa tokarahi, Nihohae matakoi, Nihoroa reimaea, and OU22262 (Tanaka and Fordyce Citation2015a, Citation2015b, Citation2014, Citation2017; Coste et al. Citation2023a, Citation2023b). Newly described dolphins from the Americas, Ediscetus osbornei, Urkudelphis chawpipacha, and Dolgopolis kinchikafiforo, are possibly related but show disparate teeth, from procumbent to toothless, across either side of the Oligocene-Miocene Boundary (Fitzgerald Citation2016; Tanaka et al. Citation2017; Albright et al. Citation2018; Viglino et al. Citation2021).
Specimen OU22553 is the holotype for a new genus and species related to other New Zealand odontocetes, here named Aureia rerehua. A. rerehua has an exquisitely preserved skull with a weak vertex and unique lateral tooth orientation, suggesting it scanned shallow waters to capture small prey in a basket of teeth. In addition to providing insights to fossil cetacean feeding methods, A. rerehua and other fossils from the region may clarify the phylogeny of odontocetes related to Waipatia.
Materials and methods
Specimen OU22553 includes a skull, teeth, ear bones, mandibles, vertebrae, and ribs. It was prepared with pneumatic chisels and hand tools, consolidated with polyvinyl-butyral, and photographed under artificial lighting.
Osteological descriptions are based on the best-preserved side, differences between sides noted as needed. Cranial anatomy follows Mead and Fordyce (Citation2009) with modifications according to terminology used with similar fossils (Tanaka and Fordyce Citation2015a; Coste et al. Citation2023a, Citation2023b), and new developments (Ichishima et al. Citation2021). Measurements follow Fordyce (Citation2002).
45 mL of sediment were collected from matrix around A. rerehua and foraminifera analysed. Foraminiferal identifications and descriptions are according to Hornibrook et al. (Citation1989).
Phylogenetic analyses in this study used a matrix based on the one from Viglino et al. (Citation2021), one of the most recent after Tanaka & Fordyce (Citation2015b), which analysed many waipatiid-like odontocetes, adding D. kinchikafiforo and an additional phylogenetic character. Two recent analyses by Coste (Citation2023a, Citation2023b), are based on Tanaka et al. (Citation2017), adding three new taxa, N. matakoi, N. reimaea, OU22262, and eight previously described taxa. This study combines Coste (Citation2023a, Citation2023b) and Viglino et al. (Citation2021) and adds three further specimens: A. rerehua, OU22709 and OU22710.
Cladistic analyses were conducted using TNT (Giribet Citation2005; Goloboff et al. Citation2008; Goloboff and Catalano Citation2016). Following previous studies for closer comparison, New Technology Search was used to recover minimum length trees one thousand times across one unweighted and two weighted analyses. Trees use Strict Consensus using a majority 50% consensus (Tanaka and Fordyce Citation2014, Citation2015a, Citation2015b, Citation2016, Citation2017). Comparisons between unweighted and weighted analyses can also test for the stability of certain relationships.
Abbreviations
Anatomy
Bo Basioccipital
Fr Frontal
Et Ethmoid
Eo Exoccipital
Hy Hyal
Jg Jugal
Lc Lacrimal
Mx Maxilla
Ns Nasal
Pe Periotic
Pl Palatine
Pr Parietal
Px Premaxilla
Sp Sphenoid
Sq Squamosal
So Supraoccipital
Tb Tympanic Bulla
Th Teeth
Vo Vomer
Institutions
OU Geology Museum, University of Otago, Dunedin, New Zealand
NMNH-TF National Museum of Natural History, Mdina, Malta
NMV P Museum Victoria Palaeontology Collection, Victoria, Australia
Geological setting
From 22 to 27 Ma, New Zealand sank, leaving between 1% exposure above sea-level and total submergence, during which marine life thrived (Cooper and Cooper Citation1995; Mildenhall et al. Citation2014)
The deposits from that time represent many of the cetaceans present in the collections of the University of Otago Geology Museum. One such is A. rerehua which was collected from a Limestone Quarry in Hakataramea Valley, South Canterbury (GPS Reference: 44°39′37.9″S 170°38′58.3″E), known as ‘Haugh's Quarry’. Related odontocetes come from the Kokoamu Greensand and Otekaike Limestone Formations in the Duntroonian or Waitakian stages on the New Zealand Geological Time Scale, equivalent to the Chattian and perhaps Aquitanian (Raine et al. Citation2015).
Previous studies on foraminiferal biostratigraphy and Strontium isotope dating place the Otekaike Limestone Formation at Haugh's Quarry between 27.3 and 21.7 Ma (Graham et al. Citation2000; Scott et al. Citation2014; Tanaka and Fordyce Citation2015b). Sediment around A. rerehua contained Globerigina woodi cf. connecta, appearing from the late Waitakian, and Globerigina euaptertura from the Whaingaroan until the mid-Waitakian, constraining A. rerehua to 22–23 Ma (Hornibrook et al. Citation1989; Tanaka and Fordyce Citation2015b).
Systematic palaeontology
Cetacea (Brisson, 1762)
Odontoceti (Flower, 1867)
Aureia rerehua gen. et sp. nov.
OU22553
Etymology
The generic name derives from Māori aurei, ‘cloak pin’, referring to the shape of the teeth. The specific name, rerehua, means ‘beautiful’, referring to its well-preserved face.
Diagnosis
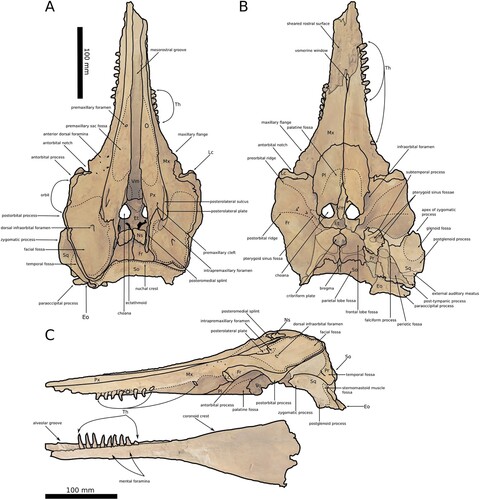
A. rerehua has features common in other stem odontocetes and some platanistoids; a subcircular fossa in the periotic fossa sensu de Muizon (Citation1987), ventrally deflected anterior processes on the periotics, anterior spines on the tympanic bullae, distinct premaxillary clefts, and well developed antorbital notches (de Muizon Citation1987; Geisler and Sanders Citation2003; Geisler et al. Citation2011, Citation2012; Murakami et al. Citation2012a, Citation2012b; Tanaka and Fordyce Citation2014; Tanaka and Fordyce Citation2015b; Gaetán et al. Citation2018). A. rerehua is most like Otekaikea and Waipatia, with an attenuated rostrum, procumbent incisors, fossa for the articular rim of the periotic, and shallow fossae for the sternomastoid muscle (Moore Citation1968; de Muizon Citation1987; Tanaka and Fordyce Citation2015b, Citation2014, Citation2017).
A. rerehua has unique basioccipital crests with posteroventral projections, a rectangular nuchal crest, flat ventral surfaces on the posterior process of the periotic, laterally splayed teeth, and a process on the subtemporal crest. Waipatiid-like odontocetes have developed vertices. The vertex of A. rerehua is less pronounced than W. maerewhenua or Otekaikea, shown in C, possessing a flatter face like Papahu taitapu (Fordyce Citation1994; Aguirre-Fernández and Fordyce Citation2014; Tanaka and Fordyce Citation2014, Citation2015b). The posterior skull is straighter and steeper than that of other waipatiid-like odontocetes, making the lateral profile of the skull triangular.
Description
Ontogeny
The skull is sutured together and epiphyses on the atlas, axis, and some thoracic vertebrae are ankylosed. Most vertebrae have loose vertebral discs, suggesting A. rerehua was a sexually mature subadult, or a class V specimen (Perrin Citation1975).
Size
Though often resulting in underestimates of longirostral taxa, bizygomatic width is the most reliable single measurement for body size estimates on incomplete cetacean skulls (Pyenson and Sponberg Citation2011; Tanaka and Fordyce Citation2015b, Citation2017). Due to damage, zygomatic width to the midline for intact material was mirrored as in previous related taxa (Tanaka and Fordyce Citation2015b, Citation2017). This measured 99.5 mm from the left zygomatic process to the midline, mirrored to 199 mm. Due to skull asymmetry, the left skull is narrower than the right and mirroring underestimates bizygomatic width, suggesting a minimum body length estimate of 1.97 m.
Rostrum
In situ teeth are laterally splayed, with teeth dorsally visible as shown in A. The splayed orientation on uncrushed teeth appears genuine. The rostrum is attenuated like other waipatiid-like odontocetes.
Premaxillae
The premaxillary foramina are anterior to the antorbital notches. The premaxillary cleft is a fine incision and the left connects with the posterolateral sulcus. The posterolateral sulcus is shallower than W. maerewhenua. The posteromedial splints extend posteriorly beyond the nasals. The posterolateral plate roofs the intrapremaxillary foramen like Otekaikea. The premaxilla covers the medial rim of the maxilla. The premaxillary sac fossa extends further down the anterior rostrum than other waipatiid-like odontocetes. The premaxillae are widest around the external bony nares, like Otekaikea. Anterior to the bony nares are two small processes, similar to those presumed to form the anterolateral sulcus in O. huata.
Maxilla
The ascending process is uniformly thick and contacts only the frontal and premaxilla. The dorsal view of the left maxilla is long and ovate and the right short and square. A thick medial lip surrounds a small oval depression on the facial fossa, delimiting the intrapremaxillary foramen. This differs from the flat lip found in W. maerewhenua and the robust lip in O. huata. Only the left maxilla has anterior dorsal infraorbital foramina. The sulcus forming the floor of the intrapremaxillary foramen rises around the posterolateral plate of the premaxilla. The maxilla forms the anterolateral wall of the bony nares and the ventromedial wall of the ventral infraorbital foramen.
Palatine
The sutures between the palatine and maxilla are almost indistinguishable. The pyramidal process contacts the ethmoid and the frontal bones, forming part of the anterior wall of the nares and the nasal spine. A tear-drop-shaped fossa on the ventral surface is analogous to the deep, semicircular fossa in O. huata, believed to articulate with the pterygoids (Tanaka and Fordyce Citation2015b). There is no fossa for the anterior pterygoid sinus.
Frontals
Viewed dorsally, the frontals have a W-shaped anterior margin. The anterior margin accommodates the nasals and right posteromedial splint of the premaxilla. The frontals roof the temporal fossa. The thick supraorbital process has a curved fan for the orbit on its ventral surface. The frontals form a wide opening for the cribriform plate and fossa at the bregma. Cerebral juga divide the internal surface with posterior portions continuous to the supraoccipital. The frontals are raised along the medial suture in the brain case.
Parietals
The parietal is absent dorsally and present in the temporal fossa. It sutures with the frontals and is separated from the alisphenoid by a winding suture. The parietal does not contact the exoccipital.
Nasals
The nasals are wide and sub-rhomboidal with concave anterior faces, with dorsal surfaces slightly lower than the frontals. A thin lateral process extends anteriorly.
Ethmoid
The ethmoid contacts the orbitosphenoid, frontals, and maxilla. The hourglass-shaped body of the mesethmoid is visible from the mesorostral groove. The orbitosphenoid forms the posterior wall of the ethmoid and the ectethmoid has a pair of large bulbs nestled on the dorsoposterior surface of the orbitosphenoid, leaving C-shaped channels. The cribriform plate is exposed by two perforations in the frontals.
Vomer
The vomer is a wide U-shaped mesotrostral groove and a thin, ventral exposure between the maxillae.
Lacrimal
The lacrimal is indistinguishable from the jugal. They are dorsally visible, forming the posterolateral section of the antorbital notch.
Sphenoid
The alisphenoid forms the anteroventral cranial wall with the frontal, parietal, and squamosal. The angular subtemporal crest has an anterolaterally directed process with a blunt distal end unseen in related taxa. The alisphenoid-squamosal suture follows the groove for the mandibular nerve, posterior to the pterygoid sinus fossa. The foramen rotundum continues to the V-shaped groove for the meningeal artery, which runs far from the alisphenoid-parietal border with a circular depression within.
Squamosal
The semi-circular zygomatic process and floor of the temporal fossa are visible dorsally and laterally. The concave ventral surface of the zygomatic process carries the mandibular fossa. The sub-temporal crest is sharp. The post-tympanic process is pointed and shorter than the rounded postglenoid process. The opening of the external auditory meatus is a wide, smooth channel. The falciform process is flat and wide. The tympanosquamosal recess has a small triangular bulge with a C-shaped sigmoidal recess. The periotic fossa has a wide suprameatal pit and broken spiny process. The foramen spinosum is near the alisphenoid. The squamosal inside the braincase has a canal running anterior to the exoccipital, easily visible as in B.
Exoccipital
Exoccipital material is a left paraoccipital process articulated to the squamosal and an isolated ring of foramen magnum with divided occipital condyles.
Supraoccipital
The nuchal crest is anteriorly oriented, with one kink where supraoccipital, premaxilla, and frontal meet and one where supraoccipital, maxilla, and parietal meet, like O. huata. The internal surface preserves a furrow towards the bregma flanked by raised edges.
Basioccipital
The basioccipital is narrow with crests separated from the exoccipital and each other by an acute angle. The anterior half of the basioccipital has a median ridge.
Periotic
Shown in A–F, the dorsal surface of the periotic is flat with a shallow transverse groove as in other waipatiid-like odontocetes. The anterior process is narrower than W. maerewhenua or O. huata. The anterior bulla facet has parallel mediolateral margins. The anterior incision is a fine groove connecting to the parabullary sulcus. The hiatus Fallopi is triangular. The parabullary sulcus connects to the anteroexternal sulcus on the left periotic but not on the right. The rim of the anteroexternal sulcus, smooth lateral tuberosity, and articular rim form three projections. The pars cochlearis is sub-rectangular and thinner dorsoventrally than Waipatia, like Otekaikea. The large acoustic meatus is tear-drop-shaped. The transverse ridge separates the area cribrosa media and the spiral cribiform tract from the superior vestibular area and the proximal opening of the facial canal. The openings of the internal acoustic meatus, cochlear and vestibular aqueducts are visible dorsally. The aperture for the vestibular aqueduct is more dorsolateral and larger than in the cochlear aqueduct. A lateral groove is dorsal to the internal acoustic meatus, with a small pit on the left periotic but not the right. The facial sulcus is elliptical. The stapedial muscle fossa is dorsal to the fenestra rotunda. The rim of the mallear fossa slightly projects. The posterior process is shorter than W. maerewhenua but similar to Otekaikea. The bulla facet of the posterior process is not as tapered as Waipatia, nor square like Otekaikea. The dorsal surface of the posterior process has a groove. An accessory ossicle forms a small plate on the anterior process.
Figure 2. The ear bones of Aureia rerehua. A, The left column displays the right periotic in dorsal. B, Ventral. C, Lateral. D, medial. E, Anterior. F, Posterior view. G, The right column displays the left tympanic bulla in dorsal. H, ventral. I, Medial. J, Lateral. K, Anterior. L, Posterior view. Note the malleus and stapes in situ.
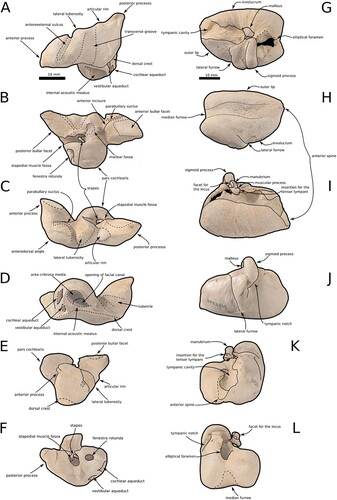
Tympanic Bullae
Typical of waipatiid-like odontocetes, the tympanic bulla is heart-shaped from a median groove, interprominential notch, and short anterior spine, as seen in G–L. The outer lip is box-like. The median furrow is wide, smooth, and almost runs the length of the tympanic bulla. The lateral furrow is long. The sigmoid process projects posterolaterally. The bulla is unfused to other bones and the sigmoid process articulates with nothing. The inner edge of the involucrum appears sigmoid dorsally. Diagonal lateral grooves and a faint ventromedial crest are on the anterior. The elliptical foramen is large and sub-triangular.
Malleus
The malleus is easily visible with the missing sigmoid process of the tympanic bulla, demonstrated in G–I–K. The manubrium and muscular processes are separated by a sigmoid groove and equally sized. The facet for the incus appears plate-like due to a thin groove. The chorda tympani obscures the lateral foramen on the bulla. The insertion for the tensor tympani tendon is on the anterodorsal surface of the muscular process with a short linear groove underneath.
Stapes
The stapes is in the right periotic, shown in A–C–F. The muscular process is rounded, and the head of the stapes is faint. The stapedial foramen is elliptical and anterior and posterior crura are longer than Tursiops. The posterior crus twists behind the anterior crus.
Mandible
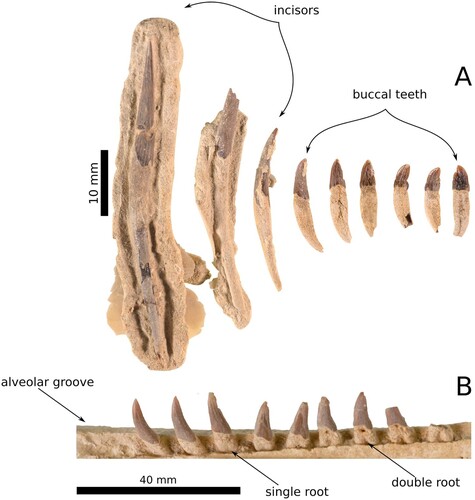
Most of the left mandible and a small fragment of the right are preserved. The mandibular symphysis is unfused and runs entirely along the midline of A. rerehua. A deep incision forms the apical groove along the medial surface, unlike the shallow one of W. maerewhenua. The apical groove exceeds the mandibular symphysis by 18.5 mm. The alveolar process is half as dorsoventrally thin as W. maerewhenua. Alveoli are not completely separated by bone from neighbouring alveoli in the alveolar groove.
Teeth
Sixty-nine individually countable teeth were recovered; twenty-four in situ with the skull and mandible and forty-five loose. Apicobasal striations cover the enamel. Two incisors with long, conical, single-roots would be procumbent, matching other delicate waipatiid-like incisors rather than robust squalodontids. Some crowns on small posterior teeth have three denticles which almost form a single point. Median teeth have bicarinate crowns, some constricted by notches in medial and distal carinae. In situ teeth point anterolaterally then bow the apices ventrally. Heterodont and single-crowned posterior teeth have ectocingula, unlike anterior teeth. Some heterodont roots are singular, some double-rooted, and some bridged by isthmi similar to Otekaikea, N. matakoi, and N. reimaea. The presence of in situ single-rooted, single-crowned teeth in the most posterior alveoli suggests the following arrangement: incisors, anterior single-rooted teeth, posterior isthmus bearing teeth, posterior double-rooted teeth, and posterior single-rooted teeth. This distribution of crown and root types is shown in and is similar to N. matakoi.
Atlas
The atlas is not fused with the axis and epiphyses are ankylosed. The condyloid facets are deep, and concave viewed dorsally. The lowest area of the condyloid facets constricts, creating a thin anterior arch compared to Otekaikea or W. maerewhenua. The fossa for the odontoid process of the axis is semi-circular and tilted dorsally. A gap separates the articular facets.
Discussion
Phylogenetic analysis
Results
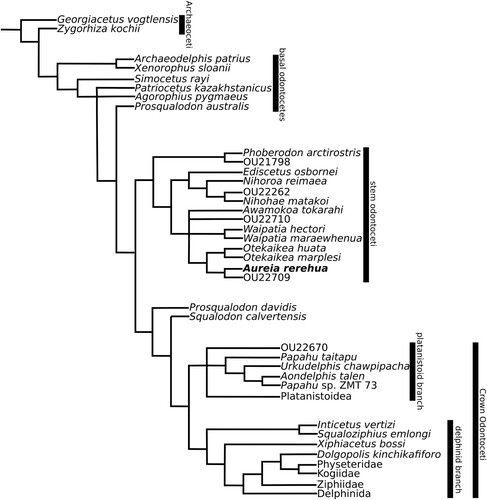
In all analyses, D. kinchikafiforo was basal to Physeteroidea, differing from Viglino et al. (Citation2021), P. taitapu and U. chawpipacha basal to Platanistoidea, differing from Tanaka et al. (Citation2017), and A. rerehua and OU22709 paired together across from Otekaikea, near A. tokarahi and OU22710.
Analysis 1 was unweighted and produced 784 trees with 2229 steps each and a fit of 129.59. Waipatiid-like odontocetes were outside of Crown Odontoceti which included Platanistoidea. Analysis 2 used an implied weight of K = 3, producing 9 trees with 2282 steps each and a fit of 131.90. Waipatiid-like odontocetes were basal to Platanistoidea as part of Crown Odontoceti. Analysis 3 used an implied weight of K = 12, producing 12 trees with 2247 steps each and a fit of 131.12. Waipatiid-like odontocetes formed a polytomy with Platanistoidea and Delphinida.
Phylogenetic relationships
Relationship to Platanistoidea
Waipatiid-like odontocetes are mostly excluded from Platanistoidea in recent studies (Boersma et al. Citation2017; Bianucci et al. Citation2020; Nelson and Uhen Citation2020; Coste et al. Citation2023a, Citation2023b), with some exceptions (Viglino et al. Citation2021). Waipatiid-like odontocetes were excluded from Crown Odontoceti in unweighted analysis, lacking low coronoid processes, premaxillary width, narrow nasals, narrowly exposed squamosals, and rectangular or weak nuchal crests. Analysis 2 (K = 3) included waipatiid-like odontocetes based on thick palatines, short posterior processes on the periotic, absent transverse ridges on the tympanic bulla, and a distally expanded acromion. Analysis 3 (K = 12) included waipatiid-like odontocetes based on narrowly exposed squamosals. U. chawpipacha was basal to Platanistoidea in all analyses unlike Tanaka et al. (Citation2017). D. kinchikafiforo was at the base of Delphinida or Physeteroidea unlike Viglino et al. (Citation2021). Unweighted and weighted analyses differ in the placement of waipatiid-like dolphins to Platanistoidea and are consistent about the position of U. chawpipacha and D. kinchikafiforo. Due to potential biases with weighted analyses, unweighted analyses will be used as the main basis for the phylogenetic position of A. rerehua and its exclusion from Platanistoidea, shown in . The weighted analyses are primarily used to illustrate the differences presented by increasing the weight or ‘value’ of characters which recurrently place specimens.
Figure 4. Phylogenetic tree produced by unweighted analysis of the combined matrix from Viglino et al. (Citation2021) and Coste (Citation2021) with the inclusion of Aureia rerehua, OU22709, and OU22710. This is the main reference study for this study.
Relationship to waipatia
All analyses grouped A. tokarahi, Otekaikea, A. rerehua, OU22710 and OU22709 together but only weighted analyses recovered a synapomorphy, the fossa for the articular rim of periotic in squamosal. N. matakoi, N. reimaea, E. osbornei and OU2262 grouped together in all analyses as in Coste (Citation2023a, Citation2023b), and unlike Albright et al. (Citation2018).
These results separate waipatiid-like dolphins into three broad groups based on cranial topology and dentition. First, ‘Waipatia-type’ dolphins have moderately developed vertices and cheek teeth with divided double roots and teeth with multiple denticles, including W. maerewhenua, W. hectori, A. tokarahi and NMV P48861 (Fordyce Citation1994; Tanaka and Fordyce Citation2015a, Citation2017; Fitzgerald Citation2016). Second, ‘Nihohae-type’ dolphins have flat cranial topologies, shark-like dental crowns, and wide mesorostral grooves, comprising N. matakoi, N. reimaea, E. osbornei and OU22262. Third, ‘Otekaikea-type’ dolphins share intrapremaxillary foramina and gracile, near-homodont dental crowns, isthmus bearing dental roots, and triangular temporal fossae. ‘Otekaikea-type’ dolphins would include O. huata, O. marplesi, A. rerehua, OU22709, and possibly NMNH-TF-01120 (Bianucci et al. Citation2011; Tanaka and Fordyce Citation2014, Citation2015b). The common features within, and the disparities between, these three groups likely indicate divergence of early common ancestors by adopting different feeding strategies to target different prey, using their teeth for different prey capture strategies or to tackle differently sized prey. This could explain the presence of so many odontocete taxa within such a short interval of time, and particularly for New Zealand taxa Waipatia, Otekaikea, A. tokarahi, N. matakoi, N. reimaea, A. rerehua, OU22602, OU22709, and OU22710, which are found in similar or the same geological formations at closely located sites. Even within the three general groups stipulated above, morphology between taxa can differ, such as the hyper-developed vertex of O. huata and the weakly developed vertex and laterally splayed teeth of A. rerehua.
The relationship between Waipatia and waipatiid-like taxa may be uncertain. While other relationships between waipatiid-like dolphins were stable across weighted and unweighted analyses, unweighted analysis grouped W. maerewhenua and W. hectori with waipatiid-like odontocetes, while weighted analyses placed Waipatia with U. chawpipacha basal to Platanistoidea. Studies analysing Platanistoidea and relatives with the matrix by Lambert et al. (Citation2014) rather than Tanaka & Fordyce (2015) also regularly recover Platanistoidea including Otekaikea and excluding Waipatia, partly due to the fewer waipatiid-like odontocetes included in their analysis (Lambert et al. Citation2014; Boersma et al. Citation2017; Nelson and Uhen Citation2020). Though not directly comparable, they may indicate that the relationship between Waipatia and related taxa may not be entirely stable yet relationships between Otekaikea and related taxa are. Otekaikea might be more phylogenetically important for other waipatiid-like odontocetes than Waipatia, creating a clade bracketed between E. osbornei and Otekaikea, possibly including Waipatia and certainly excluding D. kinchikafiforo and U. chawpipacha. Waipatiidae should retain only W. maerewhenua and W. hectori. Ranking Aureia and waipatiid-like odontocetes into families is difficult as few characters define nodes. More grades between Otekaikea and A. tokarahi and between OU22262 and N. matakoi may emerge as more taxa are added to the phylogeny and as defining characteristics are scrutinised.
Relationship to Otekaikea
Every analysis paired A. rerehua and OU22709 together, branching apart from O. huata and O. marplesi.
Every analysis distinguishes A. rerehua and OU22709 from O. huata and O. marplesi based on flanges on the basioccipital crests. Analyses 2 and 3 recovered the ligamentous connection between periotic and squamosal as another distinction for A. rerehua and OU22709 and narrow nasals and fused accessory ossicle for O. marplesi and O. huata.
A. rerehua was distinct from OU22709 based on its rectangular nuchal crest, posterior process of the periotic, and zygomatic shape.
Basicranium and sinuses
As in other waipatiid-like odontocetes, the pterygoid sinuses would likely lie against the alisphenoid in the pterygoid sinus fossa but not the orbit, further impeded by the unique subtemporal process in A. rerehua. The function of this process is unclear since related odontocetes lack it.
The periotic fits into the periotic fossa snugly despite few points of contact. The apices of both the anterior and posterior processes are free from the squamosal, creating space for soft tissue between almost the entire margin of the periotic and the periotic fossa.
Endocranial cavity
Odontoceti lineages independently lost olfactory bulbs and associated fossae on the cranial wall from the Late Oligocene (Marino et al. Citation2003; Berta et al. Citation2014). In A. rerehua, the ethmoid remains open for nerves and theoretically smell. However, the olfactory fossa is barely present, bringing any olfactory bulb nearly flush with the frontal lobe. Olfactory bulb loss does not necessarily eliminate smell, as Phocaena phocaena has olfactory cells yet no olfactory bulb (Breathnach Citation1955; Morgane et al. Citation1980; Behrmann Citation1989). Olfactory fossae and the olfactory bulb are smaller than endocasts from Prosqualodon davidi and Squalodon from the Early Miocene yet match two Oligocene odontocete endocasts (Dart and Andrrws Citation1923; Marples Citation1949; Godfrey Citation2013; Tanaka et al. Citation2022). Since Platanista gangetica and A. rerehua are separated by taxa with olfactory fossae and bulbs, stem odontocetes and Platanistoidea likely lost olfaction independently.
The sagittal ridge along the internal surface of the braincase indicates a medial sulcus, smaller than other taxa with shallow medial sulci, like OU22466, P. gangetica, or P. phocaena.
Feeding and ecology
The gracile mandible and short temporal fossa similar to O. huata, and flexible cervical vertebrae in A. rerehua indicate hunting by quick snapping (Tanaka and Fordyce Citation2015b, Citation2017). Posterior teeth are small, unsplayed, and near-homodont, resembling the teeth of Otekaikea and contrasting with the larger, more pronounced denticles in and longer temporal fossa W. maerewhenua for tackling larger prey (Fordyce Citation1994; Tanaka and Fordyce Citation2017). P. gangetica could model the hunting of A. rerehua and Otekaikea, utilising ‘grasp and suction’ to catch and swallow prey (Hocking et al. Citation2017; McCurry et al. Citation2017; McCurry et al. Citation2017). This is supported by the widely angled paraoccipital processes in A. rerehua and Otekaikea, hypothesised to provide a wide attachment for the hyoid bones (Tanaka and Fordyce Citation2014, Citation2015b).
The procumbent incisors of early odontocetes have been hypothesised to be striking apparatuses, either ramming prey like Ankylorhiza tiedemani or laterally thrashing prey like N. matakoi (Boessenecker et al. Citation2020; Coste et al. Citation2023a). The teeth of A. rerehua lack lateral occlusion and are thin and delicate. Modern river dolphins, like P. gangetica, lack procumbent incisors but another fluvial predator, the crocodilian Gavialis gangeticus, has a long rostrum and slightly procumbent anterior teeth but the majority of teeth in G. gangeticus are primarily vertical and interlock with one another (Whitaker and Basu Citation1982; Ballell et al. Citation2019). In A. rerehua, the teeth behind the teeth behind the incisors splay laterally, with the buccal sides of the teeth of A. rerehua being visible in dorsal view. Thus, the procumbent yet curved incisors in A. rerehua might extend reach rather than striking range.
For comparable examples, teeth in prehistoric marine reptiles like those of Plesiosaurus dolichodeirus, or Metriorhynchus cultridens, are adapted for piercing small or soft prey (Massare Citation1987). Dental orientation in A. rerehua resembles Paludidraco multidentatus or Morturneria seymourensis, fine-toothed, filter feeding reptiles (O'Keefe et al. Citation2017; de Miguel Chaves et al. Citation2018). Though less tightly packed than the teeth of these filter feeding reptiles, the spaced teeth of A. rerehua might still have caged small fish, innovating a unique feeding strategy among odontocetes.
A. rerehua very likely echolocated, as evidenced by skull asymmetry, auditory anatomy, and hollow mandibles, but has a weak vertex and premaxillary sac fossae more anteriorly extended than related taxa, shifting nasal sacs and possibly the melon anteroposteriorly. This may mean overlying tissue could spread lower, reducing the vertical profile of A. rerehua. However, skull morphology does not necessarily determine head morphology, demonstrated by the flat skulls and the bulbous heads of Delphinapterus leucas or Inia geoffrensis (O'Corry-Crowe Citation2009; Marx et al. Citation2016). In addition, articulation with the neck orientates the dorsal surface of the skull near horizontally, levelling the rostrum and supraorbital processes. Although A. rerehua is likely not fully grown, it is smaller than other immature waipatiid-like odontocetes, and could allow A. rerehua to swim at shallower depths (Tanaka and Fordyce Citation2014; Tanaka and Fordyce Citation2015b). This could hint A. rerehua scanned seabeds or shallows for small fish as a flexible neck and splayed teeth extended lateral reach at the cost of vertical mobility, netting prey with minimal dental impact.
Conclusion
OU22553 is the holotypes for a new species, A. rerehua, closely related to Otekaikea. It differs from other related odontocetes by its weak vertex, laterally splayed and recurved teeth, rectangular nuchal crest, and posterior flange on the basioccipital processes. Its widely splayed teeth are hypothesised to have clasped rather than strike fish. The unique dentition, small size, limited sense of smell, and dorsoventrally shallow skull illustrate a small dolphin foraging along shallow waters where speed and mobility are essential.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
A complete description of A. rerehua, additional figures, and the phylogenetic matrix from this study are available at Zenodo: https://doi.org/10.5281/zenodo.10437075.
Additional information
Funding
References
- Aguirre-Fernández G, Fordyce RE. 2014. Papahu taitapu, gen. et sp. nov., an early Miocene stem odontocete (Cetacea) from New Zealand. Journal of Vertebrate Paleontology. 34(1):195–210. doi:10.1080/02724634.2013.799069.
- Albright LB, Sanders AE, Geisler JH. 2018. An unexpectedly derived odontocete from the Ashley Formation (upper Rupelian) of South Carolina, USA. Journal of Vertebrate Paleontology. 38(4):1–15. doi:10.1080/02724634.2018.1482555.
- Ballell A, Moon BC, Porro LB, Benton MJ, Rayfield EJ. 2019. Convergence and functional evolution of longirostry in crocodylomorphs. Palaeontology. 62(6):867–887. doi:10.1111/pala.12432.
- Behrmann G. 1989. The olfactory regions in the nose of the harbour porpoise Phocoena phocoena (Linne, 1758). Aquatic Mammals. 15(3):130–133.
- Berta A, Ekdale EG, Cranford TW. 2014. Review of the cetacean nose: form, function, and evolution. The Anatomical Record. 297(11):2205–2215. doi:10.1002/ar.23034.
- Bianucci G, de Muizon C, Urbina M, Lambert O. 2020. Extensive diversity and disparity of the early Miocene platanistoids (Cetacea, Odontoceti) in the southeastern Pacific (Chilcatay Formation, Peru). Life. 10(3):27. doi:10.3390/life10030027.
- Bianucci G, Gatt M, Catanzariti R, Sorbi S, Bonavia CG, Curmi R, Varola A. 2011. Systematics, biostratigraphy and evolutionary pattern of the Oligo-Miocene marine mammals from the Maltese Islands. Geobios. 44(6):549–585. doi:10.1016/j.geobios.2011.02.009.
- Bianucci G, Urbina M, Lambert O. 2015. A new record of Notocetus vanbenedeni (Squalodelphinidae, Odontoceti, Cetacea) from the Early Miocene of Peru. Comptes Rendus Palevol. 14(1):5–13. doi:10.1016/j.crpv.2014.08.003.
- Boersma AT, McCurry MR, Pyenson ND. 2017. A new fossil dolphin Dilophodelphis fordycei provides insight into the evolution of supraorbital crests in Platanistoidea (Mammalia, Cetacea). Royal Society Open Science. 4(5):170022. doi:10.1098/rsos.170022.
- Boessenecker RW, Churchill M, Buchholtz EA, Beatty BL, Geisler JH. 2020. Convergent evolution of swimming adaptations in modern whales revealed by a large macrophagous dolphin from the Oligocene of South Carolina. Current Biology. 30(16):3267–3273. e3262. doi:10.1016/j.cub.2020.06.012.
- Breathnach A. 1955. Observations on endocranial casts of recent and fossil cetaceans. Journal of Anatomy. 89(Pt 4):532.
- Collareta A, Di Cencio A, Ricci R, Bianucci G. 2020. The shark-toothed dolphin Squalodon (Cetacea: Odontoceti) from the remarkable Montagna della Majella marine vertebrate assemblage (Bolognano Formation, central Italy). Carnets Geol. 20(02):19. doi:10.4267/2042/70716.
- Cooper A, Cooper RA. 1995. The Oligocene bottleneck and New Zealand biota: genetic record of a past environmental crisis. Proceedings of the Royal Society of London. Series B: Biological Sciences. 261(1362):293–302. doi:10.1098/rspb.1995.0150.
- Coste A. 2021. Daunting dentitions: relationships and functional morphology of three new Oligocene dolphins with tusk-like teeth from New Zealand University of Otago.
- Coste A, Fordyce RE, Loch C. 2023a. A new dolphin with tusk-like teeth from the late Oligocene of New Zealand indicates evolution of novel feeding strategies. Proceedings of the Royal Society B: Biological Sciences. 290(2000):20230873. doi:10.1098/rspb.2023.0873.
- Coste A, Fordyce RE, Loch C. 2023b. A new fossil dolphin with tusk-like teeth from New Zealand and an analysis of procumbent teeth in fossil cetaceans. Journal of the Royal Society of New Zealand. Special Issue: Fossil Vertebrates of Southern Zealandia:1–20. doi:10.1080/03036758.2023.2267456.
- Dart RA, Andrews C. 1923. The brain of the Zeuglodonlidæ (Cetacea). Proceedings of the Zoological Society of London. 93(3):615–654.
- de Miguel Chaves C, Ortega F, & Pérez-García A. 2018. New highly pachyostotic nothosauroid interpreted as a filter-feeding Triassic marine reptile. Biology Letters. 14(8):20180130. doi:10.1098/rsbl.2018.0130.
- de Muizon C. 1987. The affinities of Notocetus vanbenedeni, an early Miocene platanistoid (Cetacea, Mammalia) from Patagonia, southern Argentina. American Museum Novitates. 2904:1–27.
- de Muizon C. 2009. River dolphins, evolutionary history and affinities. In: Perrin WF, Würsig B, Thewissen JGM, editors. Encyclopedia of marine mammals. Storrs, Conneticut: Academic Press; p. 979–986.
- Fitzgerald EM. 2016. A late Oligocene waipatiid dolphin (Odontoceti: Waipatiidae) from Victoria, Australia. Memoirs of Museum Victoria. 74:117–136. doi:10.24199/j.mmv.2016.74.12.
- Fordyce RE. 1994. Waipatia maerewhenua maerewhenua, new genus and new species (Waipatiidae, new family), an archaic Late Oligocene dolphin (Cetacea: Odontoceti: Platanistoidea) from New Zealand. Proceedings of the San Diego Society of Natural History. 29:147–176.
- Fordyce RE. 2002. Simocetus rayi (Odontoceti: Simocetidae, new family): a bizarre new archaic Oligocene dolphin from the eastern North Pacific. Smithsonian Contributions to Paleobiology. 93:185–222.
- Fordyce RE. 2018. Cetacean evolution. In: Würsig B, Thewissen JGM, Kovacs KM, editors. Encyclopedia of marine mammals. Storrs, Connecticut: Academic Press; p. 180–185.
- Gaetán CM, Buono MR, Gaetano LC. 2018. Prosqualodon australis (Cetacea: Odontoceti) from the early Miocene of Patagonia, Argentina: redescription and phylogenetic analysis. Ameghiniana. 56(1):1–27. doi:10.5710/AMGH.21.11.2018.3208.
- Geisler JH, Godfrey SJ, Lambert O. 2012. A new genus and species of late Miocene inioid (Cetacea, Odontoceti) from the Meherrin River, North Carolina, USA. Journal of Vertebrate Paleontology. 32(1):198–211. doi:10.1080/02724634.2012.629016.
- Geisler JH, McGowen MR, Yang G, Gatesy J. 2011. A supermatrix analysis of genomic, morphological, and paleontological data from crown Cetacea. BMC Evolutionary Biology. 11(1):1–33. doi:10.1186/1471-2148-11-112.
- Geisler JH, Sanders AE. 2003. Morphological evidence for the phylogeny of Cetacea. Journal of Mammalian Evolution. 10(1):23–129. doi:10.1023/A:1025552007291.
- Giribet G. 2005. TNT: tree analysis using new technology. Systematic Biology. 54(1):176–178. doi:10.1080/10635150590905830.
- Godfrey SJ. 2013. On the olfactory apparatus in the Miocene odontocete Squalodon sp.(Squalodontidae). Comptes Rendus Palevol. 12(7-8):519–530. doi:10.1016/j.crpv.2013.03.002.
- Goloboff PA, Catalano SA. 2016. TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics. 32(3):221–238. doi:10.1111/cla.12160.
- Goloboff PA, Farris JS, Nixon KC. 2008. TNT, a free program for phylogenetic analysis. Cladistics. 24(5):774–786. doi:10.1111/j.1096-0031.2008.00217.x.
- Graham IJ, Morgans HE, Waghorn DB, Trotter JA, Whitford DJ. 2000. Strontium isotope stratigraphy of the Oligocene-Miocene Otekaike Limestone (Trig Z section) in southern New Zealand: age of the Duntroonian/Waitakian Stage boundary. New Zealand Journal of Geology and Geophysics. 43(3):335–347. doi:10.1080/00288306.2000.9514891.
- Hocking DP, Marx FG, Park T, Fitzgerald EM, Evans AR. 2017. A behavioural framework for the evolution of feeding in predatory aquatic mammals. Proceedings of the Royal Society B: Biological Sciences. 284(1850):20162750. doi:10.1098/rspb.2016.2750.
- Hornibrook NdB, Brazier RC, Strong CP. 1989. Manual of New Zealand Permian to Pleistocene foraminiferal biostratigraphy. Paleontological Bulletin. 56. https://pubs.geoscienceworld.org/cushmanfoundation/jfr/article-abstract/21/2/193/76512/MANUAL-OF-NEW-ZEALAND-PERMIAN-TO-PLEISTOCENE?redirectedFrom=fulltext.
- Ichishima H, Kawabe S, Sawamura H. 2021. The so-called foramen singulare in cetacean periotics is actually the superior vestibular area. The Anatomical Record. 304(8):1792–1799. doi:10.1002/ar.24585.
- Lambert O, Bianucci G, Urbina M. 2014. Huaridelphis raimondii, a new early Miocene Squalodelphinidae (Cetacea, odontoceti) from the Chilcatay Formation, Peru. Journal of Vertebrate Paleontology. 34(5):987–1004. doi:10.1080/02724634.2014.858050.
- Marino L, Uhen MD, Pyenson ND, Frohlich B. 2003. Reconstructing cetacean brain evolution using computed tomography. The Anatomical Record Part B: The New Anatomist: An Official Publication of the American Association of Anatomists. 272(1):107–117.
- Marples B. 1949. Two endocranial casts of cetaceans from the Oligocene of New Zealand. American Journal of Science. 247(7):462–471. doi:10.2475/ajs.247.7.462.
- Marx FG, Lambert O, Uhen MD. 2016. Cetacean paleobiology. Chichester, UK: John Wiley & Sons.
- Massare JA. 1987. Tooth morphology and prey preference of Mesozoic marine reptiles. Journal of Vertebrate Paleontology. 7(2):121–137. doi:10.1080/02724634.1987.10011647.
- McCurry MR, Evans AR, Fitzgerald EM, Adams JW, Clausen PD, McHenry CR. 2017. The remarkable convergence of skull shape in crocodilians and toothed whales. Proceedings of the Royal Society B: Biological Sciences. 284(1850):20162348. doi:10.1098/rspb.2016.2348.
- McCurry MR, Walmsley CW, Fitzgerald EM, McHenry CR. 2017. The biomechanical consequences of longirostry in crocodilians and odontocetes. Journal of Biomechanics. 56:61–70. doi:10.1016/j.jbiomech.2017.03.003.
- Mead JG, Fordyce RE. 2009. The therian skull: a lexicon with emphasis on the odontocetes. Smithsonian Contributions to Zoology. (627):1–249. doi:10.5479/si.00810282.627.
- Mildenhall D, Mortimer N, Bassett K, Kennedy E. 2014. Oligocene paleogeography of New Zealand: maximum marine transgression. Taylor & Francis. Vol. 57, pp. 107–109.
- Moore JC. 1968. Relationships among the living genera of beaked whales, with classification, diagnoses and keys. Fieldiana Zoology. 53:206–298.
- Morgane PJ, Jacobs MS, McFarland WL. 1980. The anatomy of the brain of the bottlenose dolphin (Tursiops truncatus). Surface configurations of the telencephalon of the bottlenose dolphin with comparative anatomical observations in four other cetacean species. Brain Research Bulletin. 5(3):1–107. doi:10.1016/0361-9230(80)90272-5.
- Murakami M, Shimada C, Hikida Y, Hirano H. 2012a. A new basal porpoise, Pterophocaena nishinoi (Cetacea, Odontoceti, Delphinoidea), from the upper Miocene of Japan and its phylogenetic relationships. Journal of Vertebrate Paleontology. 32(5):1157–1171. doi:10.1080/02724634.2012.677299.
- Murakami M, Shimada C, Hikida Y, Hirano H. 2012b. Two new extinct basal phocoenids (Cetacea, Odontoceti, Delphinoidea), from the upper Miocene Koetoi Formation of Japan and their phylogenetic significance. Journal of Vertebrate Paleontology. 32(5):1172–1185. doi:10.1080/02724634.2012.694337.
- Nelson MD, Uhen MD. 2020. A new platanistoid, Perditicetus yaconensis gen. et sp. nov.(Cetacea, Odontoceti), from the Chattian–Aquitanian Nye Formation of Oregon. Journal of Systematic Palaeontology. 18(18):1497–1517. doi:10.1080/14772019.2020.1783379.
- O'Corry-Crowe GM. 2009. Beluga whale: delphinapterus leucas. In: Perrin WF, Würsig B, Thewissen JGM, editors. Encyclopedia of marine mammals. Storrs, Connecticut: Academic Press; p. 108–112.
- O'Keefe FR, Otero RA, Soto-Acuña S, O'gorman JP, Godfrey SJ, Chatterjee S. 2017. Cranial anatomy of Morturneria seymourensis from Antarctica, and the evolution of filter feeding in plesiosaurs of the Austral Late Cretaceous. Journal of Vertebrate Paleontology. 37(4):e1347570. doi:10.1080/02724634.2017.1347570.
- Perrin WF. 1975. Distribution and differentiation of populations of dolphins of the genus Stenella in the eastern tropical Pacific. Journal of the Fisheries Board of Canada. 32(7):1059–1067. doi:10.1139/f75-125.
- Pyenson ND, Sponberg SN. 2011. Reconstructing body size in extinct crown Cetacea (Neoceti) using allometry, phylogenetic methods and tests from the fossil record. Journal of Mammalian Evolution. 18(4):269–288. doi:10.1007/s10914-011-9170-1.
- Raine JI, Beu AG, Boyes AF, Campbell HJ, Cooper RA, Crampton JS, Crundwell MP, Hollis CJ, Morgans HE, Mortimer N. 2015. New Zealand geological timescale NZGT 2015/1. New Zealand Journal of Geology and Geophysics. 58(4):398–403. doi:10.1080/00288306.2015.1086391.
- Schorr GS, Falcone EA, Moretti DJ, Andrews RD. 2014. First long-term behavioral records from Cuvier’s beaked whales (Ziphius cavirostris) reveal record-breaking dives. PLoS One. 9(3):e92633. doi:10.1371/journal.pone.0092633.
- Scott J, Lee D, Fordyce R, Palin J. 2014. A possible late Oligocene–early Miocene rocky shoreline on Otago schist. New Zealand Journal of Geology and Geophysics. 57(2):185–194. doi:10.1080/00288306.2013.814575.
- Sinha R, Sinha SK, Sharma G, Kedia D. 2010. Surfacing and diving behaviour of free-ranging Ganges River dolphin, Platanista gangetica gangetica. Current Science. 98(2):230–236.
- Tanaka Y, Abella J, Aguirre-Fernández G, Gregori M, Fordyce RE. 2017. A new tropical Oligocene dolphin from Montañita/Olón, Santa Elena, Ecuador. PLoS One. 12(12):e0188380.
- Tanaka Y, Fordyce RE. 2014. Fossil dolphin Otekaikea marplesi (latest Oligocene, New Zealand) expands the morphological and taxonomic diversity of Oligocene cetaceans. PLoS One. 9(9):e107972. doi:10.1371/journal.pone.0107972.
- Tanaka Y, Fordyce RE. 2015a. Historically significant late Oligocene dolphin Microcetus hectori Benham 1935: a new species of Waipatia (Platanistoidea). Journal of the Royal Society of New Zealand. 45(3):135–150. doi:10.1080/03036758.2015.1016046.
- Tanaka Y, Fordyce RE. 2015b. A new Oligo-Miocene dolphin from New Zealand: Otekaikea huata expands diversity of the early Platanistoidea. Palaeontologia Electronica. 18(2.23):1–71.
- Tanaka Y, Fordyce RE. 2016. Papahu-like fossil dolphin from Kaikoura, New Zealand, helps to fill the Early Miocene gap in the history of Odontoceti. New Zealand Journal of Geology and Geophysics. 59(4):551–567. doi:10.1080/00288306.2016.1211540.
- Tanaka Y, Fordyce RE. 2017. Awamokoa tokarahi, a new basal dolphin in the Platanistoidea (late Oligocene, New Zealand). Journal of Systematic Palaeontology. 15(5):365–386. doi:10.1080/14772019.2016.1202339.
- Tanaka Y, Ortega M, Fordyce RE. 2022. A new early Miocene archaic dolphin (Odontoceti, Cetacea) from New Zealand, and brain evolution of the Odontoceti. New Zealand Journal of Geology and Geophysics. 66(1):59–73. https://doi.org/10.1080/00288306.2021.2021956
- Viglino M, Buono MR, Fordyce RE, Cuitiño JI, Fitzgerald EM. 2019. Anatomy and phylogeny of the large shark-toothed dolphin Phoberodon arctirostris Cabrera, 1926 (Cetacea: Odontoceti) from the early Miocene of Patagonia (Argentina). Zoological Journal of the Linnean Society. 185(2):511–542. doi:10.1093/zoolinnean/zly053.
- Viglino M, Gaetán CM, Cuitiño JI, Buono MR. 2021. First toothless platanistoid from the early Miocene of Patagonia: the golden age of diversification of the Odontoceti. Journal of Mammalian Evolution. 28(2):337–358. doi:10.1007/s10914-020-09505-w.
- Werth A. 2000. Feeding in marine mammals. In: Feeding: form, function and evolution in tetrapod vertebrates. Storrs, Connecticut: Academic Press; p. 475–4514.
- Whitaker R, Basu D. 1982. The gharial (Gavialis gangeticus): a review. Journal of the Bombay Natural History Society. Bombay. 79(3):531–548.