Abstract
Fifteen compounds derived from the 2(3H)-benzothiazolone template with an acyl side-chain in position-6 were evaluated for their lipid-lowering action in mice. Among these compounds, 6-benzoyl-2(3H)-benzothiazolone was found to be the most potent one both in mice models receiving a hypercholesterolemic diet (for 15 days) or a standard diet (for 21 days). 6-Benzoyl-2(3H)-benzothiazolone compares favorably with fenofibrate, the standard drug, both in terms of HDL-C/Chol (High Density Lipoprotein-Cholesterol/Total Cholesterol) ratio and absence of liver hepatomegaly.
Introduction
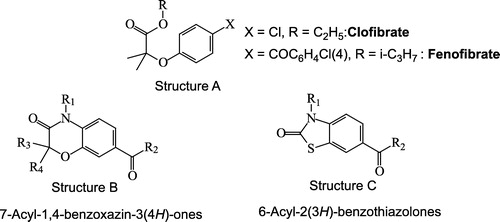
Coronary heart disease (CHD) remains the leading cause of death in the developed world. It is linked to a number of associated risk factors including hypertriglyceridemia and hypercholesterolemia. The distribution of cholesterol among the various plasma lipoproteins is commonly considered as a key index for predicting cardiovascular risk, particularly for atherosclerosis Citation1-6. For several years, the fibrates (, Structure A) have been broadly utilized for the clinical treatment of dyslipidemia and remain the current treatment of choice for patients with severe hypertriglyceridemia [Citation7,Citation8]. These agents can effectively decrease serum triglycerides and elicit increases in HDL-cholesterol concentrations in humans [Citation7]. Several studies have provided evidence that the hypolipidemic effect of this class of drugs is caused by their agonistic effect at the PPARα receptor [Citation9]. In this connection, fibrate-related compounds, which can be regarded as conformationally restrained analogues of the fibrates, incorporating a heterocyclic system instead of the oxyalkanoic moiety have been synthesized Citation10-15. Using this strategy, some 7-acyl-(2H)-1,4-benzoxazin-3(4H)-ones (, Structure B) endowed with lipid lowering properties were discovered Citation16-18. Along the same line of approach, we envisaged the design of even more rigidified heterocyclic fibrate-like compounds. As our group gained over recent years considerable expertise in the chemistry of 2(3H)-benzothiazolone [Citation19,Citation20], we thought it worthwhile to initiate a research program in which the heterocycle present in lipid-lowering benzoxazinones would be associated with a 2(3H)-benzothiazolone ring (, Structure C). In this paper, we report the synthesis and the lipid lowering properties of these 6-acyl-2(3H)-benzothiazolones in mice.
Materials and methods
Chemistry
Melting points were determined using a Büchi 530 melting point apparatus and are uncorrected. The IR spectra were recorded on a Perkin-Elmer 297 spectrometer and the 1H NMR spectra using a Bruker AC 300 spectrometer (LARMN, Universite de Lille 2). Chemical shifts are reported in ppm with tetramethylsilane as internal standard. All compounds were found homogenous in TLC (Merck silicagel 60F254, ethyl acetate/acetone, 60/40 v/v). Elemental analyses were performed by the “Service Central de Microanalyses”, CNRS, Vernaison, France.
Compounds 1–12 were synthesized according to previously described procedures [Citation19].
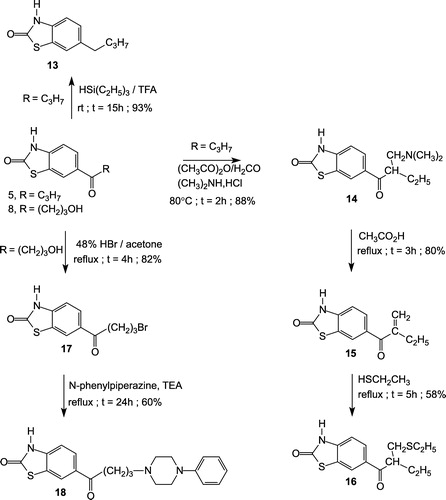
6-n-butyl-2(3H)-benzothiazolone (13)
6-(1-Butyryl)-2(3H)-benzothiazolone 5 (2.35 g, 0.01 mol) was dissolved in trifluoroacetic acid (7.4 mL, 0.1 mol). The solution was stirred at room temperature and triethylsilane (3.5 mL, 0.022 mol) was added dropwise. After 15 h, the mixture was poured onto ice. The resulting precipitate was filtered, washed with water, dried, and recrystallized from cyclohexane to give 13 as a white powder (93%), mp 127–129°C. IR (KBr), cm− 1: 3120 (NH), 1695 (CO), 1650 (CO). 1H-NMR (CDCl3), δ, ppm; J, Hz: 0.95 (t, 3H, J = 7.50, CH2–CH2–CH2–CH3), 1.00–1.75 (m, 4H, CH2–CH2–CH2–CH3), 2.50 (t, 2H, J = 7.50, CH2–CH2–CH2–CH3), 7.00–7.36 (m, 3H, H-4, H-5, H-7), 11.60 (br s, 1H, NH, exchangeable with D2O). Anal. Calcd for C11H13NOS: C, 63.73; H, 6.32; N, 6.76. Found: C, 63.82; H, 6.30, N, 6.80%.
6-[(2-dimethylaminomethyl)butyryl]-2(3H)-benzothiazolone (14)
To a pre-heated solution at 80°C of dimethylamine hydrochloride (6.11 g, 0.075 mol) and 37% aqueous formaldehyde (5.25 mL) were added cautiously and portionwise acetic anhydride (35 mL) and compound 5 (11.06 g, 0.05 mol). The reaction mixture was heated at 80°C for 2 h, and evaporated in vacuo. The residue was partitioned between ethyl acetate (20 mL) and a saturated solution of sodium hydrogencarbonate (60 mL). The resulting precipitate was filtered, washed with water, dried, and recrystallized from ethanol to give 14 as a beige powder (88%); mp 160–162°C. IR (KBr), cm− 1: 3300 (NH), 1690 (CO), 1650 (CO). 1H-NMR (CDCl3), δ, ppm; J, Hz: 0.92 (t, 3H, J = 7.65, –CH2–CH3), 1.66 (m, 2H, –CH2–CH3), 2.33 (s, 6H, N(CH3)2), 2.40 (m, 2H, –CH2–N), 3.60 (m, 1H, CH), 6.85 (d, 1H, J = 8.70, H-4), 7.77 (dd, 1H, J = 1.70, 8.70, H-5), 7.96 (d, 1H, J = 1.70, H-7), 11.00 (br s, 1H, NH, exchangeable with D2O). Anal. Calcd for C14H18N2O2S: C, 60.40; H, 6.52; N, 10.06. Found: C, 60.51; H, 6.48; N, 10.19%.
6-(2-Methylenebutyryl)-2(3H)-Benzothiazolone (15)
A solution of compound 14 (5.56 g, 0.02 mol) in acetic acid (50 mL) was refluxed for 3 h and evaporated in vacuo. The residue was taken up into 1 M HCl (100 mL). The resulting precipitate was filtered, washed with water, dried and recrystallized from toluene to give 15 as a white powder (80%); mp 151–152°C. IR (KBr), cm− 1: 3220 (NH), 1700 (CO), 1635 (CO). 1H-NMR (CDCl3), δ, ppm; J, Hz: 1.10 (t, 3H, J = 7.45, –CH2–CH3), 2.50 (m, 2H, –CH2–CH3), 5.53 (s, 1H, C = CH2), 5.81 (s, 1H, C = CH2), 7.23 (d, 1H, J = 8.50, H-4), 7.79 (dd, 1H, J = 1.40, 8.50, H-5), 7.93 (d, 1H, J = 1.40, H-7), 10.3 (br s, 1H, NH, exchangeable with D2O). Anal. Calcd for C12H11NO2S: C, 61.78; H, 4.75; N, 6.00. Found: C, 61.62; H, 4.79; N, 6.05%.
6-[(2-Ethylthiomethyl)Butyryl]-2(3H)-benzothiazolone (16)
To a freshly prepared solution of sodium ethoxide (2.04 g, 0.03 mol) in absolute ethanol, was added, under nitrogen atmosphere, ethanethiol (1.86 g, 0.03 mol). After stirring for 30 min at room temperature, a solution of compound 15 (2.3 g, 0.01 mol) in absolute ethanol (30 mL) was added dropwise. The reaction mixture was refluxed for 5 h and evaporated in vacuo. Purification was accomplished by column chromatography over silica gel with n-hexane/EtOAc (8:2) to give pure 16 as a yellow oil (58%). IR (KBr), cm− 1: 3200 (NH), 1700 (CO), 1665 (CO). 1H-NMR (CDCl3), δ, ppm; J, Hz: 0.90 (t, 3H, J = 7.55, –CH–CH2–CH3), 1.25 (t, 3H, J = 7.65, –S–CH2–CH3), 1.75 (m, 2H, –CH–CH2–CH3), 2.50 (m, 2H, –S–CH2–CH3), 2.85 (m, 2H, –CH–CH2–S–), 3.60 (m, 1H, –CH–CH2–S–), 7.25 (d, 1H, J = 8.20, H-4), 7.95 (dd, 1H, J = 1.40, 8.20, H-5), 8.10 (d, 1H, J = 1.40, H-7), 10.4 (br s, 1H, NH, exchangeable with D2O). Anal. Calcd for C14H17NO2S2: C, 56.92; H, 5.80; N, 4.74. Found: C, 57.04; H, 5.76; N, 4.62%.
6-(4-Bromobutyryl)-2(3H)-benzothiazolone (17)
To a solution of compound 8 (4.75 g, 0.02 mol) in acetone (100 mL) was added 47% hydrobromic acid (4.67 mL, 0.04 mol). The reaction mixture was refluxed for 4 h and evaporated in vacuo. The resulting precipitate was recrystallized from toluene to give 17 as a beige powder (82%); mp 126–128°C. IR (KBr), cm− 1: 3140 (NH), 1710 (CO), 1670 (CO). 1H-NMR (CDCl3), δ, ppm; J, Hz: 2.30 (m, 2H, –CH2–CH2–CH2–Br), 3.18 (t, 2H, –CH2–CH2–CH2–Br), 3.54 (t, 2H, –CH2–CH2–CH2–Br), 7.22 (d, 1H, J = 8.40, H-4), 7.95 (dd, 1H, J = 1.70, 8.40, H-5), 8.10 (d, 1H, J = 1.70, H-7), 10.10 (br s, 1H, NH, exchangeable with D2O). Anal. Calcd for C11H10BrNO2S: C, 44.01; H, 3.36; N, 4.67. Found: C, 43.87; H, 3.50; N, 4.75%.
6-[4-(4-phenylpiperazin-1-yl)butyryl]-2(3H)-benzothiazolone (18)
To a solution of 17 (6 g, 0.02 mol) in dry acetone (100 mL) were successively added triethylamine (4.2 mL, 0.03 mol), and N-phenylpiperazine (3.24 g, 0.02 mol). The reaction mixture was stirred and refluxed for 24 h, and then evaporated in vacuo. The residue was taken up with water and the resulting precipitate was collected by filtration, washed with water, dried and recrystallized from ethanol to give 17 as a white powder (60%); mp 185–187°C. IR (KBr), cm− 1: 3140 (NH), 1700 (CO), 1670 (CO). 1H-NMR (DMSO-d6), δ, ppm; J, Hz: 1.85 (m, 2H, –CH2–CH2–CH2–N–(CH2)2–(CH2)2–), 2.40–2.50 (m, 6H, –CH2–CH2–CH2–N–(CH2)2 –(CH2)2–), 3.00–3.10 (m, 6H,–CH2–CH2–CH2–N–(CH2)2–(CH2)2–), 6.80–7.40 (m, 6H, H-4, –N–C6H5), 7.85 (dd, 1H, J = 1.60, 8.50, H-5), 8.22 (d, 1H, J = 1.60, H-7), 10.40 (br s, 1H, NH, exchangeable with D2O). Anal. Calcd for C21H23N3O2S: C, 66.12; H, 6.08; N, 11.01. Found: C, 66.02; H, 6.18; N, 11.23%.
Pharmacology
Test on hyperlipemic mice [Citation25]
SWISS OF1 female mice (Iffa Credo), with initial body weight of 26–30 g, were housed in groups of 12 per cage and fed with a hypercholesterolemic diet (U.A.R. 214 B). The compounds were given daily by gastric intubation at 25 mL/kg as a suspension in 1% (w/v) carboxymethylcellulose. They were administered at 300 mg/kg per day for 2 weeks. After fasting (18 h), blood samples were obtained at the end of the treatment by decapitation. Blood was collected on EDTA (1 mg/mL) plasma separated by centrifugation (1000 × g, 15 min), and lipid determinations were made on the same day. The liver was excised, weighed and fixed for histologic examination.
Test on normolipemic mice [Citation25]
SWISS OF 1 female mice (Iffa Credo), with initial body weight of 26–30 g, were housed in groups of 12 per cage and fed with a standard diet (U.A.R. 113). The compounds to be tested were given daily by gastric intubation; they were administrated at a dose of 300 mg/Kg per day for three weeks; test compounds were diluted in carboxymethylcellulose (1% w/v). After fasting (18 h), blood samples were obtained at the end of the treatment by decapitation. Blood was collected on EDTA (1 mg/mL) plasma separated by centrifugation (1000 × g, 15 min), and lipid determinations were made on the same day. The liver was excised, weighed and fixed for histologic examination.
Analytical methods
Plasma lipids (cholesterol, triglycerides and phospholipids) were measured enzymatically, using Boehringer Mannheim kits. HDL cholesterol was measured by the same procedure after precipitating apo B containing lipoproteins with a phosphotungstate-magnesium chloride mixture [Citation26]. In addition, plasma from mice in the same group were pooled and used for lipoprotein preparation. Lipoproteins were fractionated into VLDL (d < 1,006 kg/l), IDL (1,006 < d < 1,019 kg/l), LDL (1,019 < d < 1,063 kg/l) and HDL (1,063 < d < 1,21 kg/l) by sequential ultracentrifugation (2 h, 10°C, 540960 g) in a Beckman TL 100 centrifuge with a fixed angle rotor (TLA 100.3) that holds 6 X 3.0 mL thick-walled polycarbonate tubes. Densities were adjusted with solid KB [Citation27].
Statistical analysis [Citation28,Citation29]
Data from each experiment were evaluated statistically and presented as mean ± SD. The significance of differences between the control and treated group mean was determined using the Snedecor F test and Student t test.
Hepatotoxicity
The liver weight was measured, and the degree of hepatomegaly was evaluated by comparing the weight ratio per l00 g of the body weight with that of the control group, in hyperlipemic and normolipemic mice.
Results and discussion
Chemistry
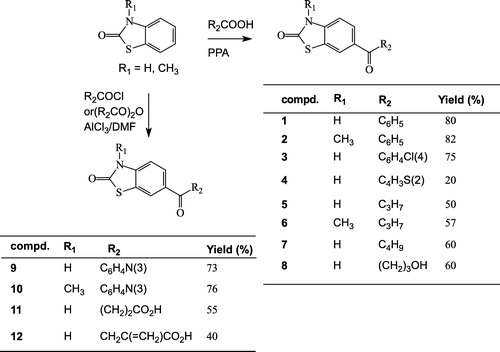
Compounds listed in were synthesized according to Schemes and . Synthesis of the starting materials, i.e. 2(3H)-benzothiazolone and 3-methyl-2(3H)-benzothiazolone, has been described previously [Citation20,Citation21]. For these compounds, two methods are usually employed to get access to the 6-acylated derivatives 1–12 (Scheme ) [Citation19]. The first method uses polyphosphoric acid (PPA) with carboxylic acids and the second one employs the AlCl3/DMF reagent in the presence of acyl chlorides or acyl anhydrides as acylating agents.
Table I. Physico-chemical properties of compounds 1–7, 9–16 and 18.
The alkane derivative 13 was obtained from compound 5 by treatment with the couple triethylsilane/trifluoroacetic acid reagent [Citation22] with 93% yield (Scheme ). The α,β-unsaturated ketone 15 (Scheme ) was prepared with good yields by heating compound 5 with formaldehyde and dimethylamine hydrochloride in acetic anhydride followed by refluxing the intermediate Mannich base 14 in acetic acid. Michaël addition of ethanethiol to the double bound of 15 gave compound 16 with moderate yield. Heating the hydroxy compound 8 in acetone in the presence of 47% aqueous HBr readily gave the bromo derivative 17. Nucleophilic substitution of the latter with phenylpiperazine and triethylamine in acetone led to the desired compound 18 in a 60% yield (Scheme ).
Pharmacology
To investigate the lipid-lowering potentials of these original structures, we used an animal model. Mice were fed either with a standard or hypercholesterolemic diet. This well-established method is fast and economical in terms of the amount of material required to complete a first assessment of the efficacy of hypolipidemic agents.
In a first study on hypercholesterolemic mice (), fifteen compounds were evaluated with regard to body weight, liver/body weight ratio, total cholesterol, triglycerides, phospholipids and HDL cholesterol/total cholesterol ratio. Decrease in total cholesterol was observed for 6 of them, namely fenofibrate (28%), compounds 1 (21%), 5 (16%), 7 (24%), 9 (21%) and 13 (8%), but only fenofibrate and compound 1 increased the ratio HDL-cholesterol/total cholesterol. However, among these products which decrease significantly cholesterolemia, only fenofibrate shows an increase of the liver/body weight ratio (49%). Except compound 9, all the compounds also significantly decrease triglyceride levels. The data reported in also permit some observations regarding the structure–activity relationships (SAR). Increasing the lipophilicity or the steric hindrance on the benzothiazolinone nitrogen with a methyl group results in a decrease in hypocholesterolemic activity. This feature is confirmed by comparing the total cholesterol rates of some pairs of analogues such as 1 and 2, 5 and 6, and 9 and 10, in which the unsubstituted derivative is always more active. As shown by comparing compounds 5 and 13, reduction of the ketone carbonyl into methylene has no effect on hypocholesterolemic activity. In a second study performed in normolipemic mice (), all the compounds, apart from fenofibrate and compound 18, significantly increase triglyceridemia. Another finding was the variability of the results concerning cholesterolemia. Fenofibrate and compounds 6, 7, 9, 10, 11, 13 and 15 significantly increase total cholesterol, whereas a decrease was observed for compounds 3, 5 and 16. Compound 1 apparently does not modify the total cholesterol compared to control. For all compounds, no significant differences considering HDL-cholesterol/total cholesterol ratio were seen. Nevertheless, all compounds except 11 increase the liver/body weight ratio. The major increase takes place upon fenofibrate administration (120%). This figure is rather high and this emphasizes the interest of compound 1, which shows a more modest increase of liver/body weight ratio (42%). In this regard, the behavior of fenofibrate in our study is not surprising since most lipid-lowering agents in rodents are known to induce hepatomegaly, proliferation of hepatic peroxisomes and, as a sequel of this proliferation, a high frequency of hepatic tumors in long term treated animals [Citation23,Citation24].
Table II. Effects of treatments (15 days) on plasma lipids, body and liver weights in mice receiving hypercholesterolemic diet.
Table III. Effects of treatments (21 days) on plasma lipids, body and liver weights in mice receiving standard diet.
Conclusion
In conclusion, among the 2(3H)-benzothiazolones explored above, compound 1 exhibits a favourable profile with a quite noticeable impact on total cholesterol, and HDL-cholesterol levels in mice fed with a hyperlipemic diet without inducing damaging hepatomegaly, and this is in contrast with fenofibrate. Lipid-lowering properties of compound 1 associated to low incidence of hepatomegaly can probably be related to an optimum in lipophilicity. Thus, 1 constitutes a lead for the design of novel normolipemic drugs with reduced hepatic toxicity, a drawback commonly encountered among hypolipemic agents such as fibrates. Studies, using binding and transactivation assays, are ongoing to determine whether the molecular mechanism of action of compound 1 involves activation of PPARα.
Acknowledgements
We would like to thank Dr M.-O PLANCKE of Institut Pasteur de Lille (SERLIA) for her help with the biological assay.
References
- Kannel B, Castelli WP, Gordon T. Ann Intern Med 1971; 74: 1–12
- Miller NE, Forde OH, Thelle DS, Mjos OD. Lancet 1977; 1: 965–968
- Goldbourt V, Medalie J. Ann J Epidemiol 1979; 109: 296–308
- Peto R, Yusuf S, Collins R. Circulation 1985; 72: 111–451
- Scott R. Clin Exp Pharmacol Physicol 1997; 24: A26–A28
- Bittner V, Simon JA, Fong J, Blumenthal RS, Newby K, Stefanick ML. Am Heart J 2000; 139: 288–296
- Chong PH, Bachenheimer BS. Drugs 2000; 60: 55–93
- Milionis HJ, Elisaf MS, Mikhailidis DP. Curr Med Res Opin 2000; 16: 21–32
- Staels B, Auwerx J. Curr Pharm Des 1997; 3: 1–14
- Witiak DT, Stratford ES, Nazareth R, Wagner G, Feller DR. J Med Chem 1971; 14: 758–766
- Newman HAI, Heilman WP, Witiak DT. Lipids 1973; 8: 378–384
- Witiak DT, Heilman WP, Sankarappa SK, Cavestri RC, Newman HAI. J Med Chem 1975; 18: 934–942
- Witiak DT, Poochikian GK, Feller DR, Kenfield NA, Newman HAI. J Med Chem 1975; 18: 992–996
- Witiak DT, Kuwano E, Feller DR, Baldwin JR, Newman HAI, Sarlkarappa SK. J Med Chem 1976; 19: 1214–1220
- Goldberg AP, Mellon WS, Witiak DT, Feller DR. Atherosclerosis 1977; 27: 15–25
- Moussavi Z, Lesieur D, Lespagnol C, Sauzières J, Olivier P. Eur J Med Chem 1989; 24: 55–60
- Moussavi Z, Plancke MO, Olivier P, Lesieur D, Fruchart JC, Sauzières J. Clin Chim Acta 1989; 180: 35–44
- Moussavi Z, Depreux P, Lesieur D, Cotelle N, Sauzières J, Plancke MO, Fruchart JC. Farmaco 1991; 46: 339–355
- Yous S, Poupaert JH, Lesieur I, Depreux P, Lesieur D. J Org Chem 1994; 59: 1574–1576
- Diouf O, Depreux P, Lesieur D, Poupaert JH, Caignard DH. Eur J Med Chem 1995; 30: 715–720
- Kanyonyo MR, Ucar H, Issa M, Carato P, Lesieur D, Renard P, Poupaert JH. J Bull Soc Chim Belg 1996; 105: 17–22
- West CT, Donelly SJ, Kooistra DA, Doyle MP. J Org Chem 1973; 38: 2675–2681
- Hansch C, Clayton JM. J Pharm Sci 1973; 62: 1–21
- Lalwani ND, Redy MK, Qureschi SA, Sirotori CR, Abiko Y, Reddy JK. Hum Toxicol 1983; 2: 27–48
- Olivier P, Plancke MO, Marzin D, Clavey V, Sauzieres J, Fruchart JC. Atherosclerosis 1988; 70: 107–114
- Burstein M, Scholnick HR, Morfin R. J Lipid Res 1970; 11: 538–595
- Plancke MO, Olivier P, Clavey V, Marzin D, Fruchart JC. Meth Find Exp Clin Pharmacol 1988; 10: 575–579
- Schwartz D, Lazar P. Eléments de statistique médicale et biologique4ème éditition. Flammarion Médecine-Sciences, Paris 1971
- Scherrer B. Biostatistique. Gaëtan Morin, Chicoutimi 1984