Abstract
Constructed wetlands are an attractive choice for removing arsenic (As) within water resources used for drinking water production. The role of substrate and vegetation in As removal processes is still poorly understood. In this study, gravel, zeolite (microporous aluminosilicate mineral), ceramsite (lightweight expanded clay aggregate) and manganese sand were tested as prospective substrates while aquatic Juncus effuses (Soft Rush or Common Rush) and terrestrial Pteris vittata L. (Chinese Ladder Brake; known as As hyperaccumulator) were tested as potential wetland plants. Indoor batch adsorption experiments combined with outdoor column experiments were conducted to assess the As removal performances and process mechanisms. Batch adsorption results indicated that manganese sand had the maximum As(V) adsorption rate of 4.55 h−1 and an adsorption capacity of 42.37 μg/g compared to the other three aggregates. The adsorption process followed the pseudo-first-order kinetic model and Freundlich isotherm equations better than other kinetic and isotherm models. Film-diffusion was the rate-limiting step. Mean adsorption energy calculation results indicated that chemical forces, particle diffusion and physical processes dominated As adsorption to manganese sand, zeolite and gravel, respectively. During the whole running period, manganese sand-packed wetland filters were associated with constantly 90% higher As(V) reduction of approximate 500 μg/L influent loads regardless if planted or not. The presence of P. vittata contributed to no more than 13.5% of the total As removal. In contrast, J. effuses was associated with a 24% As removal efficiency.
1. Introduction
The exposure of As in drinking water is a worldwide problem, because As is hazardous for human use. In some developing countries (e.g., Bangladesh, India and China), As concentrations in drinking water exceed guidelines for human health protection, causing serious poisoning and even death [Citation1]. In China, more than two million people are exposed to As through contaminated drinking water [Citation2]. The National Drinking Water Standard (GB 5749–2006) decreased the maximal As concentration to 0.01 mg/L [Citation3], which is equal to the permissible limit set by the World Health Organization (WHO) for safe drinking water [Citation4]. In order to avoid carcinogenic and many other adverse health effects, it is important to develop highly efficient, easy-to-operate, cost-effective and environmentally benign As removal techniques.
A variety of conventional and non-conventional techniques (e.g., coagulation and filtration, reverse osmosis, ion exchange, oxidation and precipitation, adsorption, and photocatalysis), their modifications and/or combinations have been tested to remove As from potential drinking water resources [Citation5–Citation7]. The aforementioned As treatment choices, however, are greatly limited to field practice with their inherent disadvantages such as relatively high costs, huge energy consumption rates and high chemical reagent utilisations [Citation7]. In contrast, less energy-consuming and solar-driving ‘green’ treatment systems like phytoremediation and constructed wetlands may be alternatives to remove As from potential drinking water resources owing to their cost-effective and eco-friendly nature.
Constructed wetlands are engineered filter systems that have been designed and constructed to utilise natural processes involving wetland vegetation, substrate and their associated microbial assemblages in treating wastewater [Citation8–Citation12]. These biological filters take advantage of physical, chemical and biological processes occurring in natural wetlands, but do so within a semi-controlled environment [Citation9]. Arsenic removal in constructed wetlands takes place because of plant uptake [Citation13], accretions of wetland soils [Citation14], microbial immobilisation [Citation15], adsorption and retention by substrates [Citation14], and precipitation in the water column [Citation16]. Although macrophytes, soil, detritus and biomass are important sinks for As in the short-term, substrate is the main sink for As in the long-term [Citation14]. In sub-surface-flow wetlands, pollutants including As are in direct contact with the substrate, and adsorption and retention are, therefore, the main pathways of As removal.
Because of the great importance of substrate in As removal, different media including limestone, zeolite, cocopeat and gravel have been studied to assess As removal in constructed wetland filters. Moreover, several industrial by-products or wastes such as blast furnace slag, red mud, fly ash and sanding wastes have been examined as potential As adsorbents in the view of waste recycling or reutilisation [Citation17–Citation20]. The common belief is that aggregates rich in iron, aluminium, manganese and/or copper containing have a strong affinity for As, because they are more prone to form different compounds besides physical adsorption [Citation21,Citation22]. Although adsorption processes of As to various adsorbent materials have been studied in detail, the efficiency of sub-surface-flow wetlands packed with different substrates, or their combinations, has not been sufficiently examined in the scientific literature. Since wetlands with conventional soil or gravel media have been commonly used to treat acid mining wastewater [Citation23], little is known about the performance of using alternative substrates purifying As-polluted drinking water resources.
Wetland plants play a critical role with regard to As removal in constructed wetland filtration systems. Macrophytes, such as Phragmites australis (Cav.) Trin. ex Steud. (Common Reed) and J. effuses, are able to accumulate As and other heavy metals in roots and shoots [Citation14]. In addition to common aquatic plants, some terrestrial plants called As ‘hyperaccumulators’ such as P. vittata and Pityrogramma calomelanos L. (Silver Fern) can also remove a formidable quantity of As from soil and store it in their fronds [Citation24,Citation25]. However, the growth and As removal performance of hyperaccumulators is questionable in sub-surface constructed wetlands because they have different humidity, water content, oxygen availability and nutrient supply conditions when compared to soil. More attention should be paid to determine whether the traditional macrophytes or hyperaccumulators are suitable for the removal of As.
1.1 Rationale, aims and objectives
Previous research indicated that As(III) is thermodynamically unstable and easily converted to As(V) in aerobic environments [Citation26]. Therefore, it is reasonable to consider only As(V) compounds when evaluating As removal in water treatment [Citation27]. Given this context, As(V) has been chosen as the target As species to evaluate the As adsorption characteristics to different wetland substrates including gravel, zeolite, ceramsite and manganese sand. After assessing As adsorption results, tap water spiked with As(V) has been used to test the As removal efficiency in wetland filters packed with ceramsite and manganese sand, and planted with J. effuses and P. vittata, respectively.
Owing to the benefits of small land occupation and relatively good oxygen availability, this study aims to use vertical wetland filters to test their performance concerning As removal, and to assess the roles of different substrates and plants involved in As reduction. The major objectives of the present study were to:
assess the As adsorption capacities and process mechanisms involving different substrates;
compare As removal performances for different substrates in column experiments with each other;
evaluate the effects of traditional macrophytes and hyperaccumulators on As removal using column experiments;
identify the main approaches to As removal in wetland filters based on an annual mass balance calculation; and
assess the roles of other water quality variables including pH, dissolved oxygen (DO) and nutrients in As removal.
2. Experimental
2.1 Materials
Commercially obtained gravel, zeolite, ceramsite and manganese sand were tested as potential wetland substrates with measured porosity values of 45%, 38.6%, 42.8% and 54%, respectively. The corresponding media compositions are shown in . It was found that silicium dioxide (SiO2) dominates the compositions for gravel, zeolite and ceramsite. Manganese sand contained mainly magnesium dioxide (MnO2), iron (III) oxide (Fe2O3) and SiO2 (). The corresponding ranking order of the percentage contents of total metal oxides (mainly MnO2, Al2O3 and Fe2O3) for different substrates was as follows: manganese sand > ceramsite > zeolite > gravel. All chemical reagents were of analytical grade and used without further purification. All aqueous solutions were prepared using ultra-pure water. An As(V) stock solution with an As concentration of 1000 mg/L was prepared using Na2HAsO4.7H2O. Parts of the stock solution were subsequently diluted to the required concentrations for conducting As adsorption and column experiments. Nutrient stock solutions containing 1000 mg/L ammonia-nitrogen (NH4-N) and 300 mg/L ortho-phosphate-phosphorus (PO4-P) were prepared using NH4Cl and KH2PO4, respectively. These solutions were used to supply the required nutrients to allow for wetland plant growth after adequate dilution. Juncus effuses and P. vittata were both bought from a local botanical garden.
Table 1. Major mineral composition of the different tested substrates.
2.2 Batch adsorption experiments
2.2.1 Outline of experiments
Batch adsorption experiments were performed to obtain kinetic and isotherm data for all substrates with grain sizes of less than 0.25 mm. The Brunauer–Emmett–Teller surface area was determined by a Nova 4200e surface area analyser obtained from Quantachrome (http://www.quantachrome.co.uk). Regardless of kinetic or isotherm adsorption, each experiment was conducted in triplicates. For all adsorption kinetic experiments, 0.5 g of substrate was placed in a 50 mL polyethene bottle containing 20 mL of 1000 μg/L As solution. Each solution was continuously shaken at 200 rpm (r = 2) within a water batch at 25°C for 0.25, 0.50, 1.00, 2.00, 4.00, 8.00, 12.00, 24.00 and 48.00 h. Suspensions were centrifuged for 10 minutes at a speed of 3500 rpm (r = 1895). The As remaining in the supernatant was determined using the atomic fluorescence spectrometer AF-610A (Beijing Rayleigh, Beijing, China) [Citation28].
For all adsorption isotherm experiments, 0.5 g of substrate was placed in a 50 mL polyethene bottle containing 20 mL of a solution with different initial As concentrations (0, 50, 100, 200, 400, 600, 800 and 1000 μg/L). Each solution was shaken at 200 rpm (r = 2) within a water batch at 25°C for 24 h. Arsenic in suspension was centrifuged and subsequently determined using the same procedure and method mentioned above. The adsorption capacity (μg/g) at equilibrium was calculated using Equation (1).
Data collected from the batch adsorption experiments were expressed as means and standard deviations (SD). The data were further fitted with various adsorption kinetic and isotherm models to help understand the As adsorption process and mechanisms to different tested substrates.
2.2.2 Adsorption kinetic simulations
Pseudo-first-order and pseudo-second-order adsorption kinetic models were applied to evaluate the kinetic order of the adsorption process. The specific rate equations are shown in Equations (2) and (3) [Citation29].
The rate-limiting step of the substrate adsorption process can be calculated using first-order kinetic data [Citation30]. Assuming spherical geometry of the adsorbent, the calculated pseudo-first-order rate constant was utilised to correlate with the pore diffusion (Equation 4) and film diffusion coefficients (Equation 5). The term is the time (s) required to bring down the As concentration to half the initial concentration.
The relationship between and
(overall reaction rate constant) can be described in Equation (6) [Citation31]. Values of
can be calculated with
obtained from Equation (2).
2.2.3 Adsorption isotherm simulations
Langmuir and Freundlich isotherms were used to simulate the adsorption isotherms of each wetland media used in the experiment. The linear forms of Langmuir and Freundlich equations can be expressed in Equations (7) and (8):
In order to characterise the type of As adsorption to different substrates, the data were applied to the Dubinin-Radushkevich (D-R) isotherm [Citation32], which can be expressed as Equations (9) and (10).
The constant kDR indicates the mean free energy of adsorption per molecule when it is transferred to the surface of the solid from the solution, and can be calculated using Equation (11), where is the mean sorption energy (kJ/mol) providing important information about the physical and chemical nature of the adsorption process.
2.3 Column experiments
After selecting the wetland substrates, ten analogous wetland filters were designed, constructed and operated predominantly to assess As removal as a function of nutrient supply between May 2012 and May 2013. All experimental wetlands were located outside on the open balcony (3 m above the ground level) of the Changjiang River Scientific Research Institute (Wuhan, China) to allow exposure to natural climatic conditions. The rig was constructed using polyethylene columns of 120 mm diameter and 750 mm height. The outlet valves were located at the centre of the bottom plate of each wetland column, and connected to 12 mm internal diameter vinyl tubing. This arrangement was used to allow for manual flow adjustment, and collection of outflow sample water at the same time.
The ten experimental constructed wetland columns were labelled either A, B, C, D, E or F. Wetlands A, B, C and D were planted and operated in duplicates, while wetlands E and F were operated as unplanted controls without duplicates. The packing orders of all wetlands were the same. Wetland columns A, B and E were filled with 0.2 m deep gravel (diameter between 10 and 50 mm) representing the bottom layer, and 0.4 m deep ceramsite (diameter between 8 and 10 mm) at the top. The compositions for columns C, D and F were the same except for the replacement of ceramsite by manganese sand (diameter between 3 and 4 mm).
The roles of J. effuses and P. vittata in removing As were also assessed. Constructed wetlands A and C were planted with J. effuses, while wetlands B and D were vegetated with P. vittata. Each tested wetland column was arranged with similar plant biomass (approximately 150 g in wet weight) of equal viability and strength.
After nearly one month of As exposure (acclimatisation period) to the wetland plants, all wetlands received 3.5 L of simulated As contaminated drinking water solution (i.e. tap water spiked with 500 μg/L As(V) and nutrients) every three days. This methodology may reduce media clogging and enhance oxygenation. The selected influent As(V) concentration of 500 μg/L is equal to the Chinese wastewater As(V) discharge concentration limit and falls within the moderate As(V) concentration range set for drinking water sources contaminated with As(V) in China [Citation33,Citation34]. The previously prepared nutrient stock solution was used as the predominant source of nutrient supply to enhance plant and microbial growth, and to improve the As removal efficiency of the wetland systems. The controlled supply of nutrients gave the research team the opportunity to assess nutrient requirements for As removal in wetland filters for the first time. The previously prepared nutrient stock solution was added to tap water spiked with As. The solution contained 2 mg/L NH4-N and 0.6 mg/L PO4-P, which was seen as a reasonable for good plant growth and As removal.
Since June 2012, all wetland filters were fully saturated and flooded to a depth of approximately 5 cm and 0.5 cm above the top level of the packing media (ceramsite and manganese sand) in order to reduce the bed media clogging probability and improve the overall oxygen availability. An As(V) loading rate of 583.3 μg/d was used for all systems. The wetlands were fully and quickly drained within a short period of time (less than 5 min) and subsequently refilled in a batch flow mode with a residence time of three days. All samples were collected from the drained water and further analysed on the same day when they were taken for the following parameters: As, NH4-N, PO4-P, pH and DO. Arsenic was determined using the atomic fluorescence spectrometer AF-610A (Beijing Rayleigh, Beijing, China) [Citation28]. The nutrients NH4-N and PO4-P were analysed according to American standard methods [Citation35]; ammonia F phenate method and automated ascorbic acid reduction method, respectively. A YSI 52 dissolved oxygen meter and a HANNA portable pH meter were used for DO and pH measurements.
Data collected from all constructed wetlands were used for statistical analysis to assess the effects of different substrates and plants on As removal and other water quality variables. All statistical tests including a one-way analysis of variance (ANOVA) were performed using the software SPSS [Citation36]. Multiple comparisons were undertaken using the least significant difference (LSD) test, homogeneity of variance test and Duncan’s multiple range test for differences between means. The selected level of significant was p < 0.05. The statistical tests were applied to assess the differences between wetland effluent As, NH4-N, PO4-P and other water quality variables such as pH and DO.
2.4 Annual arsenic mass balance evaluation
All aboveground and belowground macrophyte biomass were harvested after one year of operation to examine the role of plant uptake on As removal in wetland filters. After dividing the biomass into roots, stems, leafs (none for J. effuses) and seeds (none for P. vittata), they were subsequently air-dried to determine the dry weight and prepare sub-sample for further As concentration analysis. Sub-samples of dried fractional biomass were powdered, wet digested with a mixed solution of HNO3 and HCl (ratio of 4 to1), and analysed for As content according to the standardised atomic fluorescence spectrometric method [Citation37] with detection limit of 0.4 μg/L.
No obvious As(V) saturation phenomenon was identified during the first year of operation. In order to briefly assess the annual As retention capacity within different filter media, substrate segments were collected at 15 cm intervals and then fully mixed to obtain a homogeneous sample at the end of the experiment. All of the substrate samples were air-dried, powdered, wet-digested and measured according to Chinese standard methods [Citation38].
An As mass balance was calculated for each wetland by considering the total As-mass input, the total As-mass output including effluent discharge, substrate retention, plant uptake and other unaccountable parts such as microbial assimilation and detritus adsorption. The findings of the mass balance calculation were then used to identify the role of substrates and plants in As reduction.
3. Results
3.1 Arsenic adsorption kinetics
The adsorption rate of As(V) was found to be time-dependant as seen in . The uptake of As(V) increased with reaction time. The adsorption of As(V) was rapid in the first four hours and then slowed down as an equilibrium was reached (), which corresponds to physical and chemical adsorption processes, respectively. The substrate type impacted significant the adsorption process; gravel and zeolite were much faster in approaching the adsorption equilibrium in contrast to ceramsite and manganese sand. After 48 h, the amounts of As(V) removal were 7.58, 15.06, 26.78 and 38.67 μg/g for gravel, zeolite, ceramsite and manganese sand, respectively.
Figure 1. Pseudo-first-order kinetic model fitted for arsenic(V) adsorption to gravel, zeolite, ceramsite and manganese sand as a function of time.
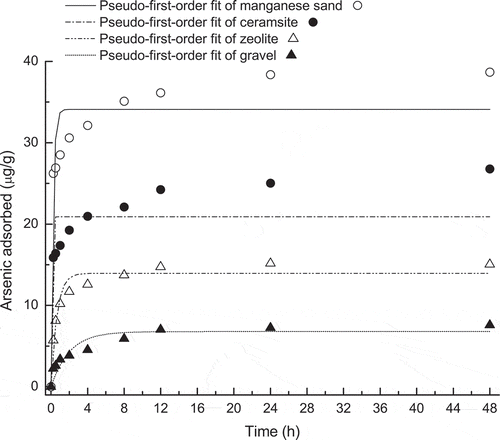
Further kinetic simulation results indicated that the pseudo-first-order equations were suitable for describing As(V) adsorption to all four tested substrates (). Moreover, the ranking order of the calculated As(V) adsorption rate constants based on pseudo-first-order fitting were as follows: gravel (0.50 h−1) < zeolite (1.56 h−1) < ceramsite (3.42 h−1) < manganese sand (4.55 h−1). The coefficients for determining the As(V) adsorption rate limiting steps are shown in . The pore diffusion constants for different substrates ranged from 0.36 × 10−8 cm2/s to 3.27 × 10−8 cm2/s, while film diffusion constants ranged between 0.23 × 10−7 cm2/s and 1.02 × 10−7 cm2/s.
Table 2. Comparison of the pseudo-first- and second- order reaction rate constants for different tested substrates.
Table 3. Calculated pore diffusion and film diffusion constants for different tested substrates.
3.2 Arsenic adsorption isotherm
The adsorption of As(V) was found to be concentration-dependant as seen in . With the initial As(V) concentration of less than 1000 μg/L, the uptake of As(V) increased with As in solution. The findings of the As(V) adsorption isotherm experiments showed that the adsorption capacities of different substrates varied considerably (); e.g., the maximum As(V) adsorption capacities (obtained by the Langmuir equation) were 42.37 μg/g for manganese sand in comparison to 12.7 μg/g for gravel.
Figure 2. Freundlich isotherm equations fitted for arsenic(V) adsorption to gravel, zeolite, ceramsite and manganese sand as a function of the equilibrium aqueous arsenic concentrations.
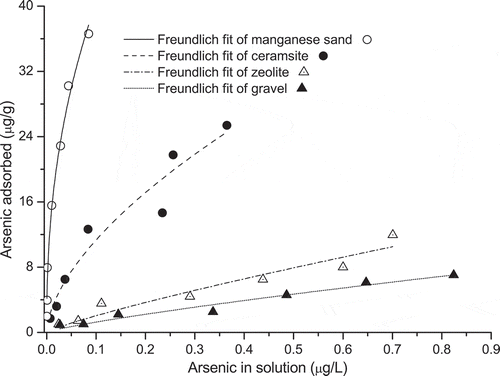
Table 4. Comparison of the correlation coefficients of the Langmuir, Freundlich and Dubinin-Radushkevich (D-R) isotherm for different tested substrates.
The ranking order for the As(V) adsorption capacity () was as follow: manganese sand > ceramsite > zeolite> gravel. After comparing the coefficients of determination (R2) values of the three adsorption isotherms, it was found that As(V) adsorption capacities of all tested substrates were best explained by the Freundlich adsorption isotherm. The corresponding correlation coefficients were 0.95, 0.92, 0.93 and 0.98 for gravel, zeolite, ceramsite and manganese sand, respectively ().
The magnitude of E of As(V) adsorption was calculated () using the Dubinin-Radushkevich (D-R) isotherm. The values of E varied greatly between different substrates; e.g., 184.43 kJ/mol for manganese sand in contrast to 4.66 kJ/mol for gravel.
3.3 Arsenic removal
During the whole experimental period, the recorded maximum precipitation intensity was no more than 200 mm. Natural water loss was attributable to evaporation. A surplus height of 10 to 14.5 cm for each column avoided the occurrence of rain water overflowing accidentally. The monthly mean As(V) removal ratios for wetlands with different substrates and plants are shown in . Throughout the entire experiment, mean removal rates were higher for wetlands with manganese sand (approximately 90%) compared to those containing ceramsite (between about 30 and 80%) as shown in .
Figure 3. Variation in monthly mean arsenic(V) removal ratios for ceramsite-packed wetland filters A (Juncus effuses planted), B (Pteris vittata L. planted) and E (unplanted), and manganese sand packed wetland filters C (Juncus effuses planted), D (Pteris vittata L. planted) and F (unplanted) between June 2012 and May 2013.
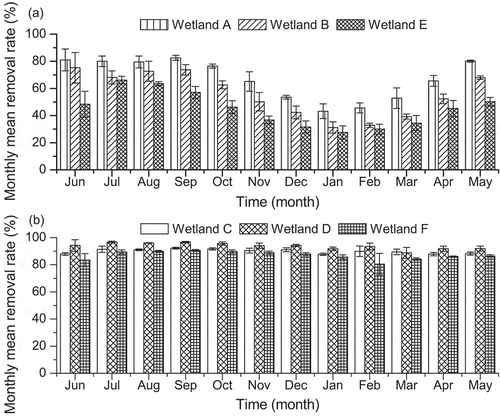
Negligible effects of operation time, seasonal temperature variation and vegetation type on As (V) removal were observed for wetland columns packed with manganese sand ( and ). As (V) was removed with mean efficiency ranges of 88–92%, 88–97% and 80–91% for columns C (planted with J. effuses), D (planted with P. vittata) and F (unplanted), respectively. Wetlands packed with manganese sand and planted with As hyperaccumulators had the most stable and efficient As(V) removal capacity in comparison to other wetland designs.
Table 5. Annual mean concentrations ±SD and pollutant removal efficiencies for Arsenic (As (V)), ammonia-nitrogen (NH4-N) and ortho-phosphorus- phosphate (PO4-P) and other variables including water temperature dissolved oxygen (DO) and pH in the influent and effluent waters of experimental ceramsite packed wetlands A (Juncus effuses planted) and B (Pteris vittata L planted) and E (unplanted) , and manganese sand packed wetlands C (Juncus effuses planted), D (Pteris vittata L planted) and F( unplanted).
In contrast to wetlands filled with manganese sand, the presence of J. effuses and P. vittata lead to an increased mean removal of As(V) by approximately 21% and 10% for wetlands A and B, respectively, if compared to the unplanted wetland E. Moreover, As(V) removal rates increased or decreased with the fluctuations of atmospheric temperature; the highest and lowest As(V) removal rates of 83% and 43% for wetland A occurred in warm September and cold January, respectively ().
3.4 Nutrient removal
Effluent NH4-N and PO4-P concentrations were evaluated to obtain an indication of the nutrient removal efficiency for wetlands treating As(V). Effluent concentrations of NH4-N and PO4-P are summarised in . The concentrations in the effluent were significantly lower than the corresponding ones in the influent, which can be expected.
Effluent NH4-N and PO4-P concentrations were significantly lower in J. effuses and P. vittata planted wetlands A, B, C and D than in the unplanted wetlands E and F (). Moreover, effluent NH4-N and PO4-P concentrations were both significantly higher in wetland F packed with manganese sand than in wetland E containing ceramsite. Regardless of the presence of ceramsite or manganese, NH4-N removal performed well; annual mean removal efficiencies were between 50% and 80%, if approximately 2 mg/L NH4-N were added. The removal of PO4-P in wetlands treating As varied considerably. Unplanted wetlands were associated with less than 40% removal if compared to the influent phosphorus load. Meanwhile, the presence of J. effuses resulted in the maximum PO4-P mean removal of 70% for wetland A packed with manganese sand ().
3.5 Other water quality variables
Changes in the online measured parameters pH and DO were also reported in . In general, the effluent pH was significantly higher in unplanted wetlands E and F than in those wetlands (A, B and C) planted with J. effuses and P. vittata. Wetlands packed with ceramsite had significantly lower pH values than corresponding wetlands packed with manganese (p < 0.05; ). The DO concentrations were significantly higher in planted wetlands than in unplanted ones. However, minor differences in effluent DO concentrations were noted for wetlands planted with the same plant species whether ceramsite or manganese sand was present.
3.6 Annual arsenic mass balance evaluation
Total As biomass production and As removal by plant harvesting is summarised in . Substrate types had little importance in wetland plant uptake of As. Juncus effuses seeds accumulated the highest As concentrations compared to the corresponding roots and stems, while As levels in P. vittata leafs were between 57 and 61 times and between 4.6 and 5.6 times higher compared the levels in roots and stems (), respectively. Total As removal showed great variability with different substrate and plant combinations (). Values of As uptake by wetland plants reached the minimum and maximal level of 41.56 mg/m2 and 804.01 mg/m2 for constructed wetlands C (J. effuses planted in manganese sand) and D (P. vittata planted in manganese sand), respectively.
Table 6. Total arsenic (As) biomass production in ceramsite packed wetlands A (Juncus effuses planted) and B (Pteris vittata L planted), manganese sand packed wetlands C (Juncus effuses planted) and D (Pteris vittata L planted), and total As accumulation by plant uptake after twelve months running.
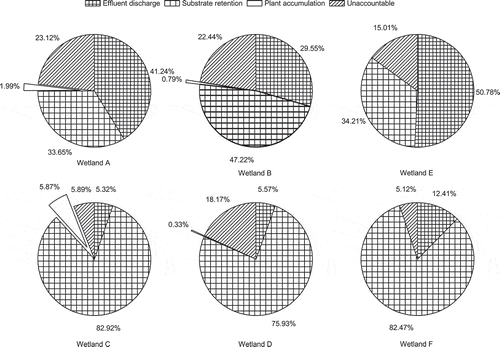
Annual As mass balance calculations were carried out for each wetland unit. As shown in , substrate adsorption and retention contributed most to As removal in manganese sand-packed wetlands with removals of 82.92%, 75.93% and 82.47% for wetlands C, D and F, respectively. Wetland plants played an insignificant role in As reduction. The highest and lowest removal rates of 5.87% and 0.33% were recorded for P. vittata-planted wetlands C and D, respectively. Except for effluent discharge, substrate retention and plant accumulation, unaccountable As removal caused by processes including microbial assimilation and detritus adsorption accounted for between 15.01% and 23.12% and between 5.12% and 5.89% of the total reduction in ceramsite and manganese packed wetlands (), respectively.
Figure 4. Mass balance of arsenic (As) evaluated as a percentage of the inflowing total As mass in the experimental wetland filters A, B, C, D, E and F.
A brief As mass balance analysis for constructed wetlands with different substrates and plant designs was made in this study. Environmental field managers may benefit from results obtained for different filter media types, packing orders and depths, macrophyte types and planting intensities, harvesting seasons, and operation parameters such as hydraulic residence time as discussed in the next section.
4. Discussion
4.1 Arsenic adsorption processes and mechanisms
From the available literature, it appears that the use of suitable specific sorbent media to enhance the removal of As within constructed wetlands has been poorly studied, although various media have been investigated for As adsorption [Citation14,Citation17–Citation22]. The first criterion in comparing the As removal performances of different substrates was usually the maximum As adsorption capacity, which can be obtained from batch adsorption experiments. In this study, calculated maximum As adsorption capacities (obtained from Langmuir isotherms; ) for different materials ranged between 12.70 μg/g and 42.37 μg/g. These values were comparable to a number of adsorbents such as zeolite of 8 μg/g [Citation39], FeCl3 treated clinoptilolite of 9.2 μg/g [Citation40], ferruginous manganese ore of 15.38 μg/g [Citation21], granular ferric hydroxide of 160 μg/g [Citation41] and laterite soil of 180 μg/g [Citation42].
The Brunauer–Emmett–Teller surface areas measured in this study ranged between 0.89 and 1.02 m2/g. No significant difference in the outer surface area for each media assessed for As adsorption was recorded. Note that a 0.25 mm mesh sieve was applied for all media. With a similar outer surface, concentrations of iron, aluminium and manganese may greatly determine As adsorption [Citation21,Citation22]. The order of metal oxides in mirrored the sequence for maximum As adsorption capacity. It follows that iron, aluminium and manganese contents can be used as effective indicators to help with a preliminarily evaluation of the As adsorption capacity.
During the As adsorption process, iron, aluminium and manganese are prone to form different compounds besides physical adsorption. Amorphous hydrous ferric oxide (FeOOH), hydrous aluminum oxide (AlOOH) and hydrous manganese oxide (MnOOH) are promising effective adsorptive materials for As removal from water [Citation5,Citation42]. The iron(III) oxide surface had a high affinity for As(V) and was capable of forming inner-sphere bidentate binuclear As(V)-Fe (III) [Citation43]. Arsenic adsorption by iron complexes occurred by ligand exchange of the As species for (OH)2 and OH- in the coordination spheres of surface structural Fe atoms [Citation44]. Manganese dioxide such as MnO2 can oxidise As(III) to As(V), and then adsorb the As(V) reaction product onto its solid phase. The most likely As(V)-MnO2 complex is a bidentate binuclear corner-sharing (bridged) complex occurring at MnO2 crystallite edges and interlayer domains. There is a potential advantage of using manganese dioxide to treat waters contaminated by As(III) and As(V) [Citation45].
Adsorption kinetics and isotherms show large dependencies on the physical and/or chemical characteristics of the sorbent material, which also influenced the adsorption mechanism [Citation46]. As shown in and , the observed good correlation coefficients indicate that the As uptake process can be approximated by the pseudo-first-order kinetic model and Freundlich isotherm equation. For film diffusion to be the adsorption rate-limiting step, the value of the film diffusion co-efficient () should be in the range of 10−6 to 10−8 cm2/s, and for pore diffusion to be rate-limiting, the pore diffusion coefficient (
) should be in the range of 10−11 to 10−13 cm2/s [Citation47]. In the present study, film diffusion appeared to be the rate-limiting step for the As adsorption kinetic process ().
The mean adsorption energy findings provide important information about the physical and chemical nature of the adsorption process. If < 8 kJ/mol, physical adsorption dominates the adsorption process. If
> 16 kJ/mol, chemical adsorption is the dominant factor. If
is between 8 and 16 kJ/mol, the adsorption process is dominated by particle diffusion [Citation48]. Based on the judgment rule mentioned above, As adsorption to ceramsite and manganese sand was dominated by chemical forces. Physical processes determined the As adsorption onto the gravel surface, while particle diffusion was the main mechanism for As adsorption onto zeolite (). When relating As maximum adsorption capacity to the mean adsorption energy, it can be shown that substrates with bigger mean adsorption energy have higher maximum adsorption capacities.
4.2 Role of substrates and plants in arsenic removal
Substrates played a key role in As removal within constructed wetlands. Adsorption, precipitation and co-precipitation of As on hydrous oxides of metals was a major sink for As fixation. Filter media with a relatively high porosity usually have larger outer surface areas and therefore higher As adsorption capacities [Citation49]. The relatively high porosity of manganese sand (54%) may partly improve As removal owing to adsorption when compared to ceramsite. In oxidising environments with high levels of As(V), precipitation of As(V) with Ca, Mg, Al and Fe(III) may occur [Citation50]. Trapping within porous filter media and trapping with Fe and Mn on the substrate surface are the major As removal mechanisms in constructed wetlands [Citation51]. Gravel is the most commonly used wetland aggregate supporting As removal. However, the As adsorption capacity is low; i.e. in the range of up to 4.3 μg/g [Citation51]. This value is smaller than 12.70 μg/g, which was obtained in this study. However, a relatively smaller grain size was applied ().
Fe can act as a co-precipitation agent for As, particularly in the oxic zones [Citation52]. In constructed wetlands, As usually adsorbs onto the surface of substrates, mineral particles, (oxy)(hydr)oxides and organic matter [Citation23]. Iron oxide tezontle has been found to remove As. The total As mass removal efficiency during the first three months was 57.7% in unplanted sub-surface-flow constructed wetlands packed with tezontle [Citation53]. In comparison to this study, ceramsite and manganese sand contributed between 33.65% and 47.22% and between 75.93% and 82.92%, respectively, to total As removal (). High As removal performances for manganese sand during the whole running period of this study confirmed that As precipitation and adsorption onto most metal (hydr) oxides (especially onto Fe and Mn oxyhydroxides) were likely the main As removal mechanisms [Citation54]. Materials rich of Fe, Al and Mn (oxy)(hydr)oxides had great potential in removing As.
The main role attributed to macrophytes in terms of As removal has been the presence of a highly diverse microbial community within the root zone, which mediates a variety of removal mechanisms. The removal efficiency of As is significantly higher (p < 0.001) in constructed wetlands planted with Phragmites australis. This is particularly the case for gravel-packed wetlands [Citation12]. Wetlands planted with J. effuses have a substantially higher As retention capacity (59–61% of the total As inflow) than wetlands without plants (only 44%) [Citation11]. This study shows that experiments also indicate that the presence of J. effuses can improve As removal efficiencies by nearly 2.64% and 21.32% () for wetlands packed with ceramsite and manganese sand, respectively. However, the introduction of the As hyperaccumulator P. vittata resulted in no more than 9.64% and 6.88% As(V) reductions when compared to corresponding unplanted wetlands. Moreover, annual As mass balance analysis results further revealed that P. vittata contributed no more than 1% to the total As removal regardless if the wetland was packed with ceramsite or manganese (). Findings imply that terrestrial As hyperaccumulators cannot exhibit their best capacity in As accumulation within fully water-saturated constructed wetlands. Although, P. vittata survived and grew normally at sufficient As exposure.
4.3 Relationship between effluent water quality and arsenic removal
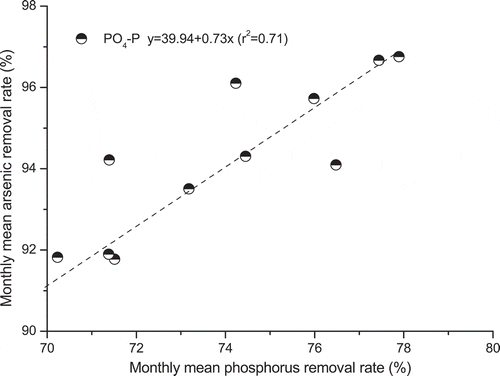
Planted wetlands removed NH4-N and PO4-P significantly from As-polluted influent, because these nutrient species are easily removed by wetland plants and microbes [Citation55,Citation56]. Furthermore, linear regression analysis results for representative wetlands D indicated that As removal was significantly and positively correlated to PO4-P reduction rates (). Adequate phosphorus supply is of particular importance to maintain the normal growth of wetland plants and associated microorganisms [Citation52]. In this study, phosphorus addition may encouraged plant and microbial growth, and thus indirectly enhanced As removal. Competitive As (V) adsorption to substrate is widely reported [Citation23].
Figure 5. Higher monthly mean arsenic(V) removal rates observed at higher ortho-phosphate-phosphorus (PO4-P) removal rates for the selected representative wetland D.
The major role of wetland plants in heavy metal removal is not indirect uptake but substrate stabilisation and bed media oxidation [Citation57]. Root oxygen release led to elevated oxygen availability within the planted wetland (), which favoured the immobilisation of As compared to the unplanted wetlands [Citation11]. Significantly higher DO levels clearly indicated that planted wetlands exhibited more oxidised conditions than unplanted wetlands since the plants were directly involved in the diffusion of oxygen via the aerechyma to the rhizomes [Citation58], although the detailed processes involving macrophytes are still debated [Citation59]. Precipitation of As with metal oxides resulted in a pH decline of wetland effluent when compared to the influent (). Similar findings were also reported previously [Citation11].
4.4 Arsenic mass balance and saturated filter media
Compared to unplanted wetlands, annual As mass balance calculations indicated that the presence of wetland plants improved As-bonding capacity [Citation11], and thus reduced the amount of As flushed out through effluent discharge (). Moreover, substrate retention contributed to the majority to As removal in wetland filters whether planted or not (). Similar finding were also reported elsewhere [Citation60] indicating that As complexation with Fe and Mn on the media surface was 31% and 38%, respectively. Moreover, As trapping within wetland substrate was 42% and 52%, respectively, of total As.
In the present study, the As content in aboveground parts including seeds, leafs and stems was higher than in the corresponding belowground parts such as roots (). This finding is in disagreement with previous studies [Citation57,Citation60], which indicate that the belowground biomass accumulated significantly more heavy metals compared to the corresponding aboveground biomass. A possible reason could be that J. effuses and P. vittata were harvested in the growing season (May) in the present study, which is associated with a high translocation efficiency from belowground biomass to aboveground parts [Citation55].
Compared to gravel, excellent As adsorption performance of ceramsite and manganese sand may reduce the importance of wetland plants in As removal. Wetland plants only contributed to less than 6% of the total As removal (), which was significantly lower than reported for gravel bed wetlands, where figures were around 50% [Citation14]. Similarly low plant uptake rates can also be found elsewhere [Citation61], indicating that only 2% of the As accumulation in the plants was obtained in an experimental constructed wetlands treating electric utility wastewater. Finally, processes including microbial assimilation, detritus adsorption, atmospheric volatilisation and pore water storage may cause the unaccountable parts of As removal [Citation14].
No obvious As saturation phenomenon was detected during the first year of operation. However, it is likely that wetland media will eventually get exhausted, and require change either by replacement with fresh media or through regeneration by reactivation of their adsorption capacity. Replacement is usually the common choice for exhausted wetland filter media while regeneration is very much in an experimental stage. Replacement is more attractive and cost-effective compared to regeneration, because wetlands are usually packed with a large amount of cheap and portable filter media. The relatively small available outer surface area of media such as gravel is indicative of a limited adsorption capacity, particularly if compared to detritus originating from long-term wetland operation. However, a dilute NaOH solution can successfully be used to regenerate media saturated with nano-particles at reasonable cost [Citation62]. For wetland media saturated with As(V), however, this technique may be economically unjustified.
5. Conclusions and further research recommendations
Owing to the small-scale and relatively short running period, the results obtained in this experimental wetland study cannot directly represent the real performance of a large-scale field operations. Nevertheless, the study discussed the As removal performance in wetlands with different substrates and plants, and provides an initial indication of overall performance for wetland designers. Moreover, the findings provide a strong indication that the use of wetland filters as a low-cost option for decontaminating As-polluted drinking water resources in developing countries is a promising alternative to conventional high cost treatment options. Results from batch adsorption kinetics and isotherm experiments indicated that aggregates containing iron, aluminium and manganese show excellent As adsorption performances. For manganese sand, the easy availability and low cost (common industrial waste), fast adsorption rate, and excellent adsorption capacity cut down operational expenditures and make it a good wetland substrate.
During the whole running period, wetlands packed with manganese sand showed more than 90% removal of influent As(V) regardless if planted or unplanted. Differences in water content, microbial community and nutrient supply processes within the root zone micro-environment of wetland filters may be the cause for the unsatisfactory removal of As from the traditional hyperaccumulator P. vittata. Therefore, J. effuses should be considered as an aquatic plant for As(V) removal in the future.
Annual As mass balance analysis indicated that substrate adsorption was the major contributor to As removal in the wetland systems. Direct wetland plant uptake played a negligible role in total As removal, but its indirect function of substrate stabilisation and bed media oxidation can significantly improve the As removal performance when compared to unplanted controls.
Wetland plants showed positive contributions to total As removal. However, further detailed research work should be conducted to identify the optimal plant harvesting season when the above biomass As concentration has reached the maximum value. Adsorption was the main As removal mechanism in the constructed wetlands. Further studies on As saturation are recommended to test the substrate lifetime with a combination of As batch adsorption experiments. The presence of As(V) and different substrates and vegetation change the microbial composition of the biomass, As species as well as their fate and distribution. Studies of the microbial population dynamics, and As translocation, accumulation and reduction mechanism should therefore be conducted. In addition, future works require the evaluation of the effect of loading rate and retention time on As removal in wetland filters.
Acknowledgements
This study was supported by the Central Public Interest Scientific Institution Research Fund (No. CKSF2012056/SH), Ministry of Water Resource, Public Interest Scientific Research Fund (No. 201101027), National Natural Science Foundation of China (No. 51209011 and 51379017), ‘948’ Imported Project of Ministry of Water Resource (No. 201208). The authors greatly appreciate the experimental support provided by Ms. Yunli Lu and Ms. Xue Feng regarding water sampling and online variables analysis.
References
- S. Srivastava, M. Shrivastava, P. Suprasanna and S.F. D’Souza, Ecol. Eng. 37, 1937 (2011).
- J.X. Guo, L. Hu, P.Z. Yand and J. Environm, Sci. Health. Part A, Toxic/Hazard. Subst. Environ. Eng. 42, 1853 (2007).
- Ministry of Health, Sanitary Standard for Drinking Water (GB5749-2006). (Ministry of Health, Beijing, China, 2006).
- World Health Organization, Guidelines for Drinking Water Quality – Recommendations (World Health Organization, Geneva, Switzerland, 2004).
- D. Mohan and C.U. Pittman, J. Hazard. Mat. 142, 1 (2007).
- Y. Liao, J. Liang and L. Zhou, Chemosphere 83, 295 (2011).
- M.I. Litter, M.E. Morgada and J. Bundschuh, Environ. Poll. 5, 1105 (2010).
- J. Vymazal, Sci. Total Environ. 380, 48 (2007).
- M. Scholz, Wetland Systems – Storm Water Management Control (Springer, Berlin, Germany, 2010).
- R.H. Kadlec and S.D. Wallace, Treatment Wetlands, 2nd ed. (CRC Press/Lewis Publishers, Boca Raton, FL, USA, 2009).
- V.A. Tsihrintzis and G.D. Gikas, Wat. Sci. Technol. 61, 2653 (2010).
- D. Zhang, R.M. Gersberg and T.S. Keat, Ecol. Eng. 35, 1367 (2009).
- L. Marchand, M. Mench, D.L. Jacob and M.L. Otte, Environ. Poll. 158, 3447 (2010).
- K.Z. Rahman, A. Wiessner, P. Kuschk, M. van Afferden, J. Mattusch and R.A. Müller, Ecol. Eng. 37, 1214 (2011).
- P. Arroyo, G. Ansola and L.E.S. Miera, Ecol. Eng. 51, 95 (2013).
- K.R. Henken and A. Hutchison, in Arsenic Environmental Chemistry, Health Threats and Waste Treatment, edited by K.R. Henken (John Wiley & Sons Ltd., Chichester, 2009).
- S.R. Kanel, H. Choi, J.Y. Kim, S. Vigneswaran and G.S. Wang, Wat. Qual. Res. J. Can. 41, 130 (2006).
- H.S. Altundogan, S. Altundogan, F. Tumen and M. Bildik, Waste Managem. 22, 357 (2002).
- V.K. Gupta, V.K. Saini and N. Jain, J. Coll. Interf. Sci. 288, 55 (2005).
- J.W. Lim, Y.Y. Chang, J.K. Yang and S.M. Lee, Coll. Surf. A: Physicochem. Eng. Asp. 345, 65 (2009).
- S. Chakravarty, V. Dureja, G. Bhattacharyya, S. Maity and S. Bhattacharjee, Wat. Res. 36, 625 (2002).
- S.K. Maji, Y.H. Kao and C.W. Liu, Desalination 280, 72 (2011).
- A.K. Lizama, T.D. Fletcher and G. Sun, Chem. Eng. J. 179, 119 (2012).
- L.Q. Ma, K.M. Komar, C. Tu, W. Zhang, Y. Cai and E.D. Kennelley, Nature 409, 579 (2001).
- P.A. Gulz, S.K. Gupta and R. Schulin, Plant Soil 272, 337 (2005).
- D. Van Halem, S.G.L. Heilman, G.L. Amy and J.C. van Dijk, Desalination 248, 241 (2009).
- Q. Li, X.T. Xu, H. Cui, J.F. Pang, Z.B. Wei, Z.Q. Sun and J.P. Zhai, J. Environm. Managem. 98, 98 (2012).
- Ministry of Water Resource, Water Quality-Determination of Arsenic-Atomic Fluorescence Spectrometric Method (SL 327.1-2005) (Ministry of Water Resource, Beijing, China, 2005).
- Y.S. Ho and G. McKay, Proc. Biochem. 34, 451 (1999).
- S.K. Maji, A. Pal and T. Pal, J. Hazard. Mat. 151, 811 (2008).
- W.E. Asher and J.F. Pankow, Environm. Sci. Technol. 25, 1294 (1991).
- S. Kundu and A.K. Gupta, Separat. Purif. Technol. 51, 165 (2006).
- Ministry of Environmental Protection, Integrated Wastewater Discharge Standard (GB8978-1996) (Ministry of Environmental Protection, Beijing, China, 1996).
- M.G. Fan, China Wat. Wastewat. 8, 46 (1999).
- APHA, Standard Methods for the Examination of Water and Wastewater. (American Public Health Association (APHA)/American Water Works Association/Water Environment Federation, Washington DC, USA, 1998).
- SPSS, Analytical Software (Statistical Package for the Social Sciences (SPSS) Headquarters, 2003).
- L. Huang, J. Analyt. Sci. 20, 109 (2004).
- Standardization Administration of the People’s Republic of China, Soil Quality Analysis of Total Mercury, Arsenic and Lead Contents in Soils. Atomic Fluorescence Spectrometry – Part 2: Analysis of Total Arsenic Contents in Soils (GB/T 22105.2-2008) (Standardization Administration of the People’s Republic of China, 2008).
- M.J. Jimenez-Cedillo, M.T. Olguin and C. Fall, J. Hazard. Mat. 163, 939 (2009).
- M.B. Baskan and A. Pala, Desalination 281, 396 (2011).
- T.,T. Viraraghavan and K.S. Subramanian, Water SA 29, 161 (2003).
- L.C. Roberts, S.J. Hug, T. Ruettimann, A.W. Khan and M.T. Rahman, Environm. Sci. Technol. 38, 307 (2004).
- T.V. Nguyen, S. Vigneswaran, H.H. Ngo and J. Kandasamy, J. Hazard. Mat. 182, 723 (2010).
- M.G. Macedo-Miranda and M.T. Olguin, J. Incl. Phenom. Macrocycl. Chem. 59, 131 (2007).
- B.A. Manning, M. Hunt, C. Amrhein and J.A. Yarmoff, Environm. Sci. Technol. 36, 5455 (2002).
- D. Ranjan, M. Talat and S.H. Hasan, J. Hazard. Mat. 166, 1050 (2009).
- L.D. Michelson, P.G. Gideon, E.G. Pace and L.H. Katal, US Department of Industry, Office Water Res. Technol. 74, 1 (1975).
- M.E. Argun, S. Dursun, C. Ozdemir and M. Karatas, J. Hazard. Mat. 141, 77 (2007).
- G. Zhang, Z. Ren, X. Zhang and J. Chen, Wat. Res. 47, 4022 (2013).
- K.R. Henken, Arsenic in natural environments, in Arsenic Environmental Chemistry, Health Threats and Waste Treatment, edited by K.R. Henken (John Wiley & Sons Ltd., Chichester, 2009).
- C. Singhakant, T. Koottatep and J. Satayavivad, Wat. Sci. Technol. 60, 1771 (2009).
- S. Buddhawong, P. Kuschk, J. Mattusch, A. Wiessner and U. Stottmeister, Eng. Life Sci. 5, 247 (2005).
- F. Zurita, C.D. Toro-Sánchez, M. Gutierrez-Lomelí, A. Rodriguez-Sahagún, O.A. Castellanos-Hernandez, G. Ramírez-Martínez and J.R. White, Ecol. Eng. 47, 101 (2012).
- P.E. Kneebone, P.A. O’Day, N. Jones and J.G. Hering, Environm. Sci. Technol. 36, 381 (2002).
- X.Q. Tang, S.L. Huang, M. Scholz and J.Z. Li, Wat. Air Soil Poll. 197, 61 (2009).
- X. Tang, S. Huang, M. Scholz and J. Li, Int. J. Environm. Analyt. Chem. 91, 727 (2011).
- A.I. Stefanakis and V.A. Tsihrintzis, J. Hazard. Mat. 213–214, 393 (2012).
- H. Brix, Wat. Sci. Technol. 35, 11 (1997).
- A. Wieβer, P. Kuschk and U. Stotmeister, Acta Biotechnologica 22, 209 (2002).
- C. Singhakant, T. Koottatep and J. Satayavivad, J. Environ. Sci. Health (Part A) 44, 163 (2009).
- Z.H. Ye, Z.Q. Lin, S.N. Whiting, M.P. De Souza and N. Terry, Chemosphere 52, 1571 (2003).
- D. Ghosh and A. Gupta, Resour. Conserv. Recycl. 61, 118 (2012).
